Abstract
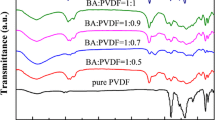
In the present study, hexafluoropropylene copolymer (P(VDF-HFP))-based “active” polymer membranes are prepared by entrapping different extent of pyrrolidinium ionic liquid-based nanofluid (ionanofluid). X-ray diffraction (XRD), differential scanning calorimetry (DSC), and Fourier transform infrared (FT-IR) spectroscopy are implemented to characterize the membranes. The study reveals that ionanofluid improves the electroactive phase nucleation of P(VDF-HFP) and suppresses the membranes’ crystallinity. SEM micrographs and butanol absorption study indicate that ionanofluid improves the electrolyte uptake ability and facilitates forming unified ion-conducting channels within the membranes. The 50 wt% ionanofluid (INF) incorporated gel-polymer electrolyte (GPE) exhibits the highest room-temperature ionic conductivity (2.33 × 10−3 S cm−1 at 25 °C), a high lithium-ion transference number (\( {t}_{{\mathrm{Li}}^{+}}\sim 0.6 \)), superior electrochemical stability window up to ~ 5.3 V (vs. Li/Li+) and excellent interfacial compatibility with the lithium electrode. The LiFePO4/Li battery comprising INF-based GPE demonstrates good C-rate performance and excellent cycling stability with a discharge capacity of ~ 156 mAh g−1 and ~ 116 mAh g−1 at C/5 and 2 C rates, respectively, and capacity retention of > 95% after 50 cycles at C/5 rate.










Similar content being viewed by others
References
Goodenough JB, Park K-S (2013) The Li-ion rechargeable battery: a perspective. J Am Chem Soc 135(4):1167–1176. https://doi.org/10.1021/ja3091438
Armand M, Tarascon JM (2008) Building better batteries. Nature 451(7179):652–657. https://doi.org/10.1038/451652a
Chu S, Cui Y, Liu N (2017) The path towards sustainable energy. Nat Mater 16(1):16–22. https://doi.org/10.1038/nmat4834
Wan Z, Lei D, Yang W, Liu C, Shi K, Hao X, Shen L, Lv W, Li B, Yang Q-H, Kang F, He Y-B (2019) Low resistance–integrated all-solid-state battery achieved by Li7La3Zr2O12 nanowire upgrading polyethylene oxide (PEO) composite electrolyte and PEO cathode binder. Adv Funct Mater 29(1):1805301. https://doi.org/10.1002/adfm.201805301
Li X, Qian K, He Y-B, Liu C, An D, Li Y, Zhou D, Lin Z, Li B, Yang Q-H, Kang F (2017) A dual-functional gel-polymer electrolyte for lithium ion batteries with superior rate and safety performances. J Mater Chem A 5(35):18888–18895. https://doi.org/10.1039/C7TA04415A
Yue L, Ma J, Zhang J, Zhao J, Dong S, Liu Z, Cui G, Chen L (2016) All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Stor Mater 5:139–164. https://doi.org/10.1016/j.ensm.2016.07.003
Hovington P, Lagacé M, Guerfi A, Bouchard P, Mauger A, Julien CM, Armand M, Zaghib K (2015) New lithium metal polymer solid state battery for an ultrahigh energy: nano C-LiFePO4 versus nano Li1.2V3O8. Nano Lett 15(4):2671–2678. https://doi.org/10.1021/acs.nanolett.5b00326
Zhang X, Wang S, Xue C, Xin C, Lin Y, Shen Y, Li L, Nan C-W (2019) Self-suppression of lithium dendrite in all-solid-state lithium metal batteries with poly(vinylidene difluoride)-based solid electrolytes. Adv Mater 31(11):1806082. https://doi.org/10.1002/adma.201806082
Kalhoff J, Eshetu GG, Bresser D, Passerini S (2015) Safer electrolytes for lithium-ion batteries: state of the art and perspectives. ChemSusChem 8(13):2154–2175. https://doi.org/10.1002/cssc.201500284
Li W, Pang Y, Liu J, Liu G, Wang Y, Xia Y (2017) A PEO-based gel polymer electrolyte for lithium ion batteries. RSC Adv 7(38):23494–23501. https://doi.org/10.1039/c7ra02603j
Perea A, Dontigny M, Zaghib K (2017) Safety of solid-state Li metal battery: solid polymer versus liquid electrolyte. J Power Sources 359:182–185. https://doi.org/10.1016/j.jpowsour.2017.05.061
Pan X, Liu T, Kautz DJ, Mu L, Tian C, Long TE, Yang P, Lin F (2018) High-performance N-methyl-N-propylpiperidinium bis(trifluoromethanesulfonyl)imide/poly(vinylidene fluoride-hexafluoropropylene) gel polymer electrolytes for lithium metal batteries. J Power Sources 403:127–136. https://doi.org/10.1016/j.jpowsour.2018.09.080
Rani MAA, Hwang J, Matsumoto K, Hagiwara R (2017) Poly(vinyl chloride) ionic liquid polymer electrolyte based on bis(fluorosulfonyl)amide for sodium secondary batteries. J Electrochem Soc 164(8):H5031–H5035
Sharma R, Sil A, Ray S (2016) Poly(methyl methacrylate) based nanocomposite gel polymer electrolytes with enhanced safety and performance. J Polym Res 23(9):194. https://doi.org/10.1007/s10965-016-1049-7
Li L, Wang F, Li J, Yang X, You J (2017) Electrochemical performance of gel polymer electrolyte with ionic liquid and PUA/PMMA prepared by ultraviolet curing technology for lithium-ion battery. Int J Hydrog Energy 42(17):12087–12093. https://doi.org/10.1016/j.ijhydene.2017.02.085
Richardson PM, Voice AM, Ward IM (2014) Two distinct lithium diffusive species for polymer gel electrolytes containing LiBF4, propylene carbonate (PC) and PVDF. Int J Hydrog Energy 39(6):2904–2908. https://doi.org/10.1016/j.ijhydene.2013.04.102
Kuo P-L, Tsao C-H, Hsu C-H, Chen S-T, Hsu H-M (2016) A new strategy for preparing oligomeric ionic liquid gel polymer electrolytes for high-performance and nonflammable lithium ion batteries. J Membr Sci 499:462–469. https://doi.org/10.1016/j.memsci.2015.11.007
Le HTT, Ngo DT, Kalubarme RS, Cao G, Park C-N, Park C-J (2016) Composite gel polymer electrolyte based on poly(vinylidene fluoride-hexafluoropropylene) (PVDF-HFP) with modified aluminum-doped lithium lanthanum titanate (A-LLTO) for high-performance lithium rechargeable batteries. ACS Appl Mater Interfaces 8(32):20710–20719. https://doi.org/10.1021/acsami.6b05301
Zhang J, Sun B, Huang X, Chen S, Wang G (2014) Honeycomb-like porous gel polymer electrolyte membrane for lithium ion batteries with enhanced safety. Sci Rep 4:6007. https://doi.org/10.1038/srep06007
Idris NH, Rahman MM, Wang J-Z, Liu H-K (2012) Microporous gel polymer electrolytes for lithium rechargeable battery application. J Power Sources 201:294–300. https://doi.org/10.1016/j.jpowsour.2011.10.141
He Z, Cao Q, Jing B, Wang X, Deng Y (2017) Gel electrolytes based on poly(vinylidenefluoride-co-hexafluoropropylene)/thermoplastic polyurethane/poly(methyl methacrylate) with in situ SiO2 for polymer lithium batteries. RSC Adv 7(6):3240–3248. https://doi.org/10.1039/C6RA25062A
Yang P, Liu L, Li L, Hou J, Xu Y, Ren X, An M, Li N (2014) Gel polymer electrolyte based on polyvinylidenefluoride-co-hexafluoropropylene and ionic liquid for lithium ion battery. Electrochim Acta 115:454–460. https://doi.org/10.1016/j.electacta.2013.10.202
Ismail I, Noda A, Nishimoto A, Watanabe M (2001) XPS study of lithium surface after contact with lithium-salt doped polymer electrolytes. Electrochim Acta 46(10):1595–1603. https://doi.org/10.1016/S0013-4686(00)00758-1
Chaudoy V, Ghamouss F, Luais E, Tran-Van F (2016) Cross-linked polymer electrolytes for Li-based batteries: from solid to gel electrolytes. Ind Eng Chem Res 55(37):9925–9933. https://doi.org/10.1021/acs.iecr.6b02287
Zhang W, Nie J, Li F, Wang ZL, Sun C (2018) A durable and safe solid-state lithium battery with a hybrid electrolyte membrane. Nano Energy 45:413–419. https://doi.org/10.1016/j.nanoen.2018.01.028
Reiter J, Vondrák J, Michálek J, Mička Z (2006) Ternary polymer electrolytes with 1-methylimidazole based ionic liquids and aprotic solvents. Electrochim Acta 52(3):1398–1408. https://doi.org/10.1016/j.electacta.2006.07.043
Hofmann A, Schulz M, Hanemann T (2013) Gel electrolytes based on ionic liquids for advanced lithium polymer batteries. Electrochim Acta 89:823–831. https://doi.org/10.1016/j.electacta.2012.10.144
Bose P, Deb D, Bhattacharya S (2019) Lithium-polymer battery with ionic liquid tethered nanoparticles incorporated P(VDF-HFP) nanocomposite gel polymer electrolyte. Electrochim Acta 319:753–765. https://doi.org/10.1016/j.electacta.2019.07.013
Li D, Zhang H, Li X (2018) Porous polyetherimide membranes with tunable morphology for lithium-ion battery. J Membr Sci 565:42–49. https://doi.org/10.1016/j.memsci.2018.08.011
Sirisopanaporn C, Fernicola A, Scrosati B (2009) New, ionic liquid-based membranes for lithium battery application. J Power Sources 186(2):490–495. https://doi.org/10.1016/j.jpowsour.2008.10.036
Li L, Wang J, Yang P, Guo S, Wang H, Yang X, Ma X, Yang S, Wu B (2013) Preparation and characterization of gel polymer electrolytes containing N-butyl-N-methylpyrrolidinium bis(trifluoromethanesulfonyl) imide ionic liquid for lithium ion batteries. Electrochim Acta 88:147–156. https://doi.org/10.1016/j.electacta.2012.10.018
Bose P, Roy A, Dutta B, Bhattacharya S (2017) Decoupling of segmental relaxation from ionic conductivity in [DEMM][TFSI] room temperature ionic liquid incorporated poly(vinylidenefluoride-co-hexafluoropropylene) membranes. Solid State Ionics 311:75–82. https://doi.org/10.1016/j.ssi.2017.09.012
Augustin S, Hennige V, Hörpel G, Hying C (2002) Ceramic but flexible: new ceramic membrane foils for fuel cells and batteries. Desalination 146(1):23–28. https://doi.org/10.1016/S0011-9164(02)00465-4
Lanceros-Méndez S, Mano JF, Costa AM, Schmidt VH (2001) FTIR and DSC studies of mechanically deformed β-PVDF films. J Macromol Sci, Part B 40(3-4):517–527. https://doi.org/10.1081/MB-100106174
Zhu Y, Wang F, Liu L, Xiao S, Chang Z, Wu Y (2013) Composite of a nonwoven fabric with poly(vinylidene fluoride) as a gel membrane of high safety for lithium ion battery. Energy Environ Sci 6(2):618–624. https://doi.org/10.1039/C2EE23564A
Kim M, Park JH (2013) Multi-scale pore generation from controlled phase inversion: application to separators for Li-ion batteries. Adv Energy Mater 3(11):1417–1420. https://doi.org/10.1002/aenm.201300235
Deb D, Dutta B, Bhattacharya S (2019) Viscosity decoupled charge transport in surface functionalized ZnS nanoparticle dispersed imidazolium ionanofluids. Mater Res Bull 116:22–31. https://doi.org/10.1016/j.materresbull.2019.03.028
Deb D, Bhattacharya S (2019) Ion transport in surface functionalized SnO2 nanoparticles dispersed imidazolium ionanofluids: decoupling from structural relaxation. J Mol Liq 285:697–706. https://doi.org/10.1016/j.molliq.2019.04.101
Deb D, Bhattacharya S (2017) Influence of ionic-liquid-tethered Al2O3 nanoparticle on the nonisothermal cold crystallization in ionic-liquid-based nanofluids. J Phys Chem C 121(12):6962–6976. https://doi.org/10.1021/acs.jpcc.6b11845
Bose P, Deb D, Bhattacharya S (2018) Ionic liquid based nanofluid electrolytes with higher lithium salt concentration for high-efficiency, safer, lithium metal batteries. J Power Sources 406:176–184. https://doi.org/10.1016/j.jpowsour.2018.10.050
Bose P, Bhattacharya S (2017) Electrochemical cycling behavior of pyrrolidinium ionic liquid tethered TiO2 nanoparticle-hybrid electrolytes: influence of grafting density. J Electrochem Soc 164(12):H788–H797. https://doi.org/10.1149/2.1331712jes
Moganty SS, Srivastava S, Lu Y, Schaefer JL, Rizvi SA, Archer LA (2012) Ionic liquid-tethered nanoparticle suspensions: a novel class of ionogels. Chem Mater 24(7):1386–1392. https://doi.org/10.1021/cm300424v
Bruce PG, Evans J, Vincent CA (1988) Conductivity and transference number measurements on polymer electrolytes. Solid State Ionics 28-30:918–922. https://doi.org/10.1016/0167-2738(88)90304-9
Abbrent S, Plestil J, Hlavata D, Lindgren J, Tegenfeldt J, Wendsjö Å (2001) Crystallinity and morphology of PVdF–HFP-based gel electrolytes. Polymer 42(4):1407–1416. https://doi.org/10.1016/S0032-3861(00)00517-6
Martins P, Lopes AC, Lanceros-Mendez S (2014) Electroactive phases of poly(vinylidene fluoride): determination, processing and applications. Prog Polym Sci 39(4):683–706. https://doi.org/10.1016/j.progpolymsci.2013.07.006
Priya L, Jog JP (2002) Poly(vinylidene fluoride)/clay nanocomposites prepared by melt intercalation: crystallization and dynamic mechanical behavior studies. J Polym Sci B Polym Phys 40(15):1682–1689. https://doi.org/10.1002/polb.10223
Mozhzhukhina N, Tesio AY, De Leo LPM, Calvo EJ (2017) In situ infrared spectroscopy study of PYR14TFSI ionic liquid stability for Li–O2 battery. J Electrochem Soc 164(2):A518–A523
Ramasundaram S, Yoon S, Kim KJ, Park C (2008) Preferential formation of electroactive crystalline phases in poly(vinylidene fluoride)/organically modified silicate nanocomposites. J Polym Sci B Polym Phys 46(20):2173–2187. https://doi.org/10.1002/polb.21550
Saikia D, Kumar A (2005) Ionic transport in P(VDF-HFP)–PMMA–LiCF3SO3–(PC + DEC)–SiO2 composite gel polymer electrolyte. Eur Polym J 41(3):563–568. https://doi.org/10.1016/j.eurpolymj.2004.10.029
Costa P, Silva J, Sencadas V, Costa CM, van Hattum FWJ, Rocha JG, Lanceros-Mendez S (2009) The effect of fibre concentration on the α to β-phase transformation, degree of crystallinity and electrical properties of vapour grown carbon nanofibre/poly(vinylidene fluoride) composites. Carbon 47(11):2590–2599. https://doi.org/10.1016/j.carbon.2009.05.011
Luo H, Huang Y, Wang D (2013) The crystallization and crystal transition of PVDF in PAN nano-tube. Polymer 54(17):4710–4718. https://doi.org/10.1016/j.polymer.2013.06.036
Sami S, Mouna B, Aymen M, Fethi G, Hassen D, Abdellatif Belhadj M (2013) Effect of PANI rate percentage on morphology, structure and charge transport mechanism in PANI?PVDF composites above percolation threshold. J Phys D Appl Phys 46(35):355101
Sharma M, Madras G, Bose S (2014) Shear induced crystallization in different polymorphic forms of PVDF induced by surface functionalized MWNTs in PVDF/PMMA blends. Phys Chem Chem Phys 16(31):16492–16501. https://doi.org/10.1039/c4cp01930j
Kundu M, Costa CM, Dias J, Maceiras A, Vilas JL, Lanceros-Méndez S (2017) On the relevance of the polar β-phase of poly(vinylidene fluoride) for high performance lithium-ion battery separators. J Phys Chem C 121(47):26216–26225. https://doi.org/10.1021/acs.jpcc.7b09227
Angell CA (1991) Relaxation in liquids, polymers and plastic crystals — strong/fragile patterns and problems. J Non-Cryst Solids 131-133(Part 1):13–31. https://doi.org/10.1016/0022-3093(91)90266-9
Caimi S, Wu H, Morbidelli M (2018) PVdF-HFP and ionic-liquid-based, freestanding thin separator for lithium-ion batteries. ACS Appl Energy Mater 1(10):5224–5232. https://doi.org/10.1021/acsaem.8b00860
Singh SK, Gupta H, Balo L, Shalu, Singh VK, Tripathi AK, Verma YL, Singh RK (2018) Electrochemical characterization of ionic liquid based gel polymer electrolyte for lithium battery application. Ionics 24(7):1895–1906. https://doi.org/10.1007/s11581-018-2458-x
Singh SK, Shalu BL, Gupta H, Singh VK, Tripathi AK, Verma YL, Singh RK (2018) Improved electrochemical performance of EMIMFSI ionic liquid based gel polymer electrolyte with temperature for rechargeable lithium battery. Energy 150:890–900. https://doi.org/10.1016/j.energy.2018.03.024
Acknowledgments
The authors thankfully acknowledge the DST-FIST scheme of the Department of Physics and DST-PURSE-II scheme of the University of Kalyani for providing necessary facilities.
Funding
Authors thankfully acknowledge the DST-SERB (Govt. of India) for the financial support under the Research Scheme No: EMR/2014/000290, dated 11.09.2015.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 286 kb)
Rights and permissions
About this article
Cite this article
Deb, D., Bose, P. & Bhattacharya, S. Gel-polymer electrolytes plasticized with pyrrolidinium-based ionanofluid for lithium battery applications. Ionics 27, 123–136 (2021). https://doi.org/10.1007/s11581-020-03807-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-020-03807-y