Abstract
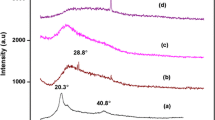
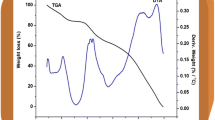
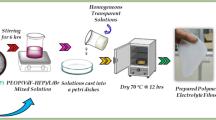
Besides commercially available synthetic polymers, the present work has been undertaken to explore the significance of poly(glycerol suberate) (PGS) polyester synthesised under lab scale in energy storage device. In this regard, a blend polymer electrolyte comprising of polyvinyl alcohol (PVA), poly(glycerol suberate) (PGS) polyester along with the various proportions of ammonium thiocyanate (NH4SCN) was prepared adopting solution casting technique. The synthesised polyester PGS was characterised by Fourier transform infrared (FT-IR) spectroscopy, 1H and 13C nuclear magnetic resonance (NMR) spectroscopy. The prepared electrolyte film was subjected to FT-IR analysis to study the complexation that has occurred within the blend. Its amorphous nature was revealed from X-ray diffraction (XRD) studies. Influence of NH4SCN on the glass transition temperature (Tg) was drawn from differential scanning calorimetry (DSC) technique. The dispersion of dopant within the polymer matrix was supported by scanning electron microscopy (SEM) followed by its elemental composition from energy dispersive spectroscopy (EDS). From the AC impedance technique, maximum conductivity of 3.01 × 10−4 S cm−1 was elicited for the optimised electrolyte (1 g PVA + 0.75 g PGS + 0.6 g NH4SCN). Frequency-dependent dielectric and modulus spectra were analysed to study the mechanism of transportation. Transport parameters evaluated by Wagner’s polarisation method proved that the conductivity was predominantly due to cations. Proton conducting battery was configured with the highest conducting electrolytic film and its cell parameters are presented.














Similar content being viewed by others
References
Long L, Wang S, Xiao M, Meng Y (2016) Polymer electrolytes for lithium polymer batteries. J Mater Chem A 4(26):10038–10069. https://doi.org/10.1039/C6TA02621D
Fergus JW (2010) Ceramic and polymeric solid electrolytes for lithium-ion batteries. J Power Sources 195(15):4554–4569. https://doi.org/10.1016/j.jpowsour.2010.01.076
Porcarelli L, Gerbaldi C, Bella F, Nair JR (2016) Super soft all-ethylene oxide polymer electrolyte for safe all-solid lithium batteries. Sci Reports 6(19892):1–14
Chai J, Liu Z, Ma J, Wang J, Liu X, Liu H, Zhang J, Cui G, Chen L (2017) In situ generation of poly (vinylene carbonate) based solid electrolyte with interfacial stability for LiCoO2 lithium batteries. Adv Sci 4(1600377):1–9
Rajeswari N, Selvasekarapandian S, Sanjeeviraja C, Kawamura J, Bahadur SA (2014) A study on polymer blend electrolyte based on PVA/PVP with proton salt. Polym Bull 71(5):1061–1080. https://doi.org/10.1007/s00289-014-1111-8
Liu Y, Gorgutsa S, Santato C, Skorobogatiy M (2012) Flexible, solid electrolyte-based lithium battery composed of LiFePO4 cathode and Li4Ti5O12 anode for applications in smart textiles. J Electrochem Soc 159(4):A349–A356
Thayumanasundaram S, Rangasamy VS, WonSeo J, Locquet JP (2017) Electrochemical performance of polymer electrolytes based on poly(vinyl alcohol)/poly (acrylic acid) blend and pyrrolidinium ionic liquid for lithium rechargeable batteries. Electrochim Acta 240:371–378
Manjuladevi R, Thamilselvan M, Selvasekarapandian S, Mangalam R, Premalatha M, Monisha S (2017) Mg-ion conducting blend polymer electrolyte based on poly(vinyl alcohol)-poly (acrylonitrile) with magnesium perchlorate. Solid State Ionics 308:90–100
Muthuvinayagam M, Gopinathan C (2015) Characterization of proton conducting polymer blend electrolytes based on PVdF-PVA. Polym 68:122–130. https://doi.org/10.1016/j.polymer.2015.05.008
Chun-ChenYang (2002) Polymer Ni–MH battery based on PEO–PVA–KOH polymer electrolyte. J Power Sources 109(1):22–31
Chatterjee J, Liu T, Wang B, Zheng JP (2010) Highly conductive PVA organogel electrolytes for applications of lithium batteries and electrochemical capacitors. Solid State Ionics 181(11–12):531–535
Ma X, Huang X, Gao J, Zhang S, Deng Z, Suo J (2014) Compliant gel polymer electrolyte based on poly(methyl acrylate-co-acrylonitrile)/poly(vinyl alcohol) for flexible lithium-ion batteries. Electrochim Acta 115:216–222
Chun-ChenYang S-JL (2002) Alkaline composite PEO–PVA–glass-fibre-mat polymer electrolyte for Zn–air battery. J Power Sources 112(2):497–503
Qiao J, Fu J, Lin R, Ma J, Liu J (2010) Alkaline solid polymer electrolyte membranes based on structurally modified PVA/PVP with improved alkali stability. Polym 51(21):4850–4859. https://doi.org/10.1016/j.polymer.2010.08.018
Tamilselvi P, Hema M (2014) Conductivity studies of LiCF3SO3 doped PVA: PVdF blend polymer electrolyte. Physica B: Condens Matter 437:53–57. https://doi.org/10.1016/j.physb.2013.12.028
Genova FKM, Selvasekarapandian S, Karthikeyan S, Vijaya N, Sivadevi S, Sanjeeviraja C (2015) Lithium ion-conducting blend polymer electrolyte based on PVA-PAN doped with lithium nitrate. Polym Plast Technol Eng 55(1):25–35
Albertsson AC, Varma IK (2002) Aliphatic polyesters: synthesis, properties and applications. Adv Polym Sci 157:1–40. https://doi.org/10.1007/3-540-45734-8_1
Albertsson AC, Varma IK (2003) Recent developments in ring opening polymerization of lactones for biomedical applications. Biomacromolecules 4(6):1466–1486. https://doi.org/10.1021/bm034247a
Jain R (1998) Controlled drug delivery by biodegradable poly (ester) devices: different preparative approaches. Drug Dev Ind Pharm 24(8):703–727. https://doi.org/10.3109/03639049809082719
Goodship V, Jacobs DK (2009) Polyvinyl alcohol: materials, processing and applications. Rapra review reports. Shrewsbury, Shropshire
Hema M, Selvasekarapandian S, Nithya H, Sakunthala A, Arunkumar D (2009) Thermal, electrical and optical studies on the poly(vinyl alcohol) based polymer electrolytes. Ionics 15(4):487–491
Vijaya N, Selvasekarapandian S, Hirankumar G, Karthikeyan S, Nithya H, Ramya CS, Prabu M (2012) Structural, vibrational, thermal, and conductivity studies on proton-conducting polymer electrolyte based on poly (N-vinyl pyrrolidone). Ionics 18(1-2):91–99. https://doi.org/10.1007/s11581-011-0589-4
Nithya S, Selvasekarapandian S, Karthikeyan S, Inbavalli D, Sikkinthar S, Sanjeeviraja C (2014) AC impedance studies on proton-conducting PAN:NH4SCN polymer electrolytes. Ionics 20:1391–1398
Vijaya N, Selvasekarapandian S, Karthikeyan S, Prabu M, Rajeswari N, Sanjeeviraja C (2013) Synthesis and characterization of proton conducting polymer electrolyte based on poly(N-vinyl pyrrolidone). J Appl Polym Sci 127(3):1538–1543. https://doi.org/10.1002/app.37765
Premalatha M, Vijaya N, Selvasekarapandian S, Selvalakshmi S (2016) Characterization of blend polymer PVA-PVP complexed with ammonium thiocyanate. Ionics 22(8):1299–1310. https://doi.org/10.1007/s11581-016-1672-7
Vinoth Pandi D, Selvasekarapandian S, Bhuvaneswari R, Premalatha M, Monisha S, Arunkumar D, Junichi K (2016) Development and characterization of proton conducting polymer electrolyte based on PVA, amino acid glycine and NH4SCN. Solid State Ionics 298:15–22
Hodge RM, Edward GH, Simon GP (1996) Water absorption and states of water in semicrystalline poly (vinyl alcohol) films. Polym. 37(8):1371–1376. https://doi.org/10.1016/0032-3861(96)81134-7
Premalatha M, Mathavan T, Selvasekarapandian S, Monisha S, Vinoth Pandi D, Selvalakshmi S (2016) Investigations on proton conducting biopolymer membranes based on tamarind seed polysaccharide incorporated with ammonium thiocyanate. J Non-Cryst Solids 453:131–140
Nirmala Devi G, Chitra S, Selvasekarapandian S, Premalatha M, Monisha S, Saranya J (2017) Synthesis and characterization of dextrin-based polymer electrolytes for potential applications in energy storage devices. Ionics 23(12):3377–3388. https://doi.org/10.1007/s11581-017-2135-5
Selvalakshmi S, Vijaya N, Selvasekarapandian S, Premalatha M (2017) Biopolymer agar-agar doped with NH4SCN as solid polymer electrolyte for electrochemical cell application. J Appl Polym Sci 134(15):1–10
Premalatha M, Mathavan T, Selvasekarapandian S, Selvalakshmi S, Monisha S (2017) Incorporation of NH4Br in tamarind seed polysaccharide biopolymer and its potential use in electrochemical energy storage devices. Org Electron 50:418–425. https://doi.org/10.1016/j.orgel.2017.08.017
Monisha S, Mathavan T, Selvasekarapandian S, Beniala AMF, Aristatilc G, Manic N, Premalatha M, Vinoth pandi D (2017) Investigation of bio polymer electrolyte based on cellulose acetate-ammonium nitrate for potential use in electrochemical devices. Carbohydr Polym 157:38–47. https://doi.org/10.1016/j.carbpol.2016.09.026
Ramaswamy M, Malayandi T, Selvasekarapandian S, Srinivasalu J, Rangaswamy M (2017) Magnesium ion conducting polyvinyl alcohol–polyvinyl pyrrolidone-based blend polymer electrolyte. Ionics 23(7):1771–1781. https://doi.org/10.1007/s11581-017-2023-z
Monisha S, Selvasekarapandian S, Mathavan T, Benial AMF, Manoharan S, Karthikeyan S (2016) Preparation and characterization of biopolymer electrolyte based on cellulose acetate for potential applications in energy storage devices. J Mater Sci Mater Electron 27(9):9314–9324
Boukamp BA (1986) A non-linear least squares fit procedure for analysis of immittance data of electrochemical systems. Solid State Ionics 20(1):31–44. https://doi.org/10.1016/0167-2738(86)90031-7
Ramaswamy M, Malayandi T, Selvasekarapandian S, Srinivasalu J, Rangaswamy M, Soundararajan V (2017) Development and study of solid polymer electrolyte based on poly vinyl alcohol : mg(ClO4)2. Polym-Plast Technol Eng 56(9):992–1002. https://doi.org/10.1080/03602559.2016.1247280
Parameswaran V, Nallamuthu N, Devendran P, Nagarajan ER, Manikandan A (2017) Electrical conductivity studies on ammonium bromide incorporated with zwitterionic polymer blend electrolyte for battery application. Physica B: Phys Condens Matter 515:89–98. https://doi.org/10.1016/j.physb.2017.03.043
Chandra MVL, Karthikeyan S, Selvasekarapandian S, Premalatha M, Monisha S (2016) Study of PVAc-PMMA-LiCl polymer blend electrolyte and the effect of plasticizer ethylene carbonate and nanofiller titania on PVAc-PMMA-LiCl polymer blend electrolyte. J Polym Eng 37(6):617–631
Boopathi G, Pugalendhi S, Selvasekarapandian S, Premalatha M, Monisha S, Aristatil G (2016) Development of proton conducting biopolymer membrane based on agar–agar for fuel cell. Ionics 23(10):2781–2790
Selvasekarapandian S, Savitha T, Malathi J, Mangalam R, Ramya SS (2009) Synthesis and characterization of nanosized materials for lithium rechargeable batteries. In: Srivastava A, Ipsita R (eds) Bionano-geo sciences: the future challenge. New Delhi
Jonscher AK (1981) A new understanding of the dielectric relaxation of solids. J Mater Sci 16(8):2037–2060. https://doi.org/10.1007/BF00542364
Dutta P, Biswas S, De SK (2002) Dielectric relaxation in polyaniline–polyvinyl alcohol composites. Mater Res Bull 37:193–200
Ramesh S, Yin TS, Liew CW (2011) Effect of dibutyl phthalate as plasticizer on high-molecular weight poly(vinyl hloride)–lithium tetraborate-based solid polymer electrolytes. Ionics 17(8):705–713. https://doi.org/10.1007/s11581-011-0568-9
Ramesh S, Arof AK (2001) Ionic conductivity studies of plasticized poly(vinyl chloride) polymer electrolytes. Mater Sci Eng B 85(1):11–15. https://doi.org/10.1016/S0921-5107(01)00555-4
Richert R, Wagner H (1998) The dielectric modulus: relaxation versus retardation. Solid State Ionics 105(1–4):167–173. https://doi.org/10.1016/S0167-2738(97)00461-X
Ramesh S, Yahaya A, Arof A (2002) Dielectric behaviour of PVC based polymer electrolytes. Solid State Ionics 152:291–294
Woo HJ, Majid SR, Arof AK (2011) Transference number and structural analysis of proton conducting polymer electrolyte based on poly (ε-caprolactone). Mater Res Innov 15(s2):s49–s54. https://doi.org/10.1179/143307511X13031890747697
Shukur MF, Kadir MFZ (2015) Electrical and transport properties of NH4Br-doped cornstarch-based solid biopolymer electrolyte. Ionics 21(1):111–124. https://doi.org/10.1007/s11581-014-1157-5
Yap KS (2012) Characteristics of PMMA-grafted natural rubber polymer electrolytes. University of Malaya
Ghosh A, Wang C, Kofinas P (2010) Block copolymer solid battery electrolyte with high Li-ion transference number. J Electrochem Soc 157(7):A846–A849. https://doi.org/10.1149/1.3428710
Arof AK, Shuhaimi NEA, Amirudin S, Kufian MZ, Woo HJ, Careem MA (2014) Polyacrylonitrile–lithium bis (oxalato) borate polymer electrolyte for electrical double layer capacitors. Polym Adv Technol 25(3):265–272. https://doi.org/10.1002/pat.3231
Majid SR, Arof AK (2005) Proton-conducting polymer electrolyte films based on chitosan acetate complexed with NH4NO3 salt. Phys B Condens Matter 355(1–4):78–82. https://doi.org/10.1016/j.physb.2004.10.025
Arof AK, Kufian MZ, Syukur MF, Aziz MF, Abdelrahman AE, Majid SR (2012) Electrical double layer capacitor using poly (methyl methacrylate)–C4BO8Li gel polymer electrolyte and carbonaceous material from shells of mata kucing (Dimocarpus longan) fruit. Electrochim Acta 74:39–45. https://doi.org/10.1016/j.electacta.2012.03.171
Bansod SM, Bhoga SS, Singh K, Tiwari RU (2007) The role of electrolyte in governing the performance of protonic solid-state battery. Ionics 13(5):329–332. https://doi.org/10.1007/s11581-007-0118-7
Lakshmi N, Chandra S (2002) Rechargeable solid-state battery using a proton-conducting composite as electrolyte. J Power Sources 108(1–2):256–260. https://doi.org/10.1016/S0378-7753(02)00021-6
Mishra K, Rai DK (2013) Studies of a plasticized PEO+NH4PF6 proton-conducting polymer electrolyte system and its application in a proton battery. J Korean Phys Soc 62(2):311–319. https://doi.org/10.3938/jkps.62.311
Broadhead J, Kuo H.C. Linden DI, Reddy TB (2001) Handbook of batteries. New York
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nusrath Unnisa, C., Chitra, S., Selvasekarapandian, S. et al. Development of poly(glycerol suberate) polyester (PGS)–PVA blend polymer electrolytes with NH4SCN and its application. Ionics 24, 1979–1993 (2018). https://doi.org/10.1007/s11581-018-2466-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2466-x