Abstract
Wetting properties of Sn-Zn and Zn-Al alloys on Cu and Al substrates were studied. Spreading tests were carried out for 3 min, in air and under protective atmosphere of nitrogen, with the use of fluxes. In the case of Zn-Al eutectic, spreading tests were carried out at 460, 480, 500, and 520 °C, and in the case of Sn-Zn eutectic at 250, 300, 350, 400, 450, and 500 °C, respectively. Solidified solder/substrate couples were cross-sectioned and subjected to microstructure examination. The spreading tests indicated that the wetting properties of eutectic Sn-Zn alloys, on copper pads do not depend on temperature (up to 400 °C), but in the lack of protective atmosphere, the solder does not wet the pads. Wettability studies of Zn-Al eutectic on aluminum and copper substrates have shown a negative effect of the protective nitrogen atmosphere on the wetting properties, especially for the copper pads. Furthermore, it was noted that with increasing temperature the solder wettability is improved. In addition, densities of liquid solders were studied by means of dilatometric technique.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The RoHS directive, issued in 2001, banished the use of lead in consumer goods with effect from July 2006. The RoHS and similar legislation in the USA and Japan fueled the worldwide search for Pb-free replacement to Pb-containing solders. Based on a great number of publications on phase diagrams, structure, and physical-chemical properties of alloys, a number of most promising eutectic alloys including Sn-Ag, Sn-Ag-Cu, Sn-Zn, and Zn-Al (Ref 1-4) were selected. Sn-Ag, Sn-Ag-Cu, and multicomponent alloys based on them were broadly studied (Ref 5, 6) and have already used been commercially. On the other hand, data for Zn-Al alloys seem fragmentary, and they do not contain details regarding wettability (Ref 7, 8).
The essence of the soldering process is the interaction between the liquid solder and the solid soldered parts. As a result, at the interface, intermetallics may form, which determine mechanical and electrical properties of a joint (Ref 9). The persistence of the solder connection to a large extent depends on the adhesion of solder to the joined metals which in turn depends on the wettability, i.e., the ability of the solder to cover the solid parts with a thin, uniform, and continuous layer of a liquid solder. The condition of good wettability is that attractive forces between molecules of solid metal and liquid solder (adhesion forces) were greater than the force of attraction between the particles of solder (cohesive forces). In general, wetting of metal by liquid solder leads to the formation of solid solutions or intermetallic phases. Metals which do not dissolve one another and do not form intermetallics do not wet one another (Ref 10). Wettability depends largely not only on the purity of the parts to be joined but also on the degree of surface oxidation of the soldered parts and the solder. Bartell and Osterhof (Ref 11), depending on changes of surface energy, proposed the following classes of wetting: spilled, immersed, and adhesive. In the case of spilled wetting, liquid spills on the surface of a solid, increasing the surface area between the solid and liquid phases. Aluminum and its alloys are difficult to join by soldering because of tight and high-melting oxide layer on their surfaces. This layer, however, can be removed by applying specially selected aggressive fluxes for soldering process (Ref 3). Although Bartell and Osterhof’s study (Ref 11) does not account for oxide layers and fluxes, we have assumed that the spilled wetting is the wetting mechanism with which we are concerned in this study.
The greatest advantage of Sn-Zn eutectic is its low melting point (198 °C), which is close to the m.p. of Sn-Pb eutectic solder (183 °C), as well as its low price per mass unit compared with Sn-Ag and Sn-Ag-Cu solders. Sn-Zn alloys have good mechanical properties and can be used to soldering of copper and aluminum, provided proper flux is used. Despite good mechanical properties, wetting properties of Sn-Zn alloys are inferior when compared to those of Sn-37Pb alloy. Moreover, Sn-Zn alloys easily oxidize, and as a result protective atmosphere is required during soldering. According to the literature survey, there are two reasons for poor wettability of Sn-Zn eutectic alloy. The first is the increase of the surface tension due to the presence of zinc (Ref 12, 13). Wetting can be improved by appropriate alloying elements lowering the surface tension, such as In or Ga (Ref 14). The second reason for poor wettability is the presence of ZnO on the border of solder/pad (Ref 15). Based on the study of Chen et al. (Ref 16), it seems that the poor wettability is caused by the presence of ZnO, which is extremely difficult to remove. Chen et al. (Ref 16) studied the effects of various alloying elements on the wetting properties of the Sn-Zn eutectic alloy. Additions of Cr, Ti, and La do not affect the improvement of wettability, and the addition of Al even worsens the wettability because of the presence of dense oxides such as Al2O3 or TiO2. Hence, it can be concluded that the more the stable and dense the oxide appearing on the surface of the solder, the worse is the wettability.
The aim of this study is to determine the effects of temperature and atmosphere on the wetting properties and microstructures of solder joints Sn-Zn/Cu, Sn-Zn/Al, Zn-Al/Cu, and Zn-Al/Al, as well as on the selection of appropriate fluxes.
Experimental
Alloys for tests were prepared by melting pure metals (Sn: 99.999%, Zn: 99.95%, and Al: 99.99%) in graphite crucibles, in a glovebox filled with Ar (99.9992%) protective atmosphere (oxygen and water vapor pressure less than 1 ppm) to avoid oxidation. Wetting tests were carried out on copper (99.95%) and aluminum pads (99.9%). Mass of each sample used for spreading tests was 0.5 g, and the dimensions of both the copper and aluminum pads were 40 × 40 × 5 mm. Copper pads were prepared in accordance with EN ISO 9455-10:2000, and aluminum pads after pre-treatment of oxides were degreased with acetone. After preliminary, unpublished wetting tests with the use of six fluxes (ALU33, AFP200, TG-3, Aluminium 700, calaphony, and ZnCl2 solution), for this study two fluxes were selected: ALU33 and Aluminium 700. Both fluxes were modified by the addition of diethylamine hydrochloride (99%, Aldrich) diluted in isopropyl alcohol. In the case of other fluxes, either no wetting or wetting only on one type of substrate was observed.
For spreading measurements, a new setup (Fig. 1) was built. It consists of a horizontal furnace, the control module, and the module collecting and archiving data. The furnace consists of two zones: cold zone in which initially the sample is placed and hot zone in which measurements are performed. It can work either in a protective atmosphere or in vacuum. Using the specially designed loading system, after reaching the predetermined temperature in air or in vacuum, the samples are transferred from the cold zone to the hot zone of the furnace. This setup can also be used to measure surface tension, density and contact angle with the sessile drop method. Images were captured with CCD camera (Canon EOS-50D) with macro lens. Image analysis was performed using the application GetArea CorelDraw12.
The measure of wettability can be expressed as the size of the area covered by the solder or the maximum height of the layer of the liquid solder, and the respective coefficients taking into account the size. Sometimes, as a measure of wetting, the wetting angle shall be present in the Young-Dupree equation, describing the second law of the theory of capillarity; or the so-called wetting force, i.e., the force driving wetting of the specimen immersed in liquid solder (Ref 17). Taking into account the available literature and procedures according to norms: IEC 61189-5, IPC TM 650 2.4.46., and EN ISO 9455-10:2004, the experimental procedure given below was developed. The following quantities were adopted as a measure of spreading (wetting):
-
the area of spreading P AV, and the coefficient of spreading:
$$ K_{\text{p}} = \left( {P_{\text{AV}} - P_{\text{o}} } \right)/P_{\text{o}} $$where P AV is the average area of spreading of the solder; and P o is the area of the projection of sphere of volume V on the flat surface, according to the equation: P o = π[(3/(4π)) × V]2/3.
-
the height of the solder layer and height coefficient:
$$ K_{\text{H}} = \left( {D_{\text{o}} --H_{\text{sr}} } \right)/D_{\text{o}} \times 100\% $$where D o is the theoretical diameter of the spherical solder drop of volume V in the case of non-wetting, according to the equation: D o = 1.24V 1/3.
It should be noted that the coefficient K p indicates by how many times the averaged absolute value of the actual surface covered by solder (P AV − P o) is greater than the surface of the base material, obscured by the projection of a sample of the solder, which under conditions of total lack of wetting takes a spherical form. In order to calculate the spreading coefficients, it is necessary to know the densities of the liquid alloys. For this purpose, densities of liquid Sn-Zn and Al-Zn eutectic alloys were studied using dilatometric technique (Ref 13) over a broad range of temperatures. The error of estimation of the spreading coefficient, expressed as standard deviation of experimental data (5-7 separate samples per each set of experimental conditions), does not exceed 10%.
Results
The results of measurements of densities of liquid alloys, together with the estimated errors of parameters A and B of equations describing the temperature dependence of densities are presented in Table 1. One could see that the densities of both alloys decrease linearly with the increasing temperature.
Zn-Al Solder
Wettability measurements were carried out using a modified Al 700 flux in air and nitrogen. Wetting time was 3 min starting from the moment the sample started to melt.
Figure 2 shows typical images of the solidified samples after spreading measurements on copper, conducted in nitrogen and in air. In nitrogen atmosphere, copper pads were not oxidized, but the solder surface is full of large craters. This is probably due to the result of interactions between the protective atmosphere of nitrogen, flux, and the solder. Spreading test results of Zn-Al eutectic on copper pads presented in Fig. 3 indicate a negligible effect of the protective atmosphere on wetting properties. However, the nitrogen seems to worsen the spreading, which can partly be explained by the adverse impact of the flux. Furthermore, it was noted that with the increasing temperature, the solder wettability is improved, and the best results were obtained at 500 °C. Figure 4 presents the microstructures of the solder joints Zn-Al/Cu, after wetting at 500 °C in air. It seems that the layers occurring at the solder-pad interface correspond to the intermetallic phases from Cu-Zn system (Ref 18). According to Tuah-Poku et al. (Ref 19), after melting the solder, the processes occurring at the solder-pad interface can be divided into three stages. The first one involves the dissolution of copper by liquid zinc, the second the crystallizations, and the third the solid-state transformations. Wolczynski et al. (Ref 20) argue that there occurs a dissolution-crystallization cycle. Zn-rich intermetallic phases are formed as a result of reactive diffusion. From the diagram (Ref 18), one can see that the reactions that have occurred are peritectic reactions accompanying crystallization. Since the process was carried out at a temperature of 500 °C, it can be concluded that these reactions were supercooled reactions. The wetting time was 3 min, and so it may be presumed that we are dealing with the third stage, i.e., transformations in the solid state. This may adversely affect the mechanical properties of joints and also significantly increase the chance of formation of brittle cracks. In the further steps, microscopic connections created after 20, 40, 60, 90, and 120 s of wetting should be tested.
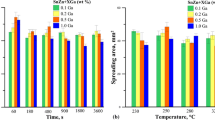
Figure 5 shows sample images from spreading measurements on aluminum conducted in nitrogen and air. Although the mass of samples in both cases was the same (0.5 g), the area of pad covered by solder is larger for the samples tested in air. This is confirmed by the spreading coefficients shown in Fig. 6. These studies have shown a negative effect of nitrogen on the solderability. Spreading tests of Zn-Al eutectic on aluminum, as in the case of copper pads, also showed an increase of wettability with the increase of measurement temperature. Considering this, we can conclude that when it comes to technology, the most optimal temperature appears to be 500 °C, and the soldering should be performed in air and not in protective nitrogen atmosphere.
The Zn-Al phase diagram shows that there are no intermetallic phases in this system. Therefore, wetting of aluminum pad by liquid Zn-Al eutectic is the result of adhesion and partial diffusion of Al from pads (Ref 11). Al-rich alloys, formed as a result of diffusion, are lighter than the liquid Zn-Al eutectic, and break away from the pads. As a result, the grain structure is formed. Al-rich grains are surrounded with eutectic matrix. Because of the Zn-Al eutectic is fragile, such structure may improve the strength of connections (Fig. 7).
Sn-Zn Solder
Spreading tests results of eutectic Sn-Zn on copper pads, performed in nitrogen protective atmosphere, are presented in Fig. 8 and 9. The results indicate that wetting properties of this solder on copper pads do not depend on temperature (up to 400 °C). Therefore, when it comes to economics, soldering temperature of 250 °C appears to be the most optimal. Preliminary, unpublished wetting tests have shown that in air atmosphere, without use of any flux, this solder does not wet copper pads. Lee and Shieu (Ref 21) studied the microstructure of the Sn-15Zn/Cu interface, depending on temperature and the type of the resulting intermetallic compound, to clarify the factors influencing the growth mechanism of intermetallic phases. After wetting at 230 °C, in solidified Sn-Zn/Cu couples, particles of β-CuZn phase were found, while after wetting at 250 and 270 °C, grains of ε-CuZn4 and γ-Cu5Zn8 were formed, respectively. Lee and Shieu (Ref 21) also found that the growth of γ-Cu5Zn8 phase is controlled by diffusions of Cu and Zn. Figure 10 shows the microstructure of the solder joints of Sn-Zn/Cu obtained at 250 °C, after a wetting duration of 3 min. Starting from the pad, as in the previous (Ref 8, 21), there is a thin strip of β-CuZn phase, further down there is a layer γ-Cu5Zn8, and from the side of solder, there is a thin strip of ε-CuZn4 phase.
Figure 11 shows the sample images from the wetting tests of Sn-Zn solder with ALU33 flux, conducted on an aluminum pad in a nitrogen atmosphere at temperatures of 250 and 400 °C. One can see that the area of pad covered by the solder is greater at 400 °C than at 250 °C. This is partly confirmed by the spreading coefficients shown in Fig. 12. As seen in Fig. 12, the spreading test results of Sn-Zn on aluminum pads are ambiguous. They show, on the one hand that the best wetting occurs at 350 and 400 °C (K p and P AV coefficients), and on the other hand, at 250 °C (K h coefficient). From the error bars shown in Fig. 12, one could see that K h coefficients for different temperatures are close to each other within the margin of error. In other words, the effect of temperature on K h coefficient is insignificant compared with other coefficients. On the other hand, K p and P AV coefficients show maximum value at midrange temperatures. This is in contradiction with results for the cases other than Sn-Zn/Al solder/substrate couples. Unfortunately, we cannot provide any reasonable explanation for the observed disagreement. Figure 13 shows the microstructures of eutectic solder joints of Sn-Zn/Al. It can be concluded that the aluminum pad is on the one hand activated by the mechanical removal of oxide layer and, on the other hand, by the action of flux. As a result, the interface is complex. The aluminum pad is dissolved in solder, and grain clusters are detached from the pad’s surface. They are lighter than the liquid solder and float toward the surface of solder (grains 1 and 2)
Conclusion
The measurements of wettability of eutectic Sn-Zn and Zn-Al alloys on the surfaces of copper and aluminum pads were conducted. The wettability problems discussed in other studies (Ref 15, 16), resulting from the presence of ZnO or Al2O3 at the solder/pad interface, were eliminated by the appropriate preparation of substrates, using an appropriate flux and atmosphere, as well as the proper choice of soldering temperature. These alloys can be used for soldering both copper and aluminum.
Spreading tests indicated that the wetting properties of eutectic solders based on Sn-Zn on copper pads do not depend on temperature (up to 400 °C), but in the lack of protective atmosphere, the solder does not wet pads. At the interface of Sn-Zn/Cu joints obtained at 250 °C, there is γ-Cu5Zn8 phase layer as observed in other studies (Ref 7, 21), and a thin strip of β-CuZn phase from the side of pad, and another thin strip of CuZn4 ε on the side of solder.
Wettability studies of Zn-Al eutectic on aluminum and copper substrates have shown a negative effect of the protective nitrogen atmosphere on wetting properties, especially for copper pads. However, the effect of atmosphere on wettability is one of many factors that should be taken into account when designing the soldering process. Final quality of the solder joint is also affected by solder composition, flux, temperature, process time, oxidation, and substrates. Furthermore, it is noted that with increasing temperature, the solder wettability is improved. Hence, it can be concluded that the soldering with Zn-Al eutectic should be conducted in the presence of flux at 500 °C, but without the protective atmosphere. The micrographs of the solidified solder/pad joints show that the liquid Zn-Al dissolves aluminum pads and causes detachment of whole grain clusters from the pads’ surface. These clusters of grains are lighter than the liquid solder, which float toward the surface.
Based on the literature (Ref 18) and the microstructures of the solder joints of Zn-Al/Cu-substrates, it can be concluded that the Zn-rich intermetallic phases are formed as a result of reactive diffusion. From the diagram of a previous study (Ref 16), it can be assumed that the reactions are peritectic reactions that have occurred accompanying crystallization. Moreover, because the process was carried out at a constant temperature of 500 °C, it can be concluded that these reactions were supercooled reactions. Next, after 3 min, we have to deal with secondary crystallization. The crystallizing grains of β phase c are arranged in a “chain” which can cause cracks. Considering this, it can be concluded that the process duration of the soldering of aluminum by Zn-Al eutectic should not be longer than 3 min.
References
M.E. Loomans, Investigation of Multi-Component Lead Free Solders, J. Electron. Mater., 1994, 23(8), p 741–765
J. Glazer, Metallurgy of Low Temperature Pb-free Solders for Electronic Assembly, Int. Mater. Rev., 1995, 40, p 65–78
H.H. Manko, Solders and Soldering, McGraw-Hill, New York, 2001
M. Abtew and G. Selvaduray, Lead-free Solders in Microelectronics, Mater. Sci. Eng. R, 2000, 27, p 95–102
H.Y. Xu, Z.F. Yuan, H. Matsuura, and F. Tsukihashi, Hysteresis Phenomenon and Wetting Characteristics of Molten Sn-3.0 wt.%Ag-0.5 wt.%Cu on Different Tilting Substrates, J. Alloys Compd., 2009, 487(1–2), p 121–125
Z. Moser, W. Gąsior, J. Pstruś, I. Ohnuma, and K. Ishida, Influence of Sb Additions on Surface Tension and Density of Sn-Sb, Sn-Ag-Sb and Sn-Ag-Cu-Sb Alloys. Experiment vs. Modeling, Int. J. Mater. R, 2006, 97, p 365–370
Y. Takaku, L. Felicia, I. Ohnuma, R. Kainuma, and K. Ishida, Interfacial Reaction Between Cu Substrates and Zn-Al Base High-Temperature Pb-Free Solders, J. Electron. Mater., 2008, 37, p 314–323
N. Kang, H.S. Na, S.J. Kim, and C.Y. Kang, Alloy Design of Zn-Al-Cu Solder for Ultra High Temperatures, J. Alloys Compd., 2009, 467, p 246–250
R. Kisiel, Podstawy technologii dla elektroników, BTC, Warszawa, 2005 (in Polish)
T. Radomski and A. Ciszewski, Lutowanie, WNT, Warszawa, 1971 (in Polish)
F.E. Bartell and H.J. Osterhof, Determination of Wettability of Solid by a Liquid, Ind. Eng. Chem., 1927, 19, p 1277–1280
S. Vaynman, G. Ghosh, and M.E. Fine, Some Fundamental Issues in the Use of Zn-Containing Lead-free Solders for Electronic Packaging, Mater. Trans., 2004, 45, p 630–636
J. Pstruś, Z. Moser, W. Gąsior, and A. Dębski, Surface Tension and Density Measurements of Liquid Sn-Zn Alloys. Experiment vs. SURDAT Database, Arch. Metall. Mater., 2006, 51, p 335–343
S. Ganesan and M. Pecht, Lead-free Electronics, Wiley-Interscience, New York, 2006
F. Hua and J. Glaze, Design and Reliability of Solders and Solder Interconnections, R.K. Mahidhara, S.M.L. Sastry, and P.K. Liaw, Ed., TMS, Warrendale, PA, 1997, p 65
Z. Chen, M. Li, X.X. Ren, A.M. Hu, and D.L. Mao, Effect of Small Additions of Alloying Elements on the Properties of Sn-Zn Eutectic Alloy, J. Electron. Mater., 2006, 35, p 1734–1739
W. Gąsior, Z. Moser, J. Pstruś, K. Bukat, R. Kisiel, and J. Sitek, Wettability Studies of the (Sn-Ag)eut+ Cu Soldering Materials Part 1, J. Phase Equilib. Diff., 2004, 25, p 115–121
N. Lebrun, Cu-Zn (Copper-Zinc), G. Effenberg, Ed., MSIT Binary Evaluation Program, in MSIT Workplace, MSI, Materials Science International Services GmbH, Stuttgart, 2003
I. Tuah-Poku, M. Dollar, and T. Massalski, A Study of the Transient Liquid Phase Bonding Process Applied to a Ag/Cu/Ag Sandwich Joint, Metall. Trans. A, 1988, 19, p 675–686
W.S. Wolczynski, T. Okane, C. Senderowski, D. Zasada, B. Kania, and J. Janczak-Rusch, Thermodynamic Justification for the Ni/AlNi Joint Formation by Diffusion Brazing, Int. J. Thermodyn., 2011, 14, p 97–105
C.S. Lee and F.S. Shieu, Growth of Intermetallic Compounds in Sn-9Zn/Cu Joint, J. Electron. Mater., 2006, 35, p 1660–1666
Acknowledgments
This study was financed under the framework of the project no. POIG.01.01.02-00-015/09, co-funded by the European Regional Development Fund (ERDF) and the Government of Poland under the Innovative Economy Program.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is an invited submission to JMEP selected from presentations at the Symposia “Wetting, soldering and brazing” and “Diffusion bonding and characterization” belonging to the Topic “Joining” at the European Congress and Exhibition on Advanced Materials and Processes (EUROMAT 2011), held September 12-15, 2011, in Montpellier, France, and has been expanded from the original presentation.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Pstruś, J., Fima, P. & Gancarz, T. Wetting of Cu and Al by Sn-Zn and Zn-Al Eutectic Alloys. J. of Materi Eng and Perform 21, 606–613 (2012). https://doi.org/10.1007/s11665-012-0174-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-012-0174-7