Abstract
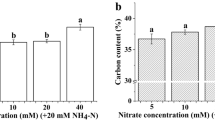
Natural nitrogen isotope composition (δ15N) is an indicator of nitrogen sources and is useful in the investigation of nitrogen cycling in organisms and ecosystems. δ15N is also used to study assimilation of inorganic nitrogen. However, the foliar δ15N of intact plants, which is a consequence of nitrate assimilation occurring in the roots and shoots, is not suited for studying nitrate assimilation in cases where nitrate is the sole nitrogen source. In this study, Orychophragmus violaceus (Ov) and Brassica napus (Bn) plantlets, in which nitrate assimilation occurred in the leaves, were used to study the relationship between foliar δ15N and nitrate assimilation. The plantlets were grown in vitro in culture media with different nitrate concentrations, and no root formation occurred for the plantlets during the multiplication stage. Nitrogen isotope fractionation occurred in both the Ov and the Bn plantlets under all treatments. Furthermore, the foliar nitrogen content of both the Ov and Bn plantlets increased with increasing nitrate concentration. Foliar nitrogen isotope fractionation was negatively correlated with foliar nitrogen content for both the Ov and Bn plantlets. Our results suggest that the foliar nitrogen isotope fractionation value could be employed to evaluate nitrate assimilation ability and leaf nitrate reductase activity. Moreover, high external nitrate concentrations could contribute to improved foliar nitrogen content and enhanced nitrate assimilation ability.



Similar content being viewed by others
References
Ahmad I, Iqbal M, Ahmad B, Ahmad G, Shah NH (2009) Maize yield, plant tissue and residual soil N as affected by nitrogen management and tillage systems. J Agric Biol Sci 1(1):19–29
Black BL, Fuchigami LH, Coleman GD (2002) Partitioning of nitrate assimilation among leaves, stems and roots of poplar. Tree Physiol 22(10):717–724
Britto DT, Kronzucker HJ (2006) Futile cycling at the plasma membrane: a hallmark of low-affinity nutrient transport. Trends Plant Sci 11(11):529–534
Campbell WH (1999) Nitrate reductase structure, function and regulation: bridging the gap between biochemistry and physiology. Annu Rev Plant Biol 50(1):277–303
Cheema MA, Malik MA, Hussain A, Shah SH, Basra SMA (2001) Effects of time and rate of nitrogen and phosphorus application on the growth and the seed and oil yields of canola (Brassica napus L.). J Agron Crop Sci 186(2):103–110
Choi WJ, Lee SM, Ro HM, Kim KC, Yoo SH (2002) Natural 15N abundances of maize and soil amended with urea and composted pig manure. Plant Soil 245(2):223–232
Comstock J (2001) Steady-state isotopic fractionation in branched pathways using plant uptake of NO3—as an example. Planta 214(2):220–234
Crawford NM (1995) Nitrate: nutrient and signal for plant growth. Plant Cell 7(7):859
Cuin TA, Shabala S (2005) Exogenously supplied compatible solutes rapidly ameliorate NaCl-induced potassium efflux from barley roots. Plant Cell Physiol 46(12):1924–1933
Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP (2002) Stable isotopes in plant ecology. Annu Rev Ecol Syst 33(1):507–559
Del Amor FM, Cuadra-Crespo P (2011) Alleviation of salinity stress in broccoli using foliar urea or methyl-jasmonate: analysis of growth, gas exchange, and isotope composition. Plant Growth Regul 63(1):55–62
Denton TM, Schmidt S, Critchley C, Stewart GR (2001) Natural abundance of stable carbon and nitrogen isotopes in Cannabis sativa reflects growth conditions. Funct Plant Biol 28(10):1005–1012
Evans RD (2001) Physiological mechanisms influencing plant nitrogen isotope composition. Trends Plant Sci 6(3):121–126
Evans RD, Bloom AJ, Sukrapanna SS, Ehleringer JR (1996) Nitrogen isotope composition of tomato (Lycopersicon esculentum Mill. cv. T-5) grown under ammonium or nitrate nutrition. Plant Cell Environ 19(11):1317–1323
George EF, Hall MA, De Klerk GJ (2008) The components of plant tissue culture media I: macro-and micro-nutrients. In: George EF (ed) Plant propagation by tissue culture, 3rd edn. Springer, Berlin, pp 65–113
Gulmon SL, Chu CC (1981) The effects of light and nitrogen on photosynthesis, leaf characteristics, and dry matter allocation in the chaparral shrub, Diplacus aurantiacus. Oecologia 49(2):207–212
Handley LL, Raven JA (1992) The use of natural abundance of nitrogen isotopes in plant physiology and ecology. Plant Cell Environ 15(9):965–985
Handley LL, Robinson D, Forster BP et al (1997) Shoot δ15N correlates with genotype and salt stress in barley. Planta 201(1):100–102
Hawkesford M, Horst W, Kichey T, Lambers H, Schjoerring J, Skrumsager MI, White P (2012) Functions of macronutrients. In: Marscher H (ed) Mineral nutrition of higher plants, 3rd edn. Academic Press, San Diego, pp 135–190
Hunt ER, Weber JA, Gates DM (1985) Effects of nitrate application on Amaranthus powellii Wats. I. Changes in photosynthesis, growth rates, and leaf area. Plant Physiol 79(3):609–613
Kaiser WM, Huber SC (2001) Post-translational regulation of nitrate reductase: mechanism, physiological relevance and environmental triggers. J Exp Bot 52(363):1981–1989
Kalcsits LA, Guy RD (2013) Quantifying remobilization of pre-existing nitrogen from cuttings to new growth of woody plants using 15N at natural abundance. Plant methods 9(1):1–9
Kalcsits LA, Buschhaus HA, Guy RD (2014) Nitrogen isotope discrimination as an integrated measure of nitrogen fluxes, assimilation and allocation in plants. Physiol Plant 151(3):293–304
Kalcsits LA, Min X, Guy RD (2015) Interspecific variation in leaf–root differences in δ15N among three tree species grown with either nitrate or ammonium. Trees 29(4):1069–1078
Kiba T, Kudo T, Kojima M, Sakakibara H (2010) Hormonal control of nitrogen acquisition: roles of auxin, abscisic acid, and cytokinin. J Exp Bot 62(4):1399–1409
Li Z, Liu HL, Luo P (1995) Production and cytogenetics of intergeneric hybrids between Brassica napus and Orychophragmus violaceus. Theor Appl Genet 91(1):131–136
Luo P, Zhou J, Wu Y, Zhang X (1995) Experimental results of the regeneration ability of different explants of Orychophragmus violaceus. Hereditas 18(1):23–25
Mariotti A, Mariotti F, Champigny ML, Amarger N, Moyse A (1982) Nitrogen isotope fractionation associated with nitrate reductase activity and uptake of NO3 − by pearl millet. Plant Physiol 69(4):880–884
Mendel RR, Alikulov ZA, Müller AJ (1982) Molybdenum cofactor in nitrate reductase-deficient tobacco mutants. III. Induction of cofactor synthesis by nitrate. Plant Sci Lett 27(1):95–101
Miyawaki K, Matsumoto-Kitano M, Kakimoto T (2004) Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate. Plant J 37(1):128–138
Moradi F, Ismail AM (2007) Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann Bot 99(6):1161–1173
Munir MA, Malik MA, Saleem MF (2007) Impact of integration of crop manuring and nitrogen application on growth, yield and quality of spring planted sunflower (Helianthus annuus L.). Pak J Bot 39(2):441–449
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Novoa R, Loomis RS (1981) Nitrogen and plant production. Plant Soil 58(1–3):177–204
Nowak B, Miczyński K, Hudy L (2007) The effect of total inorganic nitrogen and the balance between its ionic forms on adventitious bud formation and callus growth of ‘Węgierka Zwykła’ plum (Prunus domestica L.). Acta Physiol Plant 29(5):479–484
Pate JS, Stewart GR, Unkovich M (1993) Characteristics of inorganic nitrogen assimilation of plants in fire-prone Mediterranean-type vegetation. Plant Cell Environ 16(4):351–363
Piao HC, Wu YY, Hong YT, Yuan ZY (2000) Soil-released carbon dioxide from microbial biomass carbon in the cultivated soils of karst areas of southwest China. Biol Fertil Soils 31(5):422–426
Poothong S, Reed BM (2016) Optimizing shoot culture media for Rubus germplasm: the effects of NH4 +, NO3 −, and total nitrogen. In Vitro Cell Dev Biol Plant 52(3):265–275
Pritchard ES, Guy RD (2005) Nitrogen isotope discrimination in white spruce fed with low concentrations of ammonium and nitrate. Trees 19(1):89–98
Rafiq MA, Ali A, Malik MA, Hussain M (2010) Effect of fertilizer levels and plant densities on yield and protein contents of autumn planted maize. Pak J Agric Sci 47(3):201–208
Raven JA (2003) Can plants rely on nitrate? Trends Plant Sci 8:314–315
Raven JA, Handley LL, Andrews M (2004) Global aspects of C/N interactions determining plant–environment interactions. J Exp Bot 55(394):11–25
Robinson D (2001) δ15N as an integrator of the nitrogen cycle. Trends Ecol Evol 16(3):153–162
Robinson D, Handley LL, Scrimgeour CM (1998) A theory for 15N/14N fractionation in nitrate-grown vascular plants. Planta 205(3):397–406
Stewart WM, Dibb DW, Johnston AE, Smyth TJ (2005) The contribution of commercial fertilizer nutrients to food production. Agron J 97(1):1–6
Wang SJ, Liu QM, Zhang DF (2004) Karst rocky desertification in southwestern China: geomorphology, landuse, impact and rehabilitation. Land Degrad Dev 15(2):115–121
Wang YY, Hsu PK, Tsay YF (2012) Uptake, allocation and signaling of nitrate. Trends Plant Sci 17:458–467
Wu YY, Liu CQ, Wang SJ (2004) Research on the adaptation mechanisms of Orychophragmus violaceus in karst regions. Guizhou Science and Technology Press, Guiyang
Yan J, Li J, Ye Q, Li K (2012) Concentrations and exports of solutes from surface runoff in Houzhai Karst Basin, southwest China. Chem Geol 304:1–9
Yousfi S, Serret MD, Araus JL (2009) Shoot δ15N gives a better indication than ion concentration or Δ13C of genotypic differences in the response of durum wheat to salinity. Funct Plant Biol 36(2):144–155
Yousfi S, Serret MD, Araus JL (2013) Comparative response of δ13C, δ18O and δ15N in durum wheat exposed to salinity at the vegetative and reproductive stages. Plant Cell Environ 36(6):1214–1227
Yuan DX (1988) On the karst environmental system. Carsologica Sin 3:30–46
Acknowledgements
This work was supported by the National Key Research and development Program of China (2016YFC0502602), the National Natural Science Foundation of China (U1612441), and the project of high-level innovative talents of Guizhou Province [2015(4035)].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, K., Wu, Y. The δ15N response and nitrate assimilation of Orychophragmus violaceus and Brassica napus plantlets in vitro during the multiplication stage cultured under different nitrate concentrations. Acta Geochim 36, 190–197 (2017). https://doi.org/10.1007/s11631-017-0156-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-017-0156-4