Abstract
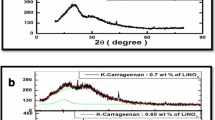
Lithium conducting materials play a major role in developing electrochemical devices. Green materials have gained much attention in order to face an energy crisis and global warming. Many researchers took effort to develop biopolymer electrolyte-based electrochemical devices instead of the synthetic polymer due to its high cost and not being environmentally green. K-carrageenan membranes with different concentrations of lithium bromide (LiBr) have been prepared by a solution casting technique and characterized by XRD, FTIR, DSC, and AC impedance technique. One gram of K-carrageenan with 0.5 wt% of LiBr has the highest conductivity as 3.43 × 10−3 Scm−1 at room temperature, and it has high amorphous nature as per the powder XRD results. FTIR confirms the complex formation between LiBr and K-carrageenan. The shift in glass transition temperature (Tg) of the membrane is observed from the DSC. The highest-conducting polymer electrolyte has a glass transition temperature of 44.55 °C. The DC polarization technique proves that the conductivity is due to ions. Lithium ion–conducting battery has been constructed using the highest-conducting biopolymer electrolyte membrane, and its output voltage is measured.














Similar content being viewed by others
References
Fergus JW (2010) Ceramic and polymeric solid electrolytes for lithium-ion batteries. J Power Sources 195:4554–4569
Meng C, Liu C, Chen L, Hu C, Fan S (2010) Highly flexible and all-solid-state paper like polymer supercapacitors. Nano Lett 10:4025–4403
Rani M, Rudhziah S, Ahmad A, Mohamed N (2014) Biopolymer electrolyte based on derivatives of cellulose from kenaf bast fiber. Polymer 6:2371–2385
Ahmad Khair AS, Arof AK (2010) Conductivity studies of starch based polymer electrolytes. Ionics 16:123–129
Selvakumar M, Bhat DK (2008) LiClO4 doped cellulose acetate as biodegradable polymer electrolyte for supercapacitors. J Appl Polym Sci 110:594–602
Vijayalekshmi V, Khastgir D (2017) Eco-friendly methane sulfonic acid and the sodium salt of dodecyl benzene sulfonic acid doped crosslinked chitosan based green polymer electrolyte membranes for fuel cell applications. J Membr Sci 523:45–59
Perumal P, Christopher Selvin P, Selvasekarapandian S (2018) Characterization of biopolymer pectin with lithium chloride and its applications to electrochemical devices. Ionics 24:3259–3270. https://doi.org/10.1007/s11581-018-2507-5
Vijaya N, Selvasekarapandian S, Sornalatha M, Sujithra KS, Monisha S (2017) Proton-conducting biopolymer electrolytes based on pectin doped with NH4X (X=Cl, Br). Ionics 23:2799–2808
Selvalakshmi S, Vijaya N, Selvasekarapandian S, Premalatha M (2017) Biopolymer agar-agar doped with NH4SCN as solid polymer electrolyte for electrochemical cell application. J Appl Polym Sci 134(15). https://doi.org/10.1002/app.44702
Selvalakshmi S, Mathavan T, Selvasekarapandian S, Premalatha M (2017) Study on NH4I composition effect in agar–agar-based biopolymer electrolyte. Ionics 23(10):2791–2797
Sampath Kumar L, Christopher Selvin P, Selvasekarapandian S, Manjuladevi R, Monisha S, Perumal P (2018) Tamarind seed polysaccharide biopolymer membrane for lithium-ion conducting battery. Ionics 24(12):1–11
Kingslin Mary Genova F, Selvasekarapandian S, Vijaya N, Sivadevi S, Premalatha M, Karthikeyan S (2017) Lithium ion-conducting polymer electrolytes based on PVA–PAN doped with lithium triflate. Ionics 23:2727–2734
Kingslin Mary Genova F, Selvasekarapandian S, Karthikeyan S, Vijaya N, Pradeepa R, Sivadevi S (2015) Study on blend polymer (PVA–PAN) doped with lithium bromide. Polym Sci Ser A 57(6):851–862
Christopher Selvin P, Perumal P, Selvasekarapandian S, Monisha S, Boopathi G, Leena Chandra MV (2018) Study of proton-conducting polymer electrolyte based on K-carrageenan and NH4SCN for electrochemical devices. Ionics 24:3535–3542. https://doi.org/10.1007/s11581-018-2521-7
Zainuddin NK, Samsudin AS (2018) Investigation on the effect of NH4Br at transport properties in K-carrageenan based biopolymer electrolytes via structural and electrical analysis. Mater Today Commun 14:199–209
Karthikeyan S, Selvasekarapandian S, Premalatha M, Monisha S, Boopathi G, Aristatil G, Arun A, Madeswaran S (2017) Proton-conducting I-carrageenan-based biopolymer electrolyte for fuel cell application. Ionics 23:2775–2780. https://doi.org/10.1007/s11581-016-1901-0
Moniha V, Alagar M, Selvasekarapandian S, Sundaresan B, Boopathi G (2018) Conductive bio-polymer electrolyte iota-carrageenan with ammonium nitrate for application in electrochemical devices. J Non-Cryst Solids 481:424–434
Shuhaimi NEA, Alias NA, Majid SR, Arof AK (2008) Electrical double layer capacitor with proton conducting K-carrageenan chitosan electrolytes. Funct Mater Lett 1:195–201
Singh R, Polu AR, Bhattacharya B, Rhee H-W, Singh CVP (2016) Perspectives for solid biopolymer electrolytes in dye-sensitized solar cell and battery application. Renew Sust Energ Rev 65:1098–1117
Liang L, Ni R, Yang S, Mao S (2014) Carrageenan and its application in drug delivery. Carbo Hydr Polym 103:1–11
Campo VL, Kowano DF, da Silva DB Jr, Carvalho I (2009) K-carrageenan: biological properties, chemical modifications, and structural analysis- a review. Carbo Hydr Polym 77:167–180
Hodge RM, Edward GH, Simon GP (1996) Water absorption and states of water in semi crystalline poly (vinyl alcohol) films. Polymer 37:1371–1376
Nithya S, Selvasekarapandian S, Karthikeyan S, Inbavalli D, Sikkanthar S, Sanjeeviraja C (2014) AC impedance studies on proton conducting PAN-NH4SCN polymer electrolytes. Ionics 20(10):1391–1398
Nithya S, Selvasekarapandian S, Karthikeyan S, Vinoth Pandi D (2015) Effect of propylene carbonate on the ionic conductivity of polyacrylonitrile-based solid polymer electrolytes. J Appl Polym Sci 132(14). https://doi.org/10.1002/app.41743
Hemalatha R, Alagar M, Selvasekarapandian S, Sundaresan B, Moniha V, Boopathi G, Christopher Selvin P (2016) Preparation and characterization of proton-conducting polymer electrolyte based on PVA, amino acid proline, and NH4Cl and its applications to electrochemical devices. Ionics 25:141–154. https://doi.org/10.1007/s11581-018-2564-9
Boopathi G, Pugalendhi S, Selvasekarapandian S, Premalatha S, Monisha S, Aristatil G (2017) Development of proton conducting biopolymer membrane based on agar–agar for the fuel cell. Ionics 23:2781–2790
Priya SS, Karthika M, Selvasekarapandian S, Manjuladevi R, Monisha (2018) Study of biopolymer I-carrageenan with magnesium perchlorate. Ionics 24:3861–3875. https://doi.org/10.1007/s11581-018-2535-1
Monisha S, Mathavan T, Selvasekarapandian S, Milton Franklin Benial A, Aristatil G, Mani N, Premalatha M (2017) Investigation of biopolymer electrolyte based on cellulose acetate-ammonium nitrate for potential use in electrochemical devices. Carbohydr Polym 157:38–47
Nirmala Devi G, Chitra S, Selvasekarapandian S, Premalatha M, Monisha S, Saranya J (2017) Synthesis and characterization of dextrin-based polymer electrolytes for potential applications in energy storage devices. Ionics 23:3377–3388. https://doi.org/10.1007/s11581-017-2135-5
Mahdavinia GR, Massoudi A, Baghban A, Shokri E (2014) Study of adsorption of cationic dye on magnetic kappa-carrageenan/ PVA nanocomposite hydrogels. J Environ Chem Eng 2:1578–1587
Boukamp BA (1986a) A nonlinear least squares fit procedure for analysis of immittance data of electrochemical systems. Solid State Ionics 20:31–44
Manjuladevi R, Thamilselvan M, Selvasekarapandian S, Christopher Selvin P, Mangalam R, Monisha S (2017) Preparation and characterization of blend polymer electrolyte film based on poly (vinyl alcohol)-poly (acrylonitrile)/MgCl2 for energy storage devices. Ionics 24:1083–1095. https://doi.org/10.1007/s11581-017-2273-9
Bhuvaneswari R, Karthikeyan S, Selvasekarapandian S, VinothPandi D, Vijaya N, Araichimani A, Sanjeeviraja C (2014) Preparation and characterization of PVA complexed with amino acid proline. Ionics 21:387–399
Vinoth Pandi D, Selvasekarapandian S, Bhuvaneswari R, Premalatha M, Monisha S, Arunkumar D, Junichi K (2016) Development and characterization of proton conducting polymer electrolyte based on PVA, amino acid glycine and NH4SCN. Solid State Ionics 298:15–22
Premalatha M, Mathavan T, Selvasekarapandian S, Monisha S, Vinoth Pandi D, Selvalakshmi S (2016) Investigations on proton conducting biopolymer membranes based on tamarind seed polysaccharide incorporated with ammonium thiocyanate. J Non-Cryst Solids 453:131–140
Manjuladevi R, Thamilselvan M, Selvasekarapandian S, Mangalam R, Premalatha M, Monisha S (2017) Mg-ion conducting blend polymer electrolyte based on poly (vinyl alcohol) - poly (acrylonitrile) with magnesium perchlorate. Solid State Ionics 308:90–100
Nurath Unnisa C, Chitra S, Selvasekarapandian S, Monisha S, Nirmala Devi G, Moniha V, Hema M (2018) Development of poly (glycerol suberate) polyester (PGS)–PVA blend polymer electrolytes with NH4SCN and its application. Ionics. https://doi.org/10.1007/s11581-018-2466-x
Ramya CS, Selvasekarapandian S, Hirankumar G, Savitha T, Angelo PC (2008) Investigation on dielectric relaxations of PVP–NH4SCN polymer electrolyte. J Non-Cryst Solids 354:1494–1502
Wagner JB, Wagner C (1957) Electrical conductivity measurements on cuprous halides. J Chem Phys 26:1597–1601
Mishra K, Rai DK (2013) Studies of a plasticized PEO + NH4PF6 proton-conducting polymer electrolyte system and its application in a proton battery. J Korean Phys Soc 62:311–319
Chitra R, Sathya P, Selvasekarapandian S, Monisha S, Moniha V, Meyvel S (2018) Synthesis and characterization of iota-carrageenan solid biopolymer electrolytes for electrochemical applications. Ionics 25:2147–2157. https://doi.org/10.1007/s11581-018-2687-z
Premalatha M, Mathavan T, Selvasekarapandian S, Monisha S, Selvalakshmi S, Vinoth Pandi D (2017) Tamarind seed polysaccharide (TSP)-based li-ion conducting membranes. Ionics 23(10):2677–2684
Subba Reddy CV, Sharma AK, Narasimha Rao VVR (2003) Conductivity and discharge characteristics of polyblend (PVP +PVA + KIO3) electrolyte. J Power Sources 114(2):338–345
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arockia Mary, I., Selvanayagam, S., Selvasekarapandian, S. et al. Lithium ion conducting membrane based on K-carrageenan complexed with lithium bromide and its electrochemical applications. Ionics 25, 5839–5855 (2019). https://doi.org/10.1007/s11581-019-03150-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-019-03150-x