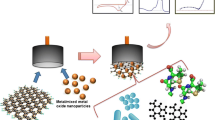
Abstract
BiPr oxide nanoparticles were successfully synthesized via a facile surfactant-free hydrothermal route using sodium bismuthate and praseodymium nitrate, and polyaniline/BiPr oxide nanoparticles were also obtained. The products were investigated by x-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy. The obtained nanoparticles with a diameter of about 50–200 nm are composed of polycrystalline structures with rhombohedral Bi0.4Pr0.6O1.5, monoclinic Bi2O3 and monoclinic Pr5O9 phases. Irregular nanoscale polyaniline particles cover the surface of the BiPr oxide. A pair of quasi-reversible cyclic voltammetry (CV) peaks are located at −0.04 V and −0.67 V, respectively, at the BiPr oxide nanoparticle-modified glassy carbon electrode (GCE) in 0.1 M KCl solution with 2 mM l-cysteine. The anodic CV peak shifts positively to +0.14 V and the cathodic CV peak shifts negatively to −0.82 V using the polyaniline/BiPr oxide nanoparticle-modified GCE. The linear range and detection limit are 0.005–2 mM and 1.18 μM, 0.0005–2 mM and 0.16 μM for the GCE modified with BiPr oxide nanoparticles and polyaniline composites, respectively. Polyaniline greatly enhances the electrochemical sensing properties of the BiPr oxide nanoparticle-modified GCE.
Graphical Abstract









Similar content being viewed by others
References
S.L.Yang, G. Li, N. Xia, P.P. Liu, Y.X. Wang, and L.B.Qu, High performance electrochemical l-cysteine sensor based on hierarchical 3D straw-bundle-like Mn-La oxides/reduced graphene oxide composite. J. Electroanal. Chem. 877, 114564 (2020).
A. Kurniawan, F. Kurniawan, F. Gunawan, and S.H. Chou. Disposable electrochemical sensor based on copper-electrodeposited screenprinted gold electrode and its application in sensing l-cysteine. Electrochim. Acta. 293, 318 (2019).
K. Atacan, CuFe2O4/reduced graphene oxide nanocomposite decorated with gold nanoparticles as a new electrochemical sensor material for l-cysteine detection. J. Alloys Compd. 791, 391 (2019).
W. Liu, J. Luo, and Y. Guo, Nanoparticle coated paper-based chemiluminescence device for the determination of l-cysteine. Talanta.120, 336 (2014).
X. Liu, L. Dong, L. Wang, H. Xu, S. Gao, L. Zhong, S. Zhang, and T. Jiang, 2-Aminopurine modified DNA probe for rapid and sensitive detection of l-cysteine. Talanta. 202, 520 (2019).
K. Tsuge, M. Kataoka, and Y. Seto, Determination of s-methyl-, s-propyl-, and spropenyl-lcysteine sulfoxides by gas chromatography-mass spectrometry after tertbutyldimethylsilylation. J. Agric. Food. Chem. 50, 4445 (2002).
A. Forgacsova, J. Galba, J. Mojzisova, P. Mikus, J. Piestansky, and A. Kovac, Ultra-high performance hydrophilic interaction liquid chromatography–triple quadrupole tandem mass spectrometry method for determination of cysteine, homocysteine, cysteinylglycine and glutathione in rat plasma. J. Pharmaceut. Biomed. 164, 442 (2019).
A. Petrova, R. Ishimatsu, K. Nakano, T. Imato, A. Vishnikin, L. Moskvin, and A. Bulatov, Flow-injection spectrophotometric determination of cysteine in biologically active dietary supplements. J. Anal. Chem. 71, 172 (2016).
A.V. Ivanov, P.O. Bulgakova, E. D. Virus, M.P. Kruglova, V.V. Alexandrin, V.A. Gadieva, B.P. Luzyanin, N.E. Kushlinskii, A.N. Fedoseev, and A.A. Kubatiev, Capillary electrophoresis coupled with chloroform-acetonitrile extraction for rapid and highly selective determination of cysteine and homocysteine levels in human blood plasma and urine. Electrophoresis. 38, 2646 (2017).
T. Matsunaga, T. Kondo, I. Shitanda, Y. Hoshi, M. Itagaki, T. Tojo, and M. Yuasa, Sensitive electrochemical detection of l-cysteine at a screen-printed diamond electrode. Carbon. 173, 395 (2021).
C. Varodi, F. Pogăcean, A. Cioriță, O. Pană, C. Leoștean, B. Cozar, T. Radu, M. Coroș, R.L.Ș. Staden, and S. M. Pruneanul, Nitrogen and sulfur Co-doped graphene as efficient electrode material for l-cysteine detection. Chemosensors. 9, 146 (2021).
T. Kokulnathan, R. Vishnuraj, T.J. Wang, E.A. Kumar, and B. Pullithadathil, Heterostructured bismuth oxide/hexagonal-boron nitride nanocomposite: a disposable electrochemical sensor for detection of flutamide. Ecotoxicol. Environ. Saf. 207, 111276 (2021).
L.Z. Pei, N. Lin, T. Wei, H.D. Liu, and H.Y. Yu, Formation of copper vanadate nanobelts and the electrochemical behaviors for the determination of ascorbic acid. J. Mater. Chem. A. 3, 2690 (2015).
L.Z. Pei, F.L. Qiu, Y. Ma, F.F. Lin, C.G. Fan, X.Z. Ling, and S.B. Zhu, Graphene/zinc bismuthate nanorods composites and their electrochemical sensing performance for ascorbic acid. Fuller. Nanotub. Car. N. 27, 58 (2019).
S. Abdollah and H. Rahman, Catalytic oxidation of thiols at preheated glassy carbon electrode modified with abrasive immobilization of multiwall carbon nanotubes: applications to amperometric detection of thiocytosine l-cysteine nd glutathione. Talanta. 66, 967 (2005).
L.Z. Pei, T. Wei, N. Lin, H. Zhang, and C.G. Fan, Bismuth tellurate nanospheres and electrochemical behaviors of l-cysteine at the nanospheres modified electrode. Russ. J. Electrochem. 54, 84 (2018).
D.R. Kumar, M.L. Baynosa, and J.J. Shim, Cu2+-1,10-phenanthroline-5,6-dione electrochemically reduced graphene oxide modified electrode for the electrocatalytic determinationof l-cysteine. Sens. Actuat. B-Chem. 293, 107 (2019).
L.Z. Pei, T. Wei, N. Lin, Z.Y. Cai, C.G. Fan, and Z. Yang, Synthesis of zinc bismuthate nanorods and electrochemical performance for sensitive determination of l-cysteine. J. Electrochem. Soc. 163:H1 (2016).
H.R. Zare, F. Jahangiri-Dehaghani, Z. Shekari, and A. Benvidi, Electrocatalytic simultaneous determination of ascorbic acid, uric acid and l-cysteine in real samples using quercetin silver nanoparticles–graphene nanosheets modified glassy carbon electrode. Appl. Surf. Sci. 375,169 (2016).
R. María-Hormigos, M.J. Gismera, and M.T. Sevilla, Straightforward ultrasound-assisted synthesis of bismuth oxide particles with enhanced performance for electrochemical sensors development. Mater. Lett. 158, 359 (2015).
L.Z. Pei, C.H. Yu, Z.Y. Xue, and Y. Zhang, A review on ternary bismuthate nanoscale materials. Recent Pat. Nanotech. 15, 142 (2021).
P. Sonström, J. Birkenstock, Y. Borchert, L. Schilinsky, P. Behrend, K. Gries, K. Müller, A. Rosenauer, and M. Bäumer, Nanostructured praseodymium oxide: correlation between phase transitions and catalytic activity. ChemCatChem. 2, 694 (2010).
Q.C. Zhang, W.L. Yan, L. Jiang, Y.G. Zheng, J.X. Wang , and R.K. Zhang, Synthesis of nano-praseodymium oxide for cataluminescence sensing of acetophenone in exhaled breath. Molecules. 24, 4275 (2019).
K. Sato, K. Imamura, Y. Kawano, S.I. Miyahara, T. Yamamoto, S. Matsumura, and K. Nagaoka, A low-crystalline ruthenium nano-layer supported on praseodymium oxide as an active catalyst for ammonia synthesis. Chem. Sci. 8, 674 (2017).
A. Doménech, N. Montoya, and J. Alarcón, Electrochemical characterization of praseodymium centers in PrxZr1-xO2 zirconias using electrocatalysis and photoelectrocatalysis. J. Solid State Electrochem. 16, 963 (2012).
S. Shrestha, F. Marken, J. Elliott, C.M.Y. Yeung, C.E. Mills, and S.C. Tsang, Electrochemical deposition of praseodymium oxide on tin-doped indium oxide as a thin sensing film. J. Electrochem. Soc. 153, C517 (2006).
M. Zidan, W.T. Tan, A.H. Abdullah, Z. Zainal, and G.J. Kheng, Electrochemical oxidation of ascorbic acid mediated by Bi2O3 microparticles modified glassy carbon electrode. Int. J. Electrochem. Sci. 6, 289 (2011).
D. Xu, K. He, R.H. Yu, Y. Tong, J.P. Qi, X.J. Sun, Y.T. Yang, H.X. Xu, H.M. Yuan, and J. Ma, Microstructure and electrical properties of praseodymium oxide doped Bi2O3 based ZnO varistor films. Mater. Technol. 30, A24 (2015).
B. Zhang, G.J. Liu, X. Yao, X.L. Li, B. Jin, L.J. Zhao, X.Y. Lang, Y.F. Zhu, and Q. Jiang, CoMoO3 nanoplate/reduced graphene oxide composites decorated with Ag nanoparticles for electrocatalytic water oxidation. ACS Appl. Nano Mater. 4, 5383 (2021).
M. Eising, C. O’Callaghan, C.E. Cava, A. Schmidt, A.J.G. Zarbin, M.S. Ferreira, and L.S. Roman, The role of carbon nanotubes on the sensitivity of composites with polyaniline for ammonia sensors. Carbon Trends. 3,100026 (2021).
F. Saadati, F. Ghahramani, H. Shayani-jam, F. Piri, and M.R. Yaftian, Synthesis and characterization of nanostructure molecularly imprinted polyaniline/graphene oxide composite as highly selective electrochemical sensor for detection of p-nitrophenol. J. Taiwan Inst. Chem. E. 86, 213 (2018).
V. Gautam, K.P. Singh, and V.L. Yadav, Preparation and characterization of green-nano-composite material based on polyaniline, multiwalled carbon nano tubes and carboxymethyl cellulose: for electrochemical sensor applications. Carbohyd. Polym. 189, 218 (2018).
X.Z. Lin, B. Guo, Y.F. Qiu , P.R. Xu, and H.B. Fan, Surfactant-free hydrothermal synthesis of CePO4 microbundles assembled by aligned nanorods. Mater. Lett. 197, 49 (2017).
W. Jiang, T.S. Huangfu, X. Yang, L. Bao, Y. Liu, G. Xu, and G.R. Han, Surfactant-free hydrothermal synthesis of hierarchical flower-like Bi2WO6 mesosphere nanostructures with excellent visible-light photocatalytic activity. CrystEngComm. 21, 6293 (2019).
W. Zhang, L.L. Feng, H.Y. Chen, and Y.Y. Zhang, Hydrothermal synthesis of SnO2 nanorod as anode materials for lithium-ion battery. NANO. 14, 1950109 (2019)
L.Z. Pei, S. Wang, Y.X. Jiang, Y. Li, Y.K. Xie, and Y.H. Guo, Single crystalline Sr germanate nanowires and their photocatalytic performance for the degradation of methyl blue. CrystEngComm. 15, 7815 (2013).
L.Z. Pei, N. Lin, T. Wei, H.D. Liu, and H.Y. Yu, Zinc vanadate nanorods and their visible light photocatalytic activity. J. Alloys Compd. 631, 90 (2015).
T. Preethi, K. Senthil, S. Ashokan, B. Sundaravel, and P. Saravanan, Structural, optical and magnetic properties of SnO2 nanoparticles synthesized via surfactant-free hydrothermal process. Mater. Today. 47, 2097 (2021).
Y.P. Luo, J.W. Chen, J. Chen, J.W. Liu, Y. Shao, X.F. Li, and D.Z. Li, Hydroxide SrSn(OH)6: a new photocatalyst for degradation of benzene and rhodamine B. Appl. Catal. B: Environ. 182, 533 (2016).
J.X. Wu, Z.M. Wang, S. Li, and M.S. Ma, In situ photoemission study of a Pr2O3 thin film on GaAs(111). J. Vac. Sci. Technol. A. 22, 594 (2004)
Z.T. Meng, Y.D. Huang, Y.C. Fang, X.C. Wang , Y. Guo, Z.J. Liu, M.R. Xi, W.Q. Su, and L. Wang. Facile preparation of praseodymium oxide coated peanut-like lithium nickel cobalt manganese oxide microspheres for lithium ion batteries with high voltage capabilities. J. Alloys Compd. 784, 620 (2019)
M. Ramezanzadeh, G. Bahlakeh, Z. Sanaei, and B. Ramezanzadeh, Interfacial adhesion and corrosion protection properties improvement of a polyester-melamine coating by deposition of a novel green praseodymium oxide nanofilm: a comprehensive experimental and computational study. J. Ind. Eng. Chem. 74, 26 (201)
J. Liao, K.D. Li, Y. Zhang, and L. Zhang, Facile synthesis of a novel ultra-low density praseodymium oxide aerogel for catalyst and adsorbent. Mater. Lett. 254, 364 (2019).
V. Kannan, M. Arredondo, F. Johann, D. Hesse, C. Labrugere, M. Maglione, and I. Vrejoiu, Strain dependent microstructural modifications of BiCrO3 epitaxial thin films. Thin Solid Films. 545, 130 (2013).
T. Unmüssig, A. Weltin, S. Urban, P. Daubinger, G.A. Urban, and J. Kieninger, Non-enzymatic glucose sensing based on hierarchical platinum micro-/nanostructures. J. Electroanal. Chem. 816, 215 (2018)
R. Nyholm, A. Berndtsson, and N. Martensson, Core level binding energies for the elements Hf to Bi (Z=72-83). J. Phys. C: Solid State Phys. 13, L1091 (1980)
C. Du, D.H. Li, Q.Y. He, J.M. Liu, W. Li, G.N. He, and Y.Z. Wang, Design and simple synthesis of composite Bi12TiO20/Bi4Ti3O12 with a good photocatalytic quantum efficiency and high production of photo-generated hydroxyl radicals. Phys. Chem. Chem. Phys. 18, 26530 (2016).
S. Palaniappan, Chemical and electrochemical polymerization of aniline using tartaric acid. Eur. Poly. J. 37, 975 (2001)
D. Boyne, N. Menegazzo, R.C. Pupillo, J. Rosenthal, and K.S. Booksh, Vacuum thermal evaporation of polyaniline doped with camphorsulfonic acid. J. Vac. Sci. Technol. A. 33, 031510 (2015)
S. Adhikari and P. Banerji, Enhanced conductivity in iodine doped polyaniline thin film formed by thermal evaporation. Thin Solid Films. 518, 5421 (2010).
S. Zinatloo-Ajabshir and M. Salavati-Niasari, Novel poly(ethyleneglycol)-assisted synthesis of praseodymium oxide nanostructures via a facile precipitation route. Ceram. Int. 41, 567 (2015).
B.M. Abu-Zied, Controlled synthesis of praseodymium oxide nanoparticles obtained by combustion route: effect of calcination temperature and fuel to oxidizer ratio. Appl. Surf. Sci. 471, 246 (2019)
X.C. Dou, Y.G. Chen, and H.F. Shi, CuBi2O4/BiOBr composites promoted PMS activation for the degradation of tetracycline: S-scheme mechanism boosted Cu2+/Cu+ cycle. Chem. Eng. J. 431, 134054 (2022).
T.R. Das, P.K. Sharma, Sensitive and selective electrochemical detection of Cd2+ by using bimetal oxide decorated graphene oxide (Bi2O3/Fe2O3@GO) electrode. Microchem. J. 147, 1203 (2019).
X. Wang, C. Yang, T. Wang, and P. Liu, Praseodymium oxide/polypyrrole nanocomposites for electrochemical energy storage. Electrochim. Acta. 58, 193 (2011).
M. Popa and M. Kakihana, Praseodymium oxide formation by thermal decomposition of a praseodymium complex. Solid State Ion. 141–142, 265 (2001)
M. Mostafavi, M.R. Yaftian, F. Piri, and H. Shayani-Jam, A new diclofenac molecularly imprinted electrochemical sensor based upon a polyaniline/reduced graphene oxide nano-composite. Biosens. Bioelectron. 122, 160 (2018).
C.U. Seo, Y. Yoon, D.H. Kim, S.Y. Choi, W.K. Park, J.S. Yoo, B. Baek, S.B. Kwon, C.M. Yang, Y.H. Song, D.H. Yoon, W.S. Yang, and S. Kim, Fabrication of polyaniline-carbon nano composite for application in sensitive flexible acid sensor. J. Ind. Eng. Chem. 64, 97 (2018).
M.M. Hasan, T. Islam, A. Imran, B. Alqahtani, S.S. Shah, W. Mahfoz, M.R. Karim, H.F. Alharbi, M.A. Aziz, and A.J.S. Ahammad, Mechanistic insights of the oxidation of bisphenol A at ultrasonication assisted polyaniline-Au nanoparticles composite for highly sensitive electrochemical sensor. Electrochim. Acta. 374, 137968 (2021).
L.Z. Pei, H.D. Liu, N. Lin, Y.K. Xie, and Z.Y. Cai, Electrochemical analysis of cyanuric acid using polyaniline/CuGeO3 nanowires as electrode modified materials. Curr. Pharm. Anal. 11, 16 (2015)
T. Osaka, S. Ogano, K. Naoi, and N. Oyama, Electrochemical polymerization of electroactive polyaniline in nonaqueous solution and its application in rechargeable lithium batteries. J. Electrochem. Soc. 136, 306 (1989).
J.E. Park, S.G. Park, A. Koukitu, O. Hatozaki, and N. Oyama Effect of adding Pd nanoparticles to dimercaptan-polyaniline cathodes for lithium polymer battery. Synthetic Met. 140, 121 (2004).
F. Cao, Y.K. Huang, F. Wang, D. Kwak, Q.C. Dong, D.H. Song, J. Zeng, and Y. Lei, A high-performance electrochemical sensor for biologically meaningful l-cysteine based on a new nanostructured l-cysteine electrocatalyst. Anal. Chim. Acta. 1019, 103 (2018).
J. Zhang, W.S. Tan, Y.X. Tao, L.H. Deng, Y. Qin, and Y. Kong, A novel electrochemical chiral interface based on sandwich-structured molecularly imprinted SiO2/AuNPs/SiO2 for enantioselective recognition of cysteine isomers. Electrochem. Commun. 86, 57 (2018).
G. Manibalan, G. Murugadoss, R. Thangamuthu, M.R. Kumar, R.M. Kumar, and R. Jayavel, CeO2-based heterostructure nanocomposite for electrochemical determination of l-cysteine biomolecule. Inorg. Chem. Commun. 113, 107793 (2020).
M. Hussain, N. Khaliq, A.A. Khan, M. Mhan, G. Ali, and M. Maqbool, Synthesis, characterization and electrochemical analysis of TiO2 nanostructures for sensing l-cysteine and hydrogen peroxide. Physica E. 128, 114541 (2021).
M. Zhou, J. Ding, L.P. Guo, and Q.K. Shang, Electrochemical behavior of l-cysteine and its detection at ordered mesoporous carbon-modified glassy carbon electrode. Anal. Chem. 79, 5328 (2007).
P. Sharma, S. Kaur, S. Chaudhary, A. Umar, and R. Kumar, Bare and non-ionic surfactant-functionalized praseodymium oxide nanoparticles: toxicological studies. Chemosphere. 209, 1007 (2018).
S. Sato, R. Takahashi, T. Sodesawa, A. Igarashi, and H. Inoue Catalytic reaction of 1,3-butanediol over rare earth oxides. Appl. Catal. A: Gen. 328, 109 (2007).
Z.N. Liu, H.C. Zhang, S.F. Hou, and H.Y. Ma, Highly sensitive and selective electrochemical detection of l-cysteine using nanoporous gold. Microchim. Acta. 177, 427 (2012).
S.D. Fei, J.H. Chen, S.Z. Yao, G. H. Deng, D.L. He, and Y.F. Kuang, Electrochemical behavior of l-cysteine and its detection at carbon nanotube electrode modified with platinum. Anal. Biochem. 339, 29 (2005).
X.J. Wang, L.L. Zhang, L.X. Miao, M.X. Kan, L.L. Kong, and H.M. Zhang, Oxidation and detection of l-cysteine using a modified Au/Nafion/glassy carbon electrode. Sci. China Chem. 54, 521 (2011).
S. Yang, G. Li, Y. Wang, G. Wang, and L. Qu, Amperometric l-cysteine sensor based on a carbon paste electrode modified with Y2O3 nanoparticles supported on nitrogen-doped reduced graphene oxide. Microchim. Acta. 183, 1351 (2016).
H. Heli, M. Hajjizadeh, A. Jabbari, and A. Moosavi-movahedia fine steps of electrocatalytic oxidation and sensitive detection of some amino acids on copper nanoparticles. Anal. Biochem. 388, 81 (2009).
Y.H. Bai, J.J. Xu, H.Y. Chen, Selective sensing of cysteine on manganese dioxide nanowires and chitosan modified glassy carbon electrodes. Biosens. Bioelectron. 24, 2985 (2009).
C.Y. Deng, J.H. Chen, X.L. Chen, M.D. Wang, Z. Nie, and S. Z. Yao, Electrochemical detection of l-cysteine using a boron-doped carbon nanotube-modified electrode. Electrochim. Acta. 54, 3298 (2009).
Y.P. Dong, L.Z. Pei, X.F. Chu, W.B. Zhang, Q.F. Zhang, Electrochemical behavior of cysteine at a CuGeO3 nanowires modified glassy carbon electrode. Electrochim. Acta. 55, 5135 (2010).
L.Z. Pei, Z.Y. Cai, Y.Q. Pei, Y.K. Xie, C.G. Fan, and D.G. Fu, Electrochemical determination of l-Cysteine using CuGeO3/polyaniline nanowires modified electrode. Russ. J. Electrochem. 50, 458 (2014).
G. Ziyatdinova, L. Grigor’eva, M. Morozov, A. Gilmutdinov, and H. Budnikov, Electrochemical oxidation of sulfur-containing amino acids on an electrode modified with multi-walled carbon nanotubes. Microchim. Acta. 165, 359 (2009).
N. Spataru, B.V. Sarada, E. Papa, D.A. Tryk, and A. Fujishima, Voltammetric determination of l-cysteine at conductive diamond electrodes. Anal. Chem. 73, 514 (2001).
M. Ahmad, C.F. Pan, and J. Zhu, Electrochemical determination of l-cysteine by an elbow shaped, Sb-doped ZnO nanowire-modified electrode. J. Mater. Chem. 20, 7169 (2010).
X.F. Tang, Y. Liu, H.Q. Hou, and T.Y. You, Electrochemical determination of l-tryptophan, l-tyrosine and l-cysteine using electrospun carbon nanofibers modified electrode. Talanta. 80, 2182 (2010).
Z. Chen, H. Zheng, C. Lu, and Y. Zu, Oxidation of l-cysteine at a fluorosurfactant-modified gold electrode: lower overpotential and higher selectivity. Langmuir. 23, 10816 (2007).
S.M. Chen, J.Y. Chen, and R. Thangamuthu, Electrochemical Preparation of brilliant-blue-modified poly(diallyldimethylammonium chloride) and nafion-coated glassy carbon electrodes and their electrocatalytic behavior towards oxygen and l-cysteine. Electroanal. 20, 1565 (2008).
A. Salimi, and R. Hallaj, Catalytic oxidation of thiols at preheated glassy carbon electrode modified with abrasive immobilization of multiwall carbon nanotubes: applications to amperometric detection of thiocytosine, l-cysteine and glutathione. Talanta. 66, 967 (2005).
Acknowledgments
Not applicable.
Funding
This work was supported by the Natural Science Foundation of Anhui Province of P. R. China (Grant No. 2008085ME172), Natural Science Foundation of Fujian Province of P. R. China (2019J01872), Open Fund of Fujian Provincial Key Laboratory of Functional Materials and Applications (fma2018010) and Scientific Research Climb Plan of Xiamen University of Technology (XPDKT19035).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, J., Cai, Z., Zhang, Y. et al. A Simple Route to Synthesize Mixed BiPr Oxide Nanoparticles and Polyaniline Composites with Enhanced l-Cysteine Sensing Properties. J. Electron. Mater. 52, 613–627 (2023). https://doi.org/10.1007/s11664-022-10033-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-022-10033-x