Summary
Plants of Diplacus aurantiacus, a successional shrub common in California chaparral, were grown under controlled conditions in which either quantum flux density or nitrogen availability was varied. Photosynthesis and leaf nitrogen content were determined on a leaf area and a leaf weight basis, and whole plant growth was monitored.
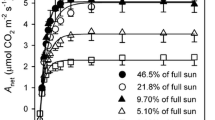
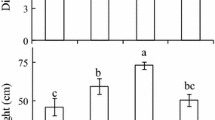
There was a direct relationship between photosynthesis and leaf nitrogen content on both area and weight bases. Reduced light intensity of the growth environment resulted in reductions in light-saturated photosynthesis and nitrogen content on an area basis, but not on a weight basis. With reduced nitrogen availability, photosynthesis and leaf nitrogen content per unit leaf weight decreased.
Resource use efficiency increased as the resource became more limiting. The results are consistent with a model of plant growth in which net carbon gain of the leaf is maximized.
Similar content being viewed by others
References
Björkman O (1973) Comparative studies on photosynthesis in higher plants. In: AC Giese (ed) Photophysiology Vol. III Academic Press New York p 1–63
Björkman O (1979) Temperature effects on photosynthesis. In: Plants and their environment. Proc British Ecological Society, March 26–30
Björkman O, Boardman NK, Anderson JM, Thorne SW, Goodchild DJ, Pyliotis NA (1972) Effect of light intensity during growth of Atriplex patula on the capacity of photosynthetic reactions, chloroplast components and structure. Carnegie Inst Wash Year Book 71:115–134
Björkman O, Nobs M, Berry J, Mooney H, Nichols F, Catanzaro B (1973) Physiological adaptations to diverse environments: approaches and facilities to study plant responses to contrasting thermal and water regimes. Carnegie Inst Wash Year Book 72:393–403
Brown RH (1978) A difference in N use efficiency in C3 and C4 plants and its implications in adaptation and evolution. Crop Sci 18:93–98
Chabot BF, Jurik TW, Chabot JF (1979) Influence of instantaneous and integrated light-flux density on leaf anatomy and photosynthesis. Am J Bot 66:940–945
Clough JM, Alberte RS, Teeri JA (1979) Photosynthetic adaptation of Solanum dulcamara L. to sun and shade environments. II. Physiological characterization of phenotypic response to environment. Plant Physiol 64:25–30
Gordon KHJ, Peoples MB, Murray DR (1978) Ageing-linked changes in photosynthetic capacity and in fraction I protein content of the first leaf of pea Pisum sativum L. New Phytol 81:35–42
Hurd RG, Thornely JHM (1974) An analysis of the growth of young tomato plants in water culture at different light integrals and CO2 concentrations. I. Physiological aspects. Ann Bot (London) 38:385–388
Isaac RA, Johnson WC (1976) Determination of total nitrogen in plant tissue, using a block digestor. J Association of Official Analytical Chemists 59:98–100
Larcher W (1975) Physiological Plant Ecology. Springer Berlin Heidelberg New York
Longstreth DJ, Nobel PS (1980) Nutrient influences on leaf photosynthesis effects of nitrogen, phosphorus, and potassium for Gossypium hirsutum L Plant Physiol 65:541–543
Medina E (1971) Effect of nitrogen supply and light intensity during growth on the photosynthetic capacity and carboxydismutase activity of leaves of Atriplex patula ssp. hastata. Carnegie Inst Wash Year Book 70:551–559
Mooney HA, Ehleringer J, Berry JA (1976) High photosynthetic capacity of a winter annual in Death Valley. Science 194:322–324
Mooney HA, Ferrar PJ, Slatyer RO (1978) Photosynthetic capacity and carbon allocation patterns in diverse growth forms of Eucalyptus. Oecologia (Berl) 36:103–111
Mooney HA, Gulmon SL (1979) Environmental and evolutionary constraints on the photosynthetic characteristics of higher plants. In: OT Solbrig, S Jain, GB Johnson, PH Raven (eds) Topics in plant population biology. Columbia University New York p 316–337
Mooney HA, Kummerow J, Johnson AW, Parsons DJ, Keeley S, Hoffman A, Hays RI, Gilberto J, Chu C (1977) The producers—their resources and adaptive responses. In: HA Mooney (ed) Convergent evolution in Chile and California—mediterranean-climate ecosystems. Dowden, Hutchinson and Ross Stroudsburg, Pennsylvania p 85–143
Nátr L (1972) Influence of mineral nutrients on photosynthesis of higher plants. Photosynthetica 6:80–99
Nevins DJ, Loomis RS (1970) Nitrogen limitation and photosynthesis in sugar beet (Beta vulgaris L.). Crop Sci 10:21–25
Nobel PS, Zaragoza LJ, Smith WK (1975) Relation between mesophyll surface area, photosynthetic rate, and illumination level during development for leaves of Plectranthus parviflorus Henckel. Plant Physiol 55:1067–1070
Oxman AM, Goodman PJ, Cooper JP (1977) The effects of nitrogen, phosphorus, and potassium on rates of growth and photosynthesis of wheat. Photosynthetica 11:66–75
Radin JW, Parker LL (1979) Water relations of cotton plants under nitrogen deficiency. I. Dependence upon leaf structure. Plant physiol 64:495–498
Ryle GJA, Hesketh JD (1969) Carbon dioxide uptake in nitrogen deficient plants. Crop Sci 9:451–454
Shimshi D (1970) The effect of nitrogen supply on some indices of plant water relations of beans (Phasoleus vulgaris L.). New Phytol 69:413–424
Takeda T (1961) Studies on the photosynthesis and production of dry matter in the community of rice plants. Japan J Bot 17:403–437
Winner WE, Mooney HA (1980a) Ecology of SO2 resistance: I. Effects of fumigations on gas exchange of deciduous and evergreen shrubs. Oecologia (Berl) 44:290–295
Winner WE, Mooney HA (1980b) Ecology of SO2 resistance: II. Photosynthetic changes of shrubs in relation to SO2 absorption and stomatal behavior. Oecologia (Berl) 44:296–302
Wong SC (1979) Elevated atmospheric partial pressure of CO2 and plant growth. I. Interactions of nitrogen nutrition and photosynthetic capacity in C3 and C4 plants. Oecologia (Berl) 44:68–74
Yoshida S, Coronel V (1976) Nitrogen nutrition, leaf resistance and leaf photosynthetic rate of the rice plant. Soil Sci Plant Nutrition 22:207–211
Author information
Authors and Affiliations
Additional information
Abbreviations. For brevity, the following set of abbreviations is used in presenting and discussing the results. P/area and N/area are, respectively, photosynthesis and leaf nitrogen content per unit leaf area. P/wt and N/wt are the same quantities per unit leaf dry weight. SLW (specific leaf weight) is dry weight per unit leaf area. RGR (relative growth rate) is the relative rate of increase in shoot dry matter per day
Rights and permissions
About this article
Cite this article
Gulmon, S.L., Chu, C.C. The effects of light and nitrogen on photosynthesis, leaf characteristics, and dry matter allocation in the chaparral shrub, Diplacus aurantiacus . Oecologia 49, 207–212 (1981). https://doi.org/10.1007/BF00349189
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00349189