Abstract
This study aimed to assess the optimal conditions for the extraction of chlorophyll and the stability of chlorophyll with postharvest storage period in Moringa Oleifera leaves harvested in 3 different years for its preservation and lifespan. For this purpose, chlorophylls a (Chla) and b (Chlb) were extracted from the leaves using acetone, methanol, N, N-dimethylformamide (DMF), and the ‘green’ solvent (ethanol). In addition, the chlorophylls were extracted under various conditions, including temperatures (4, 25, and 45 °C), and times (10, 30, and 60 min) from dry leaves that were harvested in different years (2020, 2021, and 2022). The results showed that the Chla content extracted exceeded that of Chlb in the four solvents in all temperatures and extraction times, except for acetone and ethanol extracts under 45 °C at 30 and 60-min extraction times in samples harvested in 2022. An increase in extraction time and temperature resulted in higher chlorophyll content. Overall chlorophyll content decreased with the increasing postharvest storage period, particularly in methanol and ethanol extracts. The Chlorophyll Stability Index showed that chlorophyll is stable in moringa, such that the chlorophyll content obtained in a 2-year postharvest storage period samples was also found to be high. In general, the chlorophyll obtained from this study was found to be compatible with what is used in the industrial market. This suggests that the chlorophyll from moringa is stable and can be considered a major source of chlorophyll.
Similar content being viewed by others
Introduction
One of the world’s current concerns is health and well-being, which is the third sustainable development goal (SDG 3) of the seventeen global goals set by the United Nation and is expected to be attained by 2030. It aims to reduce preventable diseases and premature deaths, ensure healthy lives and promote well-being [1]. There has been growing concern about the types of foods being consumed, particularly the health effects and toxicity of synthetic foods. Consequently, more research within the food and health industries has been directed to examining more natural products, improving overall health, and meeting consumer demand [2]. One way to achieve this objective is to assess natural pigments such as chlorophylls, as they contain high levels of vitamins with antibacterial, antioxidant, and anti-inflammatory properties. In addition, as chlorophyll is renewable, it could potentially play a major role in the sustainability of the food and health industries, as opposed to synthetic pigments, synthetic antioxidants, and synthetic dyes that are toxic to human health. The above-mentioned attributes are good for health and the prevention of certain diseases [3]. For instance, the antioxidant nature of chlorophyll can reduce the oxidation process that produces free radicals (unstable molecules) in the body. Thus, preventing free radicals that produce chain reactions that damage body cells and contribute to the development of chronic diseases. Moreso, the antioxidants neutralize free radicals and reduce oxidative damage (oxidative stress) and its consequences, such as inflammation, arthritis, diabetes, cancer, cardiovascular diseases, neurological conditions, increased pressure on the immune system, and premature ageing [4].
The use of chlorophyll in the food and health industries would depend upon its stability outside the plant cell. When exposed to oxygen, light, heat, extended time outside the plant cell, and high temperatures, it undergoes degradation which consequently results in the limitation of its functional capacity [5]. Furthermore, chlorophyll degradation usually results in a loss of pigment colour in fresh fruits and vegetables, resulting in a decrease in the commercial value of the crop. According to Manolopoulou et al. [6], consumers associate fruit colour, texture, freshness, and ripeness with the level of food safety for consumption. Furthermore, in the health industry, when chlorophyll degrades and changes its chemical structure to its derivatives it loses biological activities [7]. In addition, the instability is in the magnesium centre ion of the chlorophyll molecule where the ion becomes sensitive to degradation, leading to shorter shelf life and preservation of chlorophyll outside the life of the plant. The quicker the chlorophyll degrades, the more it loses its functional abilities [7].
Numerous studies in the literature have examined the use of different techniques and procedures for preserving the shelf life and post-harvest stability of chlorophyll [6, 8, 9]. Some of the procedures include different drying methods (freeze-drying, air-drying, spray-drying, etc.), varying storage temperature, the addition of enhancers (Mg2+, Mn2+, and Zn2+) during the extraction process, and storage of chlorophyll in powder form [6, 8, 9]. Although some of these techniques slow down degradation, plants and crops still undergo biochemical changes after being harvested and stored on supermarket shelves. Therefore, the shelf life of certain plants and crops should be determined to maintain their quality, nutritional content, and plant tissue integrity. One application of chlorophyll is to replace synthetic dyes due to their toxicity, cost and complexity of synthesis and filtration [10,11,12]. Studies have also demonstrated that synthetic dyes have neurological, behavioral, and allergic effects such as sleep disorders, irritability, aggressiveness, and hyperactivity [4, 5]. Whereas natural dyes based on plant chlorophyll are inexpensive, biocompatible, biodegradable, sustainable, and have a simple extraction process [10, 11], but the shelf life is still of concern.
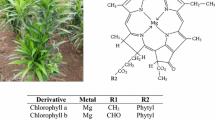
In the food industry and green plants, two types of chlorophyll exist: chlorophyll a (Chla) and chlorophyll b (Chlb). Chla is the main photosynthetic pigment, and Chlb is an accessory pigment [4]. Chla is responsible for energy absorption and conversion, whereas Chlb absorbs sunlight and transfers it to Chla for energy conversion. Chlb is known as an accessory pigment, and the ratio of Chla to Chlb in green plants is noted to be 3:1 by Manolopoulou et al. [6], Kwartiningsih et al. [13] and Ahmadi et al. [4]. The two-chlorophyll a and b differ in the type of functional group attached to the second ring in the four pyrrole rings. This leads to the variation and differences in capabilities, as Chla is bonded with a methyl group (CH3), while Chlb has a formyl group [4, 13]. However, both chlorophylls are crucial for photosynthesis, and the structural difference allows the chlorophylls to cover a wide range of visible light wavelengths, thus allowing them to absorb more sunlight [4]. Consequently, the greater the chlorophyll content, the higher the photosynthetic rate, indicating the direct relationship between the chlorophyll content and the photosynthetic rate [14].
Various extraction techniques such as sonification, water baths, maceration, microwave-assisted extraction, and ultrasound have been integrated into chlorophyll extraction from plants for a predetermined time. In this study, the evaluation of the optimal extraction process and conditions for Moringa Oleifera was studied. This includes comparing Moringa Oleifera leaves harvested during three different years (2020, 2021 and 2022) for its chlorophyll content, degradation, and stability with storage period. M. Oleifera, also commonly known as the “Miracle tree” and “Horseradish tree”, is of interest in this study due to its greenness, fast-growth rate, large biomass, and drought-tolerant properties [15]. Past research indicated that a Moringa tree can reach a height of 4 m in 6 months to a year and 6–15 m at maturity [16, 17]. In addition, the leaves of the tree can be harvested every 40 days and yield approximately 4.2–8.3 tons. Ha-1 of dry leaves [17]. The plant has been studied for various applications in various sectors, such as human nutrition, livestock feeding, human and veterinary medicine, bio-stimulant, etc. [15,16,17]. However, based on the authors’ knowledge, limited research has been conducted on its chlorophyll content, degradation with postharvest storage period, and optimal extraction process of chlorophyll. This study evaluated the effects of extraction time, extraction temperature, and the use of various organic solvents in the extraction of chlorophyll from M. Oleifera. The feasibility of a ‘green’ extraction process utilizing ethanol as a solvent is also evaluated. Furthermore, the stability of the extracted chlorophyll is assessed over time for its preservation and shelf life.
Materials and methods
Materials
Samples of M. Oleifera leaves of different postharvest storage period were utilized in this study. The postharvest storage periods of M. oleifera were 0 years (freshly harvested), one year, and two years, all harvested from Hammanskraal, Gauteng Province, South Africa (25.4132° S, 28.2578° E). The mature leaves were collected in 2020, 2021, and 2022, respectively, and all were harvested in January during the summer season. The plant material was collected from 15 individual trees and chosen randomly on the farm. The leaves were washed, air-dried for 120 h at 45 °C, covered with aluminium foil, put in a black polystyrene bag, and kept in dark conditions at room temperature until use. Before use, the dried leaves were powdered using a coffee grinder such that 80% by volume of the particle size was − 240 μm measured using a particle size analyzer (Malvern Mastersizer 2000). The experiments conducted on samples from each individual tree were replicated three times, resulting in a sample size of 45 (N = 45). Methanol, Ethanol, Acetone, and N, N-dimethylformamide (DMF) in HPLC grades (> 99.9%) were used as extracting organic solvents, all procured from Sigma Aldrich in South Africa.
Extraction method
Following pulverization, 0.5 g of the plant material was added to 50 ml of organic solvent (ethanol, methanol, acetone, DMF) and mixed together to form a uniform solution using a laboratory magnetic stirrer for 5 min. After mixing, the chlorophyll pigment was extracted using a water bath at temperatures of 4 °C, 25 °C, and 45 °C. The extraction time was also varied for 10, 30, and 60 min. After extraction, the samples were centrifuged at 2000 rpm for 5 min, and finally, the supernatant was collected.
Chlorophyll measurement
To obtain a pure chlorophyll, centrifuging is essential. Once centrifuged, the supernatant was sent for spectrophotometry at 663 nm and 645 nm for Chla and Chlb absorbance measurements, respectively, using the Genesys 10 S UV-VIS spectrophotometer (Thermo Scientific). The corresponding solvent to the sample type was used as a blank. The chlorophyll measurement content was calculated using formulas provided by Wellburn and Lichtenthaler [18] for the chlorophylls extracted with acetone, ethanol, and methanol, and by Inskeep and Bloom [19] for DMF extracts. Equations (1, 2, 3, 4, 5, 6, 7, 8, 9) represent the equations used for each solvent.
Acetone
Methanol
Ethanol
DMF
\(A\): The absorption at 663 nm and 645 nm
\({C}_{a}\): Chlorophyll a concentration (mg/l) (μg/ml)
\({C}_{b}\): Chlorophyll b concentration (mg/l) (μg/ml)
\(T\): Total chlorophyll concentration (mg/l) (μg/ml); for all other solvents except DMF, it is the summation of chlorophyll a and b.
Chlorophyll Stability Index (CSI)
The CSI was conducted following the method according to Koleyoreas [20] with modifications. According to Mohan et al. [21] the Chlorophyll Stability Index (CSI) is an indication of the stress tolerance capacity of plants. Of which, a heightened CSI value indicates that the imposed stress had minimal impact on the chlorophyll content, signifying an abundance of available chlorophyll as the plant endured the stress [21]. Essentially, it illustrates the effectiveness of chlorophyll under stressful conditions. Moreover, various forms of stressors, including high temperatures, saline soil, water scarcity, and drought, have been examined in the literature to assess chlorophyll stability through the CSI [21,22,23]. In this study, the CSI is a measure of the extent to which chlorophyll pigments undergo degradation during postharvest storage periods. This will be determined by assessing the difference between the chlorophyll content of a fresh sample and that of the same sample subjected to different postharvest storage periods. A higher CSI value denotes greater stability in chlorophyll. The CSI was determined using the harvesting period in three different years (2020, 2021, and 2022). The freshly harvested plant (2022) was the control and compared with the one-year and two-year postharvest storage periods. Equation (10) represents the equation used for CSI.
Data analysis
Statistical analysis was conducted using IBM SPSS Statistics version 23 (SPSS Inc., Chicago, IL, USA) to analyze the data. Each experiment was conducted in triplicate; thus, the results are represented as mean ± standard deviation (SD). The analysis of variance (ANOVA) was used to test for differences between the experimental conditions and was followed by a Tukey post hoc test to determine where the difference lies. The confidence level was set at P = 0.05.
Results and discussion
Chlorophyll a and b ratios based on the extracted chlorophyll
Figure 1a and c depicts the Chla: Chlb ratios for the chlorophyll extracted from M. Oleifera leaves at 4 °C for all postharvest storage periods, at the extraction times (10, 30, and 60 min), and using extracting organic solvents (methanol, ethanol, acetone, and DMF). The same parameter of extraction times and organic solvents was also applied at 25 and 45 °C extracting temperatures. The results (Figs. 1, 2, 3, 4) show that Chla content was higher as compared to Chlb in all four solvents under all extraction times and temperatures, with the exception of acetone and ethanol extracts under 45 °C at 30 and 60-min extraction times in the 2022 harvested samples. The Chla:Chlb ratio was found to increase with the increasing postharvest storage period of the leaves. This is due to the drastic decline in Chlb concentration with increasing postharvest storage period, while Chla concentration showed only a slight decline. The decline in the concentration of Chlb with the postharvest storage period of the leaves shows the deterioration of the plant. Chla concentration being abundant compared to Chlb concentration is consistent with the literature because Chla is the primary photosynthetic pigment in green plants [4, 6, 13]. Ahmadi et al. [4] evaluated the extraction of chlorophyll from alfalfa (Medicago sativa L.) and obtained that Chla was more than Chlb. Manolopoulou et al. [6] extracted the chlorophylls from green peppers and obtained more Chla compared to Chlb. Nguyen et al. [24] also obtained similar results from the Pandan leaf. The degradation of the harvested leaves with postharvest storage period may be due to factors such as environmental conditions, temperature and pH changes, heat, and oxygen because chlorophyll is unstable outside the plant cell [4]. Overall, the chlorophyll content of M. Oleifera leaves aligns with that observed in the literature for other plants. This suggests immediate use of the plant in the year of harvest to minimize the degradation of Chlb over time.
a Chla:Chlb ratios for all postharvest storage periods extracted at 4 °C and 10-mins extraction time. The bar graphs follow the mean ± SE (n = 3) standard, with the symbols representing a significant difference between the extracting conditions (P < 0.05). The double symbols represent a non-significant difference (P > 0.05) between the Chla:Chlb ratios and solvents per postharvest storage period. b Chla:Chlb ratios for all postharvest storage periods ages extracted at 4 °C and 30-mins extraction time. The bar graphs follow the mean ± SE (n = 3) standard, with the symbols representing a significant difference between the extracting conditions (P < 0.05). The double symbols represent a non-significant difference (P > 0.05) between the Chla:Chlb ratios and solvents per postharvest storage period. c Chla:Chlb ratios for all postharvest storage periods extracted at 4 °C and 60-min extraction time. The bar graphs follow the mean ± SE (n = 3) standard, with the symbols representing a significant difference between the extracting conditions (P < 0.05). The double symbols represent a non-significant difference (P > 0.05) between the Chla:Chlb ratios and solvents per postharvest storage period
Effect of extracting solvents on the concentration of extracted chlorophyll
Figures 2, 3, and 4 demonstrate the results of chlorophyll extraction from leaves with different postharvest storage periods, extraction times (10, 30, and 60 min), extraction temperatures (4, 25, and 45 °C), and extracting organic solvents (methanol, ethanol, acetone, and DMF). The results show that each solvent had its strengths and uniqueness. Overall, methanol was observed as the most efficient solvent for extracting Chla pigment, followed by DMF, ethanol-and-acetone, independently. Ethanol and acetone yielded similar results, with DMF illustrating a constant Chla concentration extraction per plant year. Furthermore, based on the variance analysis, there was no significant difference between ethanol and acetone for the Chla concentration (P > 0.05). Contrary to Chla extraction, Methanol was the least efficient solvent for Chlb extraction (Fig. 2a and c), followed by ethanol, acetone-and-DMF, interchangeably with extraction time. Consequently, DMF possesses the highest total chlorophyll concentration (TCC), which is the sum of the chlorophylls (Chla + Chlb), followed by acetone, ethanol, and methanol, respectively. This is because DMF and acetone could dissolve Chla and Chlb efficiently compared to alcohols. In addition, the variance analysis indicated that there was a significant difference between all solvents for the TCC concentration (P < 0.05).
The solvent strength does have an impact on the concentration of both Chla and Chlb. For the fresh leaves, all the solvents extracted relatively good amounts of both Chla and Chlb but decreased as the postharvest storage period increased. The decrease in Chlb was more pronounced than that of Chla, with the extracted amount decreasing by approximately 50% for DMF and acetone in the 1-year postharvest storage period samples. In the alcohol solvents, the reduction of Chlb was significant such that there was no trace of Chlb extracted by methanol. For the 2-year postharvest storage period samples, in all the figures, a similar trend to that of the 1-year postharvest storage period samples was observed. Chla extracted by all solvents was relatively high but there was a drop in Chlb extraction for DMF and Acetone, with no trace of Chlb extracted by either of the alcohols (Methanol or Ethanol), as seen in Fig. 2a and c. Acetone and DMF demonstrated their effectiveness as they were able to extract chlorophyll even from samples with a two-year postharvest storage period in Moringa leaves. The ability of an organic solvent to extract chlorophyll is due to its ability to penetrate the chloroplast’s membrane and dissolve the lipids and lipoproteins in the plant [29]. Therefore, extraction is governed by the polarity and chemical structure of the solvent and pigment to be extracted, which must be aligned. Essentially, ‘like dissolves like’, polar dissolves polar, and non-polar dissolves non-polar. Thus, the extracting solvent of choice must be compatible with the polarity of the structure. The structure of chlorophyll is composed of a phytol hydrophobic tail (non-polar) and a macrocycle hydrophilic head (polar) [9]. Overall, chlorophyll is a polar molecule because of the ester groups and cyclopentanone [9]; therefore, it is extracted through polar solvents such as methanol, ethanol, acetone, DMF and others.
The results obtained in this study suggest that the optimum solvents for chlorophyll extraction were polar aprotic solvents, DMF and Acetone. These solvents have the ability to dissolve the polar and nonpolar parts of the chlorophyll pigment. Only a few studies have been conducted on chlorophyll extraction using DMF, and this is because of its toxic nature. However, the stability of Chla concentration in DMF and its affinity for higher recovery of total chlorophyll recovery led to its utilization [19, 25]. In a study by Suzuki and Ishimaro [26] and Seibeneicher et al. [27] in different plants, the stability of DMF during chlorophyll extraction has been confirmed. From the study conducted by Tomsone and Kruma [28], acetone was reported as the best solvent because it could extract both chlorophylls from the frozen horseradish leaves and frozen horseradish leaves by-products. The authors ascertain that the chemical structures and polarity of the solvents greatly influence the degree of extraction of these pigments.
a Chlorophyll extraction for all postharvest storage periods at 4 °C and 10-min extraction time. The bar graphs follow the mean ± SE (n = 3) standard, with the symbols representing a significant difference between the extracting conditions (P < 0.05). The double symbols represent a non-significant difference, (P > 0.05) between the extracting conditions and solvents per postharvest storage period. b Chlorophyll extraction for all postharvest storage periods at 25 °C and 10-min extraction time. The bar graphs follow the mean ± SE (n = 3) standard, with the symbols representing a significant difference between the extracting conditions (P < 0.05). The double symbols represent a non-significant difference (P > 0.05) between the extracting conditions and solvents per postharvest storage period. c Chlorophyll extraction for all postharvest storage periods at 45 °C and 10-min extraction time. The bar graphs follow the mean ± SE (n = 3) standard, with the symbols representing a significant difference between the extracting conditions (P < 0.05). The double symbols represent a non-significant difference (P > 0.05) between the extracting conditions and solvents per postharvest storage period
Effect of temperature and time on the extraction of chlorophyll
Figures 2, 3 and 4 illustrate all the evaluated experimental conditions for all the postharvest storage periods, including extraction temperatures and times. From the graphs presented (Figs. 3a, c and 4a, c), for the newly harvested leaves, Chla showed an inverse relationship to temperature, and Chlb showed a direct proportional relationship with temperature. Thus, a rise in temperature caused less Chla and more Chlb content produced. However, the decline in Chla was not significant, but the increase in Chlb was significant, so Chlb had a larger impact on the total chlorophyll produced. This suggests that for the fresh leaves, higher temperatures are ideal for high amounts of chlorophyll, but there are limitations. For the 1-year and 2-year postharvest storage periods leaf samples, a similar trend was observed, with the highest temperature producing high amounts of both Chla and Chlb concentrations and the total chlorophyll concentration. However, it is essential to note that the difference in the chlorophyll concentrations with increasing temperature is minimal.
Likewise, in terms of temperature, an increase in extraction time also led to an increase in chlorophyll production. As the temperature and extraction time increased, more Chlb was produced and therefore more total chlorophyll (Chla + Chlb). However, relatively longer extraction times (30 and 60 min) and elevated temperatures (45 °C) combined led to chlorophyll degradation. This was observed in Figs. 3c and 4c, where more Chlb was extracted compared to Chla for acetone and ethanol, whereas DMF and methanol nearly produced more Chlb for those conditions as well. Several authors have also evaluated the impact of temperature on chlorophyll [9, 13]. From the study conducted by Ly et al. [9] on Centella asiatica L at 15 °C, 30 °C, and 60 °C, the author reported that an increase in temperature led to a higher concentration of total chlorophyll. Kwartiningsih et al. [13] also study the effects of temperature on chlorophyll extraction from Katuk leaves with temperatures ranging from 10 to 100 °C. The author reported optimal chlorophyll extraction and chlorophyll pigment yield at 60 °C and a significant decrease subsequently between 60 and 100 °C. The degradation noted above 60 °C is due to the accelerated rate of mass transfer of cellular components to the solvent and the increase in the chlorophyll derivative [29]. With the increase in the chlorophyll derivative, the chlorophyll’s centre magnesium ions are eliminated, leading to loss of the pigment colour and a decrease in the chlorophyll content [4].
Longer extraction times have also been reported to aid the extraction process by allowing the solvents to dissolve the phytochemicals. Siebeneicher et al. [27] reported for Acacia mangium that an increase in extraction time led to the extraction of a greater amount of chlorophyll, but that prolonged exposure duration led to pigment degradation. Marti-Quijal et al. [30] also reported high pigment extraction from Arthospira platensis chlorophyll extraction during longer extraction periods.
a Chlorophyll extraction for all postharvest storage periods at 4 °C and 30-min extraction time. The bar graphs follow the mean ± SE (n = 3) standard, with the symbols representing a significant difference between the extracting conditions (P < 0.05). The double symbols represent a non-significant difference (P > 0.05) between the extracting conditions and solvents per postharvest storage period. b Chlorophyll extraction for all postharvest storage periods at 25 °C and 30-min extraction time. The bar graphs follow the mean ± SE (n = 3) standard, with the symbols representing a significant difference between the extracting conditions (P < 0.05). The double symbols represent a non-significant difference (P > 0.05) between the extracting conditions and solvents per postharvest storage period. c Chlorophyll extraction for all postharvest storage periods at 45 °C and 30-min extraction time. The bar graphs follow the mean ± SE (n = 3) standard, with the symbols representing a significant difference between the extracting conditions (P < 0.05). The double symbols represent a non-significant difference (P > 0.05) between the extracting conditions and solvents per postharvest storage period
a Chlorophyll extraction for all postharvest storage periods at 4 °C and 60-minute extraction time. The bar graphs follow the mean ± SE (n = 3) standard, with the symbols representing a significant difference between the extracting conditions (P < 0.05). The double symbols represent a non-significant difference (P > 0.05) between the extracting conditions and solvents per postharvest storage period. b Chlorophyll extraction for all postharvest storage periods at 25 °C and 60-min extraction time. The bar graphs follow the mean ± SE (n = 3) standard, with the symbols representing a significant difference between the extracting conditions (P < 0.05). The double symbols represent a non-significant difference (P > 0.05) between the extracting conditions and solvents per postharvest storage period. c Chlorophyll extraction for all postharvest storage periods at 45 °C and 60-min extraction time. The bar graphs follow the mean ± SE (n = 3) standard, with the symbols representing a significant difference between the extracting conditions (P < 0.05). The double symbols represent a non-significant (P > 0.05) difference between the extracting conditions and solvents per postharvest storage period
Chlorophyll content of Moringa
The chlorophyll content of plants varies with different types of plants, algae, and plant species. This is a result of various factors, such as different stages of growth and development, and response to environmental factors such as light and nutrient availability [6]. However, the chlorophyll content of plants is important because it impacts its use in industries. Consequently, plants with high chlorophyll content are preferred and may degrade at a slower rate than those with low chlorophyll concentrations. According to Table 1, the maximum chlorophyll extracted from M. Oleifera was \(63 \pm 0. 524\) mg/l, \(46\pm 0.069\) mg/l, and \(32\pm 0.022\) mg/l while the minimum was \(41\pm 0.560\) mg/l, \(26\pm 0.052\) mg/l, and \(14\pm 0.156\) mg/l from the freshly harvested, 1-year, and 2-year leaves, respectively. The results show that the chlorophyll content of the leaves is relatively high compared to some of the results reported in the literature. From the study conducted by Varaprasad et al. [31] highest total chlorophyll of 25.8 mg/l and 23.0 mg/l using ethanol extracts from C. reinhardtii and C. vulgaris, respectively, was obtained. In another investigation by Nguyen et al. [24] a total chlorophyll content of 13.67 mg/l from the Pandanus amaryllifolius Roxb leaf using microwave-assisted extraction (MAE) with an acetone solvent was achieved. Kwartiningsih et al. [13] also obtained total chlorophyll of 15.16 mg/l from Katuk leaves using supercritical carbon dioxide extraction.
This study shows that with acetone and DMF as extraction solvents, relatively high chlorophyll content was obtained in a 2-year-old M. Oleifera, compared with other freshly harvested plants. Indicating the optimal chlorophyll stability of M. Oleifera and therefore promoting the use of the plant in the food and health industries due to its lifespan, longevity and preservation of chlorophyll. However, while DMF extracts more chlorophyll, acetone is recommended for the food and health industries because DMF is toxic when ingested. The use of DMF chlorophyll extracts may be explored in other industries such as optoelectronic and next-generation photovoltaic. Furthermore, the ‘green’ solvent (ethanol), extracted a relatively high chlorophyll content of \(62\pm 0.139\) mg/l at a temperature of 45 °C and a 60-min extraction period for the freshly harvested plant. Thus, it is always preferred in the food and pharmaceutical industries because of its low cost, non-toxicness, and ease of separation [29].
The chlorophyll content obtained from the Moringa used in this study for all postharvest storage periods is compatible with what is used in the market. Further surpassing some of the chlorophyll content of the plants currently used in the market, such as spinach, alfalfa, etc. [26]. For instance, Nugroho et al. [32] evaluated the chlorophyll content of microgreen red and green spinach, obtaining an average total chlorophyll content of 15.203 mg/l and 12.998 mg/l, with ranges of 7.539–21.795 mg/l and 5.721–20.320 mg/l, respectively. Thus, this establishes Moringa as a major source of chlorophyll with potential for large-scale commercial production.
Rate of chlorophyll content reduction at different extraction times and CSI
Figure 5a and c illustrate the reduction rate of average total chlorophyll concentrations for freshly harvested, 1-year, and 2-year postharvest storage periods of M. Oleifera at different extraction times. Tables 2, 3, 4 present the chlorophyll stability index (CSI) for all the evaluated conditions. From Fig. 5, the graphs illustrate a consistent rate of chlorophyll reduction with DMF extracts for postharvest storage periods in comparison with other solvents. It’s more pronounced at a 10-min extraction time. DMF extracts degraded consistently from year to year, with chlorophyll levels declining by approximately 20% each year, and showed little decrease in percentage variation with increasing extraction time. For the 2-year postharvest storage period moringa, the percentage drop for DMF extracts was still below 50%, which thus shows that chlorophyll is relatively constant in DMF. Ethanol and acetone extracts showed a 40% decrease in the chlorophyll content of the 1-year (2021) plant of the freshly harvested (2022) plant. Followed by an approximately 50% decrease in the chlorophyll content of the 2-year (2020) leaves of the freshly harvested leaves (2022) for acetone extracts only. Methanol and ethanol showed little variation in reduction rates between postharvest storage periods, as Chlb content was not detected in the 1-year-old/2-year postharvest storage periods and 2-year postharvest storage period extracts for methanol and ethanol, respectively, whereas Chla showed little variation.
Similarly, with the chlorophyll reduction results, the chlorophyll stability test conducted with the chlorophyll stability index illustrated that the highest levels of CSI were obtained using DMF extracts compared to the other solvents. Followed by acetone extracts, and the results of the alcohol extracts are invalid because little to no amounts of Chlb were extracted using them. The highest percentages of CSI for DMF and acetone extracts were 87% and 73%, respectively, during the one-year postharvest storage period, and 65% and 58%, respectively, during the two-year postharvest storage period of the plants. Thus, the high percentages indicate that the total chlorophyll content was not greatly affected by the postharvest storage period of the leaves and that chlorophyll from moringa is stable.
Overall, the findings suggest that alcohol extracts are not recommended for longer preservation of the chlorophyll pigment, particularly methanol. The results also show that the chlorophyll pigments degrade faster in the first year than in the second year moringa. Furthermore, the results show that the chlorophyll content in M. Oleifera decreases with increasing postharvest storage period.
a Rate of chlorophyll content reduction for all postharvest storage periods at 10-min extraction time. The line graph follows the mean ± SE (n = 3) standard. b Rate of chlorophyll content reduction for all postharvest storage periods at 30-min extraction time. The line graph follows the mean ± SE (n = 3) standard. c Rate of chlorophyll content reduction for all postharvest storage periods at 60-min extraction time. The line graph follows the mean ± SE (n = 3) standard
Conclusion
According to the results obtained in this study, the chlorophyll content of M. Oleifera is relatively high, stable, and compatible with other plants currently used in the food and pharmaceuticals industries. The study demonstrated that the chlorophyll extracted from a 2-year postharvest storage period M. Oleifera leaves was stable, and the chlorophyll content was still compatible with that of other freshly harvested plants. As a result, this plant can be considered a significant source of chlorophyll due to its shelf life, longevity, and chlorophyll preservation. Increased extraction time and temperature resulted in increased chlorophyll production, but an increase in both conditions resulted in chlorophyll degradation. Methanol extracted the highest Chla concentrations but the lowest Chlb concentrations, thereby total chlorophyll (Chla + Chlb). Acetone and DMF demonstrated the highest efficiency in chlorophyll extraction from the harvested leaves and maintained this trend even with an increasing postharvest storage period of the leaves. The optimal extraction conditions for ethanol and methanol were achieved by using a 10-min and 30-min extraction time at 45 °C, resulting in maximum chlorophyll concentrations of 56 ± 0.193 mg/l and 60 ± 0.572 mg/l, respectively. Similarly, for acetone and DMF, the best results were obtained at 45 °C with extraction times of 10 and 60 min, resulting in maximum chlorophyll concentrations of 56 ± 0.550 mg/l and 63 ± 0.524 mg/l.
Regarding the degradation of chlorophyll concentrations, when acetone and ethanol were used at 45 °C with extraction times of 30 and 60 min, the resulting values were 65 ± 0.653 mg/l, 61 ± 0.118 mg/l, and 63 ± 0.324 mg/l, 62 ± 0.139 mg/l, respectively. Acetone and ethanol are recommended for chlorophyll use in the food and pharmaceutical industries, but ethanol should be used for chlorophyll extraction only on freshly harvested plants. That is because the alcohols (methanol and ethanol) degraded at a faster rate compared to the other solvents with the increasing postharvest storage period.
In general, this study established that the chlorophyll content and stability of M. Oleifera will benefit different industries according to the solvent used in its extraction. The plant’s ability to produce a large biomass, with its drought-tolerant properties and rapid growth rate, can support large-scale production.
References
U. Nations, United Nations, (2021) SDG goal 3: Good health and well-being, UNICEF DATA. https://data.unicef.org/sdgs/goal-3-good-health-wellbeing/#:~:text=SDG%203%20aims%20to%20prevent,and%20regions%20are%20priority%20areas. Accessed 28 February 2023
J.S. Ribeiro, M.J.M.C. Santos, L.K.R. Silva, L.C.L. Pereira, I.A. Santos, da S.C. Silva Lannes, da M.V. Silva, Natural antioxidants used in meat products: a brief review. Meat Sci. 148, 181–188 (2019). https://doi.org/10.1016/j.meatsci.2018.10.016
U.M. Lanfer-Marquez, R.M.C. Barros, P. Sinnecker, Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 38(8), 885–891 (2005). https://doi.org/10.1016/j.foodres.2005.02.012
A. Ahmadi, S.-A. Shahidi, R. Safari, A. Motamedzadegan, A. Ghorbani-HasanSaraei, Evaluation of stability and antibacterial properties of extracted chlorophyll from alfalfa (Medicago sativa L.). Food Chem. Toxicol.: Int. J. British Indust. Biol. Res. Assoc. 163, 112980 (2022). https://doi.org/10.1016/j.fct.2022.112980
C.-J. Hsiao, J.-F. Lin, H.-Y. Wen, Y.-M. Lin, C.-H. Yang, K.-S. Huang, J.-F. Shaw, Enhancement of the stability of chlorophyll using chlorophyll-encapsulated polycaprolactone microparticles based on droplet microfluidics. Food Chem. 306, 125300 (2020). https://doi.org/10.1016/j.foodchem.2019.125300
E. Manolopoulou, T. Varzakas, A. Petsalaki, Chlorophyll determination in green pepper using two different extraction methods. Curr. Res. Nutr. Food Sci. J. 4, 52–60 (2016). https://doi.org/10.12944/CRNFSJ.4.Special-Issue1.05
D.I.A.S. Indrasti, N.U.R.I. Andarwulan, E.K.O.H.A.R.I. Purnomo, N.U.R. Wulandari, Stability of chlorophyll as natural colorant: a review for Suji(Dracaena angustifolia (Medik.) roxb.) leaves’ case. Curr. Res. Nutr. Food Sci. J. 6(3), 609–625 (2018). https://doi.org/10.12944/crnfsj.6.3.04
K. Östbring, I. Sjöholm, M. Rayner, C. Erlanson-Albertsson, Effects of storage conditions on degradation of chlorophyll and emulsifying capacity of thylakoid powders produced by different drying methods. Foods. 9(5), 669 (2020). https://doi.org/10.3390/foods9050669
C. Hoang Thanh et al., Optimization of chlorophyll extraction in Centella asiatica L. Jilin Daxue Xuebao (Gongxueban) (2022). https://doi.org/10.17605/OSF.IO/Z5ECR
A. Mahapatra, P. Kumar, A.K. Behera, A. Sen, B. Pradhan, Comparative study of natural dye-sensitized solar cells using inedible extracts from kumkum, kamala and malabar spinach fruits. J. Photochem. Photobiol. 436, 114385 (2023). https://doi.org/10.1016/j.jphotochem.2022.114385
P. Sabarikirishwaran, Y. Unpaprom, R. Ramaraj, Effects of natural dye solvent extraction on the efficiency of dye-sensitive solar cells from the leaf biomass of Sandoricum koetjape and Syzygium samarangense. Waste Biomass Valoriz. (2023). https://doi.org/10.1007/s12649-022-02030-2
S.C. Yadav, M.K. Tiwari, A. Kanwade, H. Lee, A. Ogura, P.M. Shirage, Butea monosperma, crown of thorns, red lantana camara and royal poinciana flowers extract as natural dyes for dye sensitized solar cells with improved efficiency. Electrochim. Acta. 441, 141793 (2023). https://doi.org/10.1016/j.electacta.2022.141793
E. Kwartiningsih, A.N. Ramadhani, N.G.A. Putri, V.C.J. Damara, Chlorophyll extraction methods review and chlorophyll stability of Katuk leaves (Sauropus androgynous). J. Phys.: Conference Series, 1858(1), 012015. (2021). https://doi.org/10.1088/1742-6596/1858/1/012015
R. Latifa, S. Hadi, E. Nurrohman, The exploration of chlorophyll content of various plants in city forest of Malabar Malang. BIOEDUKASI 17(2), 50 (2019). https://doi.org/10.19184/bioedu.v17i2.14091
Z. Islam, S.M.R. Islam, F. Hossen, K. Mahtab-ul-Islam, M.R. Hasan, R. Karim, Moringa oleifera is a prominent source of nutrients with potential health benefits. Int. J. Food Sci. (2021). https://doi.org/10.1155/2021/6627265
P.G. Milla, R. Peñalver, G. Nieto, Health benefits of uses and applications of Moringa oleifera in bakery products. Plants (Basel, Switzerland) 10(2), 318 (2021). https://doi.org/10.3390/plants10020318
C.V. Mashamaite, B.L. Ngcobo, A. Manyevere, I. Bertling, O.A. Fawole, Assessing the usefulness of Moringa oleifera leaf extract as a biostimulant to supplement synthetic fertilizers: a review. Plants (2022). https://doi.org/10.3390/plants11172214
H.K. Lichtenthaler, Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148, 350–382 (1987). https://doi.org/10.1016/0076-6879(87)48036-1
W.P. Inskeep, P.R. Bloom, Extinction coefficients of chlorophyll a and b in N,N-dimethylformamide and 80% acetone 1. Plant Physiol. 77(2), 483–485 (1985). https://doi.org/10.1104/pp.77.2.483
S.A. Kaloyereas, A new method of determining drought resistance. Plant Physiol. 33(3), 232–233 (1958). https://doi.org/10.1104/pp.33.3.232
M.M. Mohan, S.L. Narayanan, S.M. Ibrahim, Chlorophyll stability index (CSI): its impact on salt tolerance in rice. Int. Rice Res. Notes. 25(2), 38–39 (2000)
S. Nahakpam, Chlorophyll stability: a better trait for grain yield in rice under drought. Indian J. Ecol. 44(4), 77–82 (2017)
D. Purushothaman, R. Kesavan, T. Marimuthu, G. Oblisami, Chlorophyll stability index (CSI) of certain algae. Curr. Sci. 43(5), 159–161 (1974)
N.H.K. Nguyen, N.T.D. An, P.K. Anh, T.T. Truc, Microwave-assisted extraction of chlorophyll and polyphenol with antioxidant activity from Pandanus amaryllifolius Roxb. In Vietnam. IOP Conference Series: Materials Science and Engineering, 1166(1), 012039. (2021). https://doi.org/10.1088/1757-899X/1166/1/012039
C. Osório, S. Machado, J. Peixoto, S. Bessada, F.B. Pimentel, C. Alves, M.B.P.P. Oliveira, Pigments content (chlorophylls, fucoxanthin and phycobiliproteins) of different commercial dried algae. Separations (2020). https://doi.org/10.3390/separations7020033
R. Suzuki, T. Ishimaru, An improved method for the determination of phytoplankton chlorophyll using N, N-dimethylformamide. J. Oceanogr. Soc. Jpn. 46(4), 190–194 (1990). https://doi.org/10.1007/BF02125580
S. Siebeneichler, J. Barbosa, A. Cruz, M. Ramos, H. Fernandes, V. Nascimento, Comparison between extraction methods of photosynthetic pigments in Acacia mangium. Commun. Plant Sci. 1, 1–5 (2019). https://doi.org/10.26814/cps2019001
L. Tomsone, Z. Kruma, Spectrophotometric analysis of pigments in horseradish by using various extraction solvents. Foodbalt (2019). https://doi.org/10.22616/FoodBalt.2019.023
S. Sarkar, M.S. Manna, T.K. Bhowmick, K. Gayen, Extraction of chlorophylls and carotenoids from dry and wet biomass of isolated Chlorella thermophila: optimization of process parameters and modelling by artificial neural network. Process Biochem. 96, 58–72 (2020). https://doi.org/10.1016/j.procbio.2020.05.025
F.J. Martí-Quijal, F. Ramon-Mascarell, N. Pallarés, E. Ferrer, H. Berrada, Y. Phimolsiripol, F.J. Barba, Extraction of antioxidant compounds and pigments from spirulina (Arthrospira platensis) assisted by pulsed electric fields and the binary mixture of organic solvents and water. Appl. Sci. 11(16), 7629 (2021). https://doi.org/10.3390/app11167629
S. Parveen, V. Duddela, R. Narravula, T. Chandrasekhar, Effect of various solvents on chlorophyll and carotenoid extraction in green algae: Chlamydomonas reinhardtii and Chlorella vulgaris. Ann. Plant Soil Res. 21, 341–345 (2019)
E. Nugroho, A.M. Zahra, R.E. Masithoh, H.K. Simatupang, A.N. Sinaga, N.D. Pitaloka, M.F. Pahlawan, L. Rahmawati, Determination of green and red spinach microgreen chlorophyll content using visible spectroscopy and wavelength selection. IOP Conference Series: Earth and Environmental Science, 1183(1), 012049. (2023). https://doi.org/10.1088/1755-1315/1183/1/012049
Funding
Open access funding provided by University of the Witwatersrand. The authors acknowledge the financial support from the Competitive Support for Unrated Researchers from the National Research Foundation (CSUR-NRF) (Grant No. 138007).
Author information
Authors and Affiliations
Contributions
SN: Writing the original draft, methodology and lab work, software, data acquisition. IR: Supervision, conceptualization, validation, and revising the manuscript. AMU: Supervision, conceptualization, validation, and revising the manuscript. SOB: Supervision, conceptualization, validation, revising the manuscript, Project administration and Funding acquisition.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ngcobo, S., Bada, S.O., Ukpong, A.M. et al. Optimal chlorophyll extraction conditions and postharvest stability in Moringa (M. Oleifera) leaves. Food Measure 18, 1611–1626 (2024). https://doi.org/10.1007/s11694-023-02271-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-02271-2