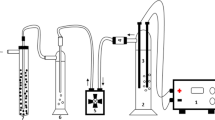
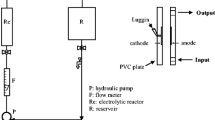
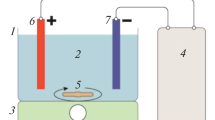
Abstract
The potential of the novel electro-chlorination system assembled with graphite anodes for its application in drinking water supply was explored. The process parameter optimization was carried out using response surface methodology (RSM) approach and the optimal conditions for highest yield of active chlorine were arrived. The effect of the process variables were investigated using Box-Behnken design. The experimentally observed results were correlated and integrated to derive a mathematical model. The derived RSM model predicted active chlorine production was validated using various statistical parameters i.e., coefficient of determination (R2), adjusted R2 (R2adj), and predicted R2 (R2pred). The experimental results were fitted well with the quadratic model suggested by the software and the R2 value obtained was 0.9828. The study concluded that active chlorine formation can be optimized and modeled using RSM approach and can be effectively implemented.






Similar content being viewed by others
References
Cantor KP, Lynch CF, Hildesheim M, Dosemeci M, Lubin J, Alavanja M, Craun G (1998) Drinking water source and chlorination byproducts I. Risk of bladder cancer. Epidemiology 9(1):21–28. https://doi.org/10.1097/00001648-199801000-00007
White GC (2010) Handbook of chlorination and alternative disinfectants. van Nostrand Reinhold, New York, pp 212–287
USEPA (1986) Guidelines for carcinogen risk assessment. U.S. Environmental Protection Agency. EPA, Washington, DC (/600/8-87/045)
USEPA (1999) Guidelines for carcinogen risk assessment. U.S. Environmental Protection Agency. EPA, Washington, DC (NCEA-F-0644) (revised draft) risk assessment forum
USEPA (2002) Integrated risk information system (electronic database). US. Environmental Protection Agency. EPA, Washington, DC 〈http://www.epa.gov/iris〉
Lee SC, Guo H, Lam SMJ, Lau SLA (2004) Multi-pathway risk assessment on disinfection by-products of drinking water in Hong Kong. Environ Res 94(1):47–56. https://doi.org/10.1016/S0013-9351(03)00067-7
Kumari M, Gupta SK, Mishra BK (2015) Multi-exposure cancer and non-cancer risk assessment of trihalomethanes in drinking water supplies—a case study of eastern region of India. Ecotoxicol Environ Saf 113:433–438. https://doi.org/10.1016/j.ecoenv.2014.12.028
Kristiana I, Gallard H, Joll C, Croué JP (2009) The formation of halogen-specific TOX from chlorination and chloramination of natural organic matter isolates. Water Res 43(17):4177–4186. https://doi.org/10.1016/j.watres.2009.06.044
Rajeshwar KIJG, Ibanez JG, Swain GM (1994) Electrochemistry and the environment. J Appl Electrochem 24(11):1077–1091
Venczel LV, Arrowood M, Hurd M, Sobsey MD (1997) Inactivation of Cryptosporidium parvum oocysts and Clostridium perfringens spores by a mixed-oxidant disinfectant and by free chlorine. Appl Environ Microbiol 63(4):1598–1601
Butterfield IM, Christensen PA, Curtis TP, Gunlazuardi J (1997) Water disinfection using an immobilised titanium dioxide film in a photochemical reactor with electric field enhancement. Water Res 31(3):675–677. https://doi.org/10.1016/S0043-1354(96)00391-0
Hayfield PCS (1998) Development of the noble metal/oxide coated titanium electrode. Platin Met Rev 42(2):46–55
Kraft A (2007) Doped diamond: a compact review on a new, versatile electrode material. Int J Electrochem Sci 2(5):355–385
Patil RS, Juvekar VA, Naik VM (2014) A polarity switching technique for the efficient production of sodium hypochlorite from aqueous sodium chloride using platinum electrodes. Ind Eng Chem Res 53(50):19426–19437. https://doi.org/10.1021/ie503084m
Kuhn AT, Wright PM (1973) The behaviour of platinum, iridium and ruthenium electrodes in strong chloride solutions. J Electroanal Chem Interfacial Electrochem 41(3):329–349
Patil RS, Juvekar VA, Naik VM (2011) Oxidation of chloride ion on platinum electrode: dynamics of electrode passivation and its effect on oxidation kinetics. Ind Eng Chem Res 50(23):12946–12959. https://doi.org/10.1021/ie200663a
Kodera F, Umeda M, Yamada A (2005) Determination of free chlorine based on anodic voltammetry using platinum, gold, and glassy carbon electrodes. Anal Chim Acta 537(1):293–298. https://doi.org/10.1016/j.aca.2005.01.053
Murata M, Ivandini TA, Shibata M, Nomura S, Fujishima A, Einaga Y (2008) Electrochemical detection of free chlorine at highly boron-doped diamond electrodes. J Electroanal Chem 612(1):29–36. https://doi.org/10.1016/j.jelechem.2007.09.006
Song S, Liang Y, Li Z, Wang Y, Fu R, Wu D, Tsiakaras P (2010) Effect of pore morphology of mesoporous carbons on the electrocatalytic activity of Pt nanoparticles for fuel cell reactions. Appl Catal B Environ 98(3):132–137. https://doi.org/10.1016/j.apcatb.2010.05.021
Yi L, Liu L, Liu X, Wang X, Yi W, He P, Wang X (2012) Carbon-supported Pt–Co nanoparticles as anode catalyst for direct borohydride-hydrogen peroxide fuel cell: electrocatalysis and fuel cell performance. Int J Hydrog Energy 37(17):12650–12658. https://doi.org/10.1016/j.ijhydene.2012.06.065
Seetharaman S, Balaji R, Ramya K, Dhathathreyan KS, Velan M (2014) Electrochemical behaviour of nickel-based electrodes for oxygen evolution reaction in alkaline water electrolysis. Ionics 20(5):713–720. https://doi.org/10.1007/s11581-013-1032-9
Saha, J, Gupta, SK (2017) A novel electro-chlorinator using low cost graphite electrode for drinking water disinfection. Ionics 23(7):1903–1913
Chen MJ, Chen KN, Lin CW (2005) Optimization on response surface models for the optimal manufacturing conditions of dairy tofu. J Food Eng 68(4):471–480. https://doi.org/10.1016/j.jfoodeng.2004.06.028
Karacan F, Ozden U, Karacan S (2007) Optimization of manufacturing conditions for activated carbon from Turkish lignite by chemical activation using response surface methodology. Appl Therm Eng 27(7):1212–1218. https://doi.org/10.1016/j.applthermaleng.2006.02.046
Sharma S, Malik A, Satya S (2009) Application of response surface methodology (RSM) for optimization of nutrient supplementation for Cr (VI) removal by Aspergillus lentulus AML05. J Hazard Mater 164(2):1198–1204. https://doi.org/10.1016/j.jhazmat.2008.09.030
Aghav RM, Kumar S, Mukherjee SN (2011) Artificial neural network modeling in competitive adsorption of phenol and resorcinol from water environment using some carbonaceous adsorbents. J Hazard Mater 188(1):67–77. https://doi.org/10.1016/j.jhazmat.2011.01.067
Pal S, Mukherjee S, Ghosh S (2014) Optimum phenol sorption in peat by the response surface method. Environ Geotechnics 1(3):142–151. https://doi.org/10.1680/envgeo.13.00017
Myers RH, Montgomery DC, Anderson-Cook CM (2016) Response surface methodology: process and product optimization using designed experiments. John Wiley & Sons
Ravikumar K, Pakshirajan K, Swaminathan T, Balu K (2005) Optimization of batch process parameters using response surface methodology for dye removal by a novel adsorbent. Chem Eng J 105(3):131–138. https://doi.org/10.1016/j.cej.2004.10.008
Körbahti BK, Aktaş N, Tanyolaç A (2007) Optimization of electrochemical treatment of industrial paint wastewater with response surface methodology. J Hazard Mater 148(1):83–90. https://doi.org/10.1016/j.jhazmat.2007.02.005
Körbahti BK (2007) Response surface optimization of electrochemical treatment of textile dye wastewater. J Hazard Mater 145(1):277–286. https://doi.org/10.1016/j.jhazmat.2006.11.031
Körbahti BK, Tanyolaç A (2008) Electrochemical treatment of simulated textile wastewater with industrial components and Levafix blue CA reactive dye: optimization through response surface methodology. J Hazard Mater 151(2):422–431. https://doi.org/10.1016/j.jhazmat.2007.06.010
Madaria PR, Mohan N, Rajagopal C, Garg BS (2004) Application of carbon aerogel for electrolytic removal of mercury from aqueous solutions. CSIR
Baek K, Shin HJ, Lee HH, Jun YS, Yang JW (2002) Statistical modeling of electrochemical removal of sodium in fermented food composts. Korean J Chem Eng 19(4):627–631. https://doi.org/10.1007/BF02699308
Rana P, Mohan N, Rajagopal C (2004) Electrochemical removal of chromium from wastewater by using carbon aerogel electrodes. Water Res 38(12):2811–2820. https://doi.org/10.1016/j.watres.2004.02.029
Rana-Madaria P, Nagarajan M, Rajagopal C, Garg BS (2005) Removal of chromium from aqueous solutions by treatment with carbon aerogel electrodes using response surface methodology. Ind Eng Chem Res 44(17):6549–6559. https://doi.org/10.1021/ie050321p
Chakraborty S, Dasgupta J, Farooq U, Sikder J, Drioli E, Curcio S (2014) Experimental analysis, modeling and optimization of chromium (VI) removal from aqueous solutions by polymer-enhanced ultrafiltration. J Membr Sci 456:139–154. https://doi.org/10.1016/j.memsci.2014.01.016
Fradj AB, Hamouda SB, Ouni H, Lafi R, Gzara L, Hafiane A (2014) Removal of methylene blue from aqueous solutions by poly (acrylic acid) and poly (ammonium acrylate) assisted ultrafiltration. Sep Purif Technol 133:76–81. https://doi.org/10.1016/j.seppur.2014.06.038
Dang TTH, Li CW, Choo KH (2016) Comparison of low-pressure reverse osmosis filtration and polyelectrolyte-enhanced ultrafiltration for the removal of co and Sr from nuclear plant wastewater. Sep Purif Technol 157:209–214. https://doi.org/10.1016/j.seppur.2015.11.019
Gardiner WP, Gettinby G (1998) Experimental design techniques in statistical practice: a practical software-based approach. Elsevier. https://doi.org/10.1533/9780857099785
Cojocaru C, Zakrzewska-Trznadel G, Jaworska A (2009) Removal of cobalt ions from aqueous solutions by polymer assisted ultrafiltration using experimental design approach. Part 1: optimization of complexation conditions. J Hazard Mater 169(1):599–609. https://doi.org/10.1016/j.jhazmat.2009.03.145
Zuorro A, Fidaleo M, Lavecchia R (2013) Response surface methodology (RSM) analysis of photodegradation of sulfonated diazo dye reactive green 19 by UV/H2O2 process. J Environ Manag 127:28–35. https://doi.org/10.1016/j.jenvman.2013.04.023
Federation WE (2012) Standard methods for the examination of water and wastewater. American Public Health Association (APHA), Washington, DC
Choi J, Shim S, Yoon J (2013) Design and operating parameters affecting an electrochlorination system. Ind Eng Chem Res 19(1):215–219. https://doi.org/10.1016/j.jiec.2012.08.004
Khelifa A, Moulay S, Hannane F, Benslimene S, Hecini M (2004) Application of an experimental design method to study the performance of electrochlorination cells. Desalination 160(1):91–98. https://doi.org/10.1016/S0011-9164(04)90021-5
Bergmann MEH, Koparal AS (2005) Studies on electrochemical disinfectant production using anodes containing RuO2. J Appl Electrochem 35(12):1321–1329. https://doi.org/10.1007/s10800-005-9064-0
Badruzzaman M, Oppenheimer J, Adham S (2009) Innovative beneficial reuse of reverse osmosis concentrate using bipolar membrane electrodialysis and electrochlorination processes. J Membr Sci 326(2):392–399. https://doi.org/10.1016/j.memsci.2008.10.018
Rajab M, Heim C, Letzel T, Drewes JE, Helmreich B (2015) Electrochemical disinfection using boron-doped diamond electrode–the synergetic effects of in situ ozone and free chlorine generation. Chemosphere 121:47–53. https://doi.org/10.1016/j.chemosphere.2014.10.075
Kraft A, Blaschke M, Kreysig D, Sandt B, Schröder F, Rennau J (1999) Electrochemical water disinfection. Part II: hypochlorite production from potable water, chlorine consumption and the problem of calcareous deposits. J Appl Electrochem 29(8):895–902. https://doi.org/10.1023/A:1003654305490
Kraft A, Stadelmann M, Blaschke M, Kreysig D, Sandt B, Schröder F, Rennau J (1999) Electrochemical water disinfection part I: hypochlorite production from very dilute chloride solutions. J Appl Electrochem 29(7):859–866. https://doi.org/10.1023/A:1003650220511
Kerwick MI, Reddy SM, Chamberlain AHL, Holt DM (2005) Electrochemical disinfection, an environmentally acceptable method of drinking water disinfection? Electrochim Acta 50(25):5270–5277. https://doi.org/10.1016/j.electacta.2005.02.074
Mezule L, Denisova V, Briedis A, Reimanis M, Ozolins J, Juhna T (2015) Disinfection effect of electrochemically generated chlorine on surface associated Escherichia coli in a drinking water system. Desalin Water Treat 53(13):3704–3710. https://doi.org/10.1080/19443994.2013.873742
Krstajić N, Nakić V, Spasojević M (1991) Hypochlorite production II. Direct electrolysis in a cell divided by an anionic membrane. J Appl Electrochem 21(7):637–641. https://doi.org/10.1007/BF01024853
Acknowledgements
The authors thank the financial support from Indian Institute of Technology (ISM), Dhanbad, funded by the Ministry of Human Resource Development (MHRD), Government of India, New Delhi, for carrying out this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• Study on a novel electro-chlorination reactor using low-cost graphite anodes and stainless steel cathodes for drinking water treatment.
• The study was conducted with electrolytes at low chloride concentration occurring naturally in the surface water.
• The optimization process was carried out using designs of experiment-based response surface methodology (RSM).
• Box-Behnken design was used for evaluating the impacts of the process factors on the response.
• The experimentally determined response levels conformed satisfactorily with the model assumed theoretical values at an R2 value of 0.98.
Rights and permissions
About this article
Cite this article
Saha, J., Gupta, S.K. Application of response surface methodology for optimization of an onsite electro-chlorinator for drinking water treatment. Ionics 24, 3237–3248 (2018). https://doi.org/10.1007/s11581-017-2430-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2430-1