Abstract
New solder, based on Sn-Zn, with the addition of 1 at.% Ag, 1 at.% Al, and 0.5 at.% Li (SZAAL) was prepared. The influence of alloying elements on mechanical and electrical properties, microstructure, and melting point was investigated. The tensile test showed enhanced mechanical properties of SZAAL alloy compared to Sn-Zn eutectic and Sn-Zn-Ag. Investigated Al and Li additions improve the mechanical properties of the alloy, tensile strength and solder plasticity. Another positive effect is that those elements caused a decrease in the melting point, compared to Sn-Zn with 1 at.% Ag. Microstructural investigation revealed a fine Sn-Zn eutectic structure with the present epsilon phase (AgZn3). The addition of lithium and silver decreased the coefficient of linear expansion of the tested alloys. Li addition reduced significantly both the surface and interfacial tension, which translates into improved solder wettability. The surface tension of all solders decreased with temperature, but the interfacial tension increased with temperature. That is due to the weakening effect of the flux. Thus, the soldering temperature in the presence of the ALU33® flux should not exceed 250 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Electronics is nowadays one of the industry's most important branches. Its development determines the progress of the world economy. That is possible through enhancements, especially miniaturization and increasing connection density. Moor’s law, where the number of transistors doubles every two years, is not implementable anymore because of many factors. One of them is materials limitations, according to the Basic Research Needs for Microelectronics Report (Ref 1). That is why solders need to be improved to allow computing advances.
In the past, alloys based on lead were applied, because of their low melting point. Also, at that time, their mechanical and electrical properties were satisfactory. However, research to find a replacement for Pb-based solders is still in progress since the announcement of the RoHS directive by the European Union (Ref 2). Lead restrictions are also in the US (Ref 3) and Japan (Ref 4). Four main requirements for Pb-free solders are low melting point, wettability, availability, and relatively low cost (Ref 5). The expectations for the alloys are constantly growing since the cross-section of the interconnects is forced to become smaller. Thus, the interconnect mechanical properties need to be accordingly enhanced. Two of the SAC (Sn-Ag-Cu) alloys, candidates for lead-based materials replacement, are even mass-produced and recommended to use by the Association of the US, like Sn-3.0Ag-0.5Cu (produced by Japanese), Sn-3.9Ag-0.6Cu (USA) (Ref 6). However, the addition of silver makes them more expensive. Nevertheless, there still is a need for new solder materials research and development.
Due to the low melting point (198 °C for eutectic composition) and comparatively low cost, Sn-Zn alloys are also a candidate for Pb-based alloys replacement. Unfortunately, there are still some problems with the implementation and performance of those solders. Due to the fast oxidation process, protective atmospheres have to be applied while soldering. The other concerns are low corrosion resistance in acidic environments (Ref 7, 8), damage by heat exposure (Ref 6), and poor wettability (Ref 9, 10). Thus, adding other elements to the solder in low concentration is being studied and developed to solve those problems (Ref 11,12,13,14).
Recently, alloys based on (Sn-Zn) eutectic with different additions: elements, nanoparticles, and other compounds, like oxides, have been studied (Ref 15). However, there are no reports about the Li additions, which may enhance the unsatisfactory wettability of the solder. Taking into consideration the above, the influence of already known alloying elements as well as a lack of knowledge about Li addition to Sn-Zn eutectic, this paper aims to determine the properties of Sn-Zn eutectic alloy with 1 at.% the addition of Ag, Al, and 0.5 at.% at Li, as alloying elements (SZAAL solder). Lithium and Silver are added to Sn-Zn alloys to improve the wettability and mechanical properties, such as tensile strength and ductility. Additionally, Li and Al increases the corrosion resistance of Sn-Zn alloys (Ref 16). Silver also enhances the alloy's electrical properties, and Li increase the spreading velocity.
2 Experimental Section
The conditions of pure metals melting were already described before (Ref 17). The purity of used metals was as follows: Sn-99.999%, Zn-99.95%, Ag-99.999%, Al-99.95%, and Li-99.95%. The solders (SZAAL as well as other solders produced to determine the influence of alloying elements) were prepared in a glove-box under a protective atmosphere of high purity argon (99.9999%), with water vapor, nitrogen and oxygen concentration lower than 0.1 ppm. The atmosphere in the glove-box was additionally purified by Ti shavings, working at 950 °C, with a circulation flow rate of 35 m3/h to avoid the oxidation of the materials as well as the possibility of forming lithium nitrides. This procedure ensured high purity of the obtained alloys. In all cases, tin was melted at T = 400 °C in a graphite crucible, followed by the addition of the remaining alloy elements. Next, the alloys were homogenized under coverage for t = 2 h and cast into experiment–specific molds. For microstructure characterization, solidified droplets were cut and prepared accordingly to the Scanning Electron Microscopy (SEM) characterization technique. SEM imaging was carried out using FEI E-SEM XL30 microscope in BSE mode (20 keV, WD = 10 mm), and the chemical analysis was performed with EDX spectrometer X EDAX GEMINI 4000. The melting point of alloys was obtained from the DSC heating curves. The Differential Scanning Calorimetry method was performed on a DiPoint μcalorimeter, in an argon atmosphere, with a heating rate of 10 °C/ min.
The electrical resistivity measurements of the alloys with temperature were performed using the standard four-point probe method (Ref 18). Tensile tests were conducted on an INSTRON 6025 testing machine modernized by the Zwick/Roell conditions, according to EN ISO 6892-1:2009, with a strain rate of 0.00025, at room temperature. The thermal expansion coefficient was estimated using Misura® 3 FLEX-ODLT horizontal dilatometer. Samples of SZAAL were cast to obtain 50 × 50 × 500 mm rods and put into a dilatometer furnace. The heating rate was 5 °C/min up to 190 °C. Experiments were performed in the atmosphere of the air. Details of the measurement could be found elsewhere (Ref 19). For statistical purposes, the experiment was repeated 4 times. Density measurements were carried out with the dilatometric, and surface tension measurements with the maximum bubble pressure (MBP) method (Ref 20). Density and surface tension measurements were performed under an Ar15%H2 protective atmosphere. Both for density and surface tension measurement, low-porosity graphite crucibles were used. Molybdenum capillaries were used for surface tension measurement. The interfacial tension method was measured using wetting balance method (WBT), described in details elsewhere (Ref 21, 22). The formula for calculation interfacial tension from WBT experiments is as follows:
where \(\gamma_{{{\text{LV}}}}\) is the interfacial tension, \(P\) is the area of the part of the rod immersed in the investigated alloy, \(F_{{\text{r}}}\) is the value of the resultant force, where the force-depth relationship is linear, \(\rho\) denotes the alloy density, \(l\) states for the samples’ perimeter in the meniscus area, g is the gravity, and \(h\) states for immersion depth.
The chemical compositions of the investigation solders provided in Table 1.
3 Results and Discussion
3.1 Melting Behavior
The melting point (TM) is one of the basic properties of solder and a factor in the development of new solder alloys. The reference point for applications in electronics is the Sn-Pb eutectic alloy [183 °C (Ref 23)], while the most commonly used alloy is SAC [217 °C for Sn-3.0Ag-0.5Cu, and 220 °C for Sn-3.9Ag-0.6Cu (Ref 6)]. Therefore, it seems that the melting points of the most promising substitutes for lead solders should be in the range of 183-217 °C. The melting points of investigated alloys, compared with the melting points of solders used in electronics (Ref 24,25,26,27,28,29), are shown in Fig. 1. The solders proposed in this work have melting points lower than the commonly used SACs. Generally, the additions of Ag and Al to the Sn-Zn eutectic increase the melting point and the addition of Li lowers it. However, none of the investigated configurations achieved as low melting points as alloys with the additions of Bi (Ref 6) or In (Ref 23).
3.2 Structural Characterization
Figure 2-6 presents the microstructures of the SnZn, SnZn1.0Ag, SnZn1.0Ag1.0Al, SnZn1.0Ag0.5Li and SnZn1.0Ag1.0Al0.5Li as-cast alloys. The microstructures of the investigated alloys consist of a two phases. In the case of the Sn-Zn eutectic alloy (Fig. 2), SEM-BSE images show a two-phase structure: bright one, which is mainly composed of tin (approx. 98 at.% Sn), as well as a darker, needle-like phase, formed by α-zinc, which can be seen from the EDS maps. This type of structure was already widely reported in the literature (Ref 30,31,32).
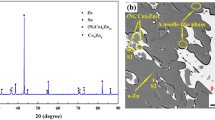
Figure 3 shows the microstructure of as-cast (SnZn)eut + 1 at.% Ag solder, which consists of a eutectic, fine-grained structure. Small lamellar precipitations of the Zn-rich phase and Sn-rich β phase are visible. The addition of silver to the alloy caused the formation of another phase, also previously described (Ref 33). Those dark regions, based on the EDS investigations, correspond to Ag and Zn-rich phases, suggesting the presence of an AgZn3 intermetallic compound. However, the contrast can be seen in the outer and inner parts of this phase. Darker contours suggest a higher concentration of lighter element, which was confirmed using EDS–the outer part had a higher concentration of Zn than the inner area of the precipitation (82.39 at.% compared to 77.34 at.% in the inner part). Analysis showed small amounts of Ag. Microstructure also consists of rounded shape precipitates of AgZn3, similar to the previous variant (Sn-Zn-Ag). AgZn3 was already found in previous studies for Sn-Zn-Ag alloys (Ref 34).
For the modification of Sn-Zn-Ag alloy with Li (Fig. 4), the microstructure did not show great changes compared to previously described samples, except for the fact that the imaged structure is refined. The eutectic area, created out of Sn-rich light regions and alpha-zinc precipitates, was selected for the chemical composition analysis. The result was that the mean value of the zinc and tin composition was around 11.5 and 87.5 at.%, respectively. The analysis also showed small amounts of Ag. The microstructure consists of rounded shape precipitates of AgZn3, similar to the previous variant (Sn-Zn-Ag). Additionally, areas of dezincification could be observed for the eutectic matrix.
Modification of the Sn-Zn-Ag alloy with the addition of Al did change the microstructure (Fig. 5)-the images show comparatively bigger precipitates of alpha-zinc and Ag-rich phase than for the previous alloys. Despite this fact, the BSE-SEM images still show a bright phase consisting mainly of tin, needle-like alpha zinc phase, as well as rounded shape AgZn3 phase. Additionally, there is another place, dark in contrast corresponding to Al-rich spots. EDS analysis showed over 90 at.% of Al, nearly 9 at.% of Zn, and trace amounts of the remaining elements. Literature studies also confirm the segregation of aluminum atoms in the Zn-rich precipitations (Ref 11, 33).
For the SZAAL alloy (Sn-Zn-Ag-Al-Li), microstructure analysis, shown in Fig. 6, revealed similar precipitations as in the case of the previous alloys. The behavior of the elements is similar–tin creates a bright-imaged phase, a darker one identified as α-Zn in the shape of rods, as well as rounded AgZn3. Area EDS analysis showed a selective distribution of Al, with two spots exhibiting a dense concentration of this element, near Zn-rich precipitates. Those spots in BSE mode are the darkest places in the microstructure. Due to analytical and time limitations, the analysis of Li in 1 at.% Li samples is impracticable (Ref 35). The authors (Ref 36) showed that the ease of saturation with lithium is greater for Sn than for Zn, therefore lithium prefers to be incorporated into Sn structures. With the above in mind, it can be assumed that the lithium atoms are evenly distributed in the matrix, which is also confirmed by the finely divided structure of the SnZnAg solder after adding lithium (Fig. 3 versus Figure 4 and 6). Further work, including TEM observation, is needed to determine Li and Al's positions.
3.3 Mechanical and Thermophysical Properties
The effect of alloying additives on the mechanical properties can be observed in the strain–stress curves, shown in Fig. 7. The addition of Ag to Sn-Zn eutectic enhanced the tensile strength of the alloy, due to the formation of the AgZn3 phase (Fig. 3). Aluminum in SZAAL improved the plasticity of solder with slightly higher values of obtained tensile strength. However, alloying elements changed the behavior of the samples after reaching tensile strength. In the case of Sn-Zn with no alloying elements, there is still some elongation at the end of the curve, while for cases with present additions, samples did not show any plastic effect. This indicates a different mechanism driving that process, probably connected with the formation of intermetallic phases visible when silver is added. Interestingly, elongation values obtained were the highest with the addition of Al and Li. In light of this reasoning, the formation of fine Al and Li precipitates is possible. In that case, enhancement of mechanical properties, as well as grain refinement, could take place, which leads to the increase of alloys’ ductility. However, future investigations, including TEM observations need to be carried out to confirm this hypothesis.
The alloys studied in this investigation expand linearly over the 20-190 °C temperature range, as can be seen in Fig. 8. The addition of Al, oppositely to Li, increased the linear expansion of the samples. Linear expansion coefficient CTE for Cu equals17,1 × 10−6 °C−1 between 20 and 300 °C (Ref 37). To obtain better reliability of the solder joint against thermal fatigue, the CTE of the solder alloy should be close to the materials being soldered. From this point of view, SnZnAg and SnZnAgLi alloys seem to be the best candidate for connections with copper exposed to thermal cycling. In contrast to Al, the additions of both Ag and Li significantly reduce the linear expansion coefficient of the Sn-Zn eutectic alloy. This increase in the CTE of Sn-Zn-Ag-Al and Sn-Zn-Ag-Al alloys can be correlated with the higher Al's CTE than for the eutectic Sn-Zn alloy.
Figure 9 plots the result from electric resistance tests for (Sn-Zn)eut with various alloying additions. Nearly all the solders exhibit the linear characteristic of the resistivity–temperature relationship. There is a visible correlation between the presence of additional elements in the solder to the performed resistance. As a general rule, the higher amount of additions in the alloy, the higher value of electrical resistivity. This phenomenon is connected with lattice distortion. However, at the lowest investigated temperature, for the alloy with the highest amount of alloying elements (Sn-Zn + 1 at.% Ag + 1 at.% Al + 0.5 at.% Li), resistivity is only a bit higher than Sn-Zn eutectic. What is more, the steeper curve is observed for the same alloy, which means the greatest increase in resistance value with temperature. A similar tendency is visible in Sn-Zn alloy with Ag and Al. It seems like aluminum is the element which changes the slope of resistivity–temperature relationship. Sn-Zn with Ag alloy, in comparison to an alloy with Li addition to the same composition, has a slightly lower value of resistivity throughout the whole investigated temperature range. Compared to the other reported results for Sn-Zn eutectic alloy (Ref 18), very good agreement is observed. Moreover, the authors published also results for Sn-Pb alloy. Thus, a conclusion can be made that all of the investigated alloys have a lower electrical resistivity in the whole temperature range than lead-based alloys. For the Ag addition, reports confirm that silver increases the electrical conductivity for tin-zinc eutectic (Ref 38,39,40). The authors stress the importance of the formation AgZn3 phase in the phenomenon.
Based on the electrical resistivity results measurements, the thermal conductivity was calculated (Ref 40, 41). Results are indicated in Fig. 10. For all alloys, thermal conductance increased with the decrease in temperature. When the temperature rises, the electron mobility is also higher, which promotes a conductivity increase. However, in that case, lattice vibrations also contribute to the overall conductivity. The highest thermal conductivity was noted for alloys with Ag and Li additions. A similar tendency (decrease of thermal conductance with temperature) for Sn-Zn and eutectic with the addition of silver was reported by Şahin et al. (Ref 40). Inversely, in the case of samples with Al, the conductance was the lowest above all investigated samples. Additionally, in light of this reasoning, a conclusion can be made that, for alloys containing Al, an increase of electron mobility with temperature has a smaller contribution to the overall result than lattice vibrations, which leads to energy dissipation and a decrease in thermal conduction.
Figure 11 presents the temperature-density dependence of liquid solders. For all alloys, the density decreases with temperature. The solders density is necessary to calculate the surface (Fig. 12) and interfacial (Fig. 13) tensions obtained from wetting balance method.
Sn-Zn-based solders were also investigated in terms of surface tension. Results are plotted in Fig. 12. In all cases, the value of surface tension decreased with temperature. However, additions to Sn-Zn eutectic caused a change in the decrease rate. For base alloy, the decrease is slower than for its modifications. Comparing SZAAL solder to Sn-Zn eutectic, the addition of Al, Li, and Ag, caused a decrease in surface tension in all the temperature points. On the other side, the addition of just silver increased the value of surface tension. Thus, a conclusion can be made that the combination of aluminum and lithium has a greater impact on the surface tension value than silver.
Finally, the solders: Sn-Zn + 1 at.% Ag, Sn-Zn + 1 at.% Ag + 0.5 at.% Li and Sn-Zn + 1 at.% Ag + 1 at.% Al + 0.5 at.% Li were tested with the wetting balance method (WBT) to determine their interfacial tension. Figure 13 shows the results of interfacial tension measurements, compared to surface tension at three temperatures: 250, 275 and 300 °C. Generally, lowering the surface tension of liquid alloys improves wettability. The measurement results presented in the article showed that the addition of lithium reduces both surface tension and interfacial tension. Thus, reducing this value by applying specific alloying elements or process conditions (like temperature or atmosphere) is beneficial. Usually, there is a correlation tendency that some factor lowers the surface and interfacial tension.
The interfacial tension (in the presence of ALU33® flux) of the tested Sn-Zn alloys decreased with the increase in the number of alloy additions. The greatest decrease in interfacial tension was observed for the temperature of 250 °C. For higher temperatures (275 and 300 °C), the influence of alloying elements on the reduction of interfacial tension was smaller and decreased with temperature—for 300 °C it was disappearing but still visible. However, as the temperature increased, the interfacial tension increased, and the surface tension decreased.
The ALU33® flux used for the WBT tests could have contributed to the interfacial tension increase with the temperature. The effect of the flux could weaken with increasing temperature because of the degradation of its components.
4 Conclusions
New solder based on the Sn-Zn eutectic with 1 at.% Ag, 1 at.% Al, and 0.5 at.% Li was investigated. Experiments show that Al and Li additions enhance the properties of the alloy, tensile strength, and solder plasticity. Another positive effect is that those elements caused a decrease in the melting point, compared to Sn-Zn with 1 at.% Ag. Microstructural investigation revealed a fine Sn-Zn eutectic structure with the present epsilon phase (AgZn3). The addition of lithium and silver decreased the coefficient of linear expansion of the tested alloys. From that point of view, SnZnAg and SnZnAgLi alloys ought to be the best candidates for Cu soldering when exposed to thermal cycles.
The addition of lithium significantly reduced both the surface and interfacial tension, which translates into improved solder wettability. The surface tension of all solders decreased with temperature, but the interfacial tension increased with temperature. That is due to the weakening effect of the flux. Thus, the soldering temperature in the presence of the ALU33® flux should not exceed 250 °C.
References
C. Murray, S. Guha, D. Reed, and G. Herrara, Basic Research Needs for Microelectronics, Rep. Off. Sci. Work. Basic Res. Needs Microelectron. p 5–8 (2018)
EU Parliament, Directive 2011/65/EU of the European Parliment and of the council on the Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment (RoHS) - recast, Off. J. Eur. Union, 2011, pp. 88–110
Office of Law Revision Counsel, Ch. 53 Toxic Substances Control, United States Code, Title 15—Commerce and Trade. subchapter IV- lead exposure reduction, 2020, §2681–§2692
K.J. Puttlitz and K.A. Stalter, Handbook of Lead-Free Solder Technology for Microelectronic Assemblies. In Marcel Dekker, 2004, p 141–145
C.M.L. Wu, D.Q. Yu, C.M.T. Law, and L. Wang, Properties of Lead-Free Solder Alloys with Rare Earth Element Additions, Mater. Sci. Eng. R Rep., 2004, 44(1), p 1–44.
K.N. Subramanian, Lead-Free Electronic Solders: A Special Issue of the Journal of Materials Science: Materials in Electronics, Lead-Free Electronic Solders: A Special Issue of the Journal of Materials Science: Materials in Electronics (2007)
Ž Gerhátová, P. Babincová, M. Drienovský, M. Pašák, I. Černičková, L. Ďuriška, R. Havlík, and M. Palcut, Microstructure and Corrosion Behavior of Sn-Zn Alloys, Materials (Basel), 2022, 15(20), 7210.
J.C. Liu, G. Zhang, S. Nagao, J.T. Jiu, M. Nogi, T. Sugahara, J.S. Ma, and K. Suganuma, Metastable Pitting and Its Correlation with Electronic Properties of Passive Films on Sn-XZn Solder Alloys, Corros. Sci., 2015, 99, p 154–163.
S. Vaynman, G. Ghosh, and M.E. Fine, Some Fundamental Issues in the Use of Zn-Containing Lead-Free Solders for Electronic Packaging, Mater. Trans., 2004, 45(3), p 630–636.
J. Pstruś, Z. Moser, W. Gasior, and A. Debski, Surface Tension and Density Measurements of Liquid Sn-Zn Alloys Experiment versus SURDATdatabase of Pb-Free Solders, Arch. Metall. Mater., 2006, 51(3), p 335–343.
X. Chen, M. Li, X.X. Ren, A.M. Hu, and D.L. Mao, Effect of Small Additions of Alloying Elements on the Properties of Sn-Zn Eutectic Alloy, J. Electron. Mater., 2006, 35(9), p 1734–1739.
L. Zhang, S.B. Xue, L.L. Gao, Z. Sheng, H. Ye, Z.X. Xiao, G. Zeng, Y. Chen, and S.L. Yu, Development of Sn-Zn Lead-Free Solders Bearing Alloying Elements, J. Mater. Sci. Mater. Electron., 2010, 21(1), p 1–15.
S. Liu, S. Bai Xue, P. Xue, and D. Xue Luo, Present Status of Sn-Zn Lead-Free Solders Bearing Alloying Elements, J. Mater. Sci. Mater. Electron., 2015, 26(7), p 4389–4411.
T. Gancarz, Physical, Thermal, Mechanical Properties, and Microstructural Characterization of Sn-9Zn-XGa Alloys, Metall. Mater. Trans. A Phys. Metall. Mater. Sci., 2016, 47(1), p 326–333.
K. Xu, L. Zhang, L. Gao, N. Jiang, L. Zhang, and S. Zhong, Review of Microstructure and Properties of Low Temperature Lead-Free Solder in Electronic Packaging, Sci. Technol. Adv. Mater., Taylor & Francis, 2020, 0(0).
Z. Moser, W. Gąsior, J. Pstruś, K. Kujawa-Bukat, J. Sitek, and J. Kościelski, Lead-free, modified tin-and-zinc solder for soft soldering, Patent nr PL216987-B1 (2014).
J. Pstruś, Early Stages of Wetting of Copper by Sn–Zn Eutectic Alloy, J. Mater. Sci. Mater. Electron., 2018, 29(24), p 20531–20545.
T. Gancarz, P. Fima, and J. Pstruś, Thermal Expansion, Electrical Resistivity, and Spreading Area of Sn-Zn-In Alloys, J. Mater. Eng. Perform., 2014, 23(5), p 1524–1529.
S. Terlicka, A. Dębski, A. Budziak, M. Zabrocki, and W. Gąsior, Structural and Physical Studies of the Ag-Rich Alloys from Ag-Li System, Thermochim Acta, 2019, 673, p 185–191.
Z. Moser, W. Gąsior, J. Pstruś, I. Ohnuma, and K. Ishida, Influence of Sb Additions on Surface Tension and Density of Sn-Sb, Sn-Ag-Sb and Sn-Ag-Cu-Sb Alloys. Experiment versus Modeling, Int. J. Mater. Res., 2006, 97, p 365–370.
M. Miyazaki, M. Mizutani, T. Takemoto, and A. Matsunawa, Conditions for the Measurement of Surface Tension of Solders with a Wetting Balance Tester (Materials, Metallurgy & Weldability), Trans. JWRI, 1997, 26(1), p 81–84.
G. Kumar, and K.N. Prabhu, Review of Non-Reactive and Reactive Wetting of Liquids on Surfaces, Adv. Colloid Interface Sci., 2007, 133(2), p 61–89.
M. McCormack and S. Jin, New, Lead-Free Solders, J. Electron. Mater., 1994, 23(7), p 635–640.
M.L. Huang, Y.Z. Huang, H.T. Ma, and J. Zhao, Mechanical Properties and Electrochemical Corrosion Behavior of Al/Sn-9Zn−XAg/Cu Joints, J. Electron. Mater., 2011, 40(3), p 315–323.
P. Fima, K. Berent, J. Pstrus, and T. Gancarz, Wetting of Al Pads by Sn-8.8Zn and Sn-8.7Zn-1.5(Ag, In) Alloys, J. Mater. Sci., 2012, 47(24), p 8472–8476.
A.K. Gain, and L. Zhang, Interfacial Microstructure, Wettability and Material Properties of Nickel (Ni) Nanoparticle Doped Tin–Bismuth–Silver (Sn–Bi–Ag) Solder on Copper (Cu) Substrate, J. Mater. Sci. Mater. Electron., 2016, 27(4), p 3982–3994.
A.A. El-Daly, A.M. El-Taher, and S. Gouda, Development of New Multicomponent Sn-Ag-Cu-Bi Lead-Free Solders for Low-Cost Commercial Electronic Assembly, J. Alloys Compd., 2015, 627, p 268–275.
M. Şahin and E. Çadirli, The Effects of Temperature Gradient and Growth Rate on the Microstructure of Directionally Solidified Sn-3.5Ag Eutectic Solder, J. Mater. Sci. Mater. Electron., 2012, 23(2), p 484–492.
A.K. Gain, L. Zhang, and M.Z. Quadir, Thermal Aging Effects on Microstructures and Mechanical Properties of an Environmentally Friendly Eutectic Tin-Copper Solder Alloy, Mater. Des., 2016, 110, p 275–283.
J. Pstruś, P. Fima, and T. Gancarz, Wetting of Cu and Al by Sn-Zn and Zn-Al Eutectic Alloys, J. Mater. Eng. Perform., 2012, 21(5), p 606–613.
P. Pandey, C.S. Tiwary, and K. Chattopadhyay, Effects of Cu and In Trace Elements on Microstructure and Thermal and Mechanical Properties of Sn-Zn Eutectic Alloy, J. Electron. Mater., 2019, 48(5), p 2660–2669.
D.A. Zernitsa and V.G. Shepelevich, The Structure Formation of Rapidly Solidified Foil of the Eutectuc Alloy Sn-8, 8 Wt. % Zn, Phys. Chem. Asp. Study Clust. Nanostruct. Nanomater., 2020, 12, p 601–608.
M.L. Huang, X.L. Hou, N. Kang, and Y.C. Yang, Microstructure and Interfacial Reaction of Sn-Zn-x(Al, Ag) near-Eutectic Solders on Al and Cu Substrates, J. Mater. Sci. Mater. Electron., 2014, 25(5), p 2311–2319.
J.E. Lee, K.S. Kim, M. Inoue, J. Jiang, and K. Suganuma, Effects of Ag and Cu Addition on Microstructural Properties and Oxidation Resistance of Sn-Zn Eutectic Alloy, J. Alloys Compd., 2008, 454(1–2), p 310–320.
R. Mateus, M.B. Costa, L.C. Alves, M. Guedes, E. Alves, and A.C. Ferro, Lithium Dilution in Li-Sn Alloys, Nucl. Mater. Energy, 2020, 25, p 100783.
B.T. Heligman, K.J. Kreder, and A. Manthiram, Zn-Sn Interdigitated Eutectic Alloy Anodes with High Volumetric Capacity for Lithium-Ion Batteries, Joule, 2019, 3(4), p 1051–1063.
W.F. Gale and T.C. Totemeier, Smithells Metals Reference Book, 8th ed. Elsevier, 2003, p 2003
T. Gancarz and J. Pstruś, Characteristics of Sn-Zn Cast Alloys with the Addition of Ag and Cu, Arch. Metall. Mater., 2015, 60(3A), p 1603–1607.
M. Kamal, M.S. Meikhail, A.B. El-Bediwi, and E.S. Gouda, Study of Structural Changes and Properties for Sn-Zn9 Lead-Free Solder Alloy with Addition of Different Alloying Elements, Radiat. Eff. Defects Solids, 2005, 160(1–2), p 45–52.
M. Şahin, E. Çadırlı, U.M. Bayram, and P.A. Esener, Investigation of the Thermoelectrical Properties of the Sn91.2−x-Zn88-Agx Alloys, J. Therm. Anal. Calorim., 2018, 132(1), p 317–325.
G.S. Kumar, G. Prasad, and R.O. Pohl, Experimental Determinations of the Lorenz Number, J. Mater. Sci., 1993, 28(16), p 4261–4272.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is an invited submission to the Journal of Materials Engineering and Performance selected from presentations at the 10th International Conference on High Temperature Capillarity (HTC 2022) held September 12–16, 2022, in Kraków, Poland. It has been expanded from the original presentation. The issue was organized by Prof. Natalia Sobczak of the Polish Academy of Sciences.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dybeł, A., Pstruś, J. New Solder Based on the Sn-Zn Eutectic with Addition of Ag, Al, and Li. J. of Materi Eng and Perform 32, 5710–5722 (2023). https://doi.org/10.1007/s11665-023-08103-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-023-08103-0