Abstract
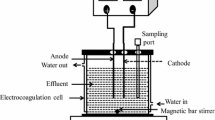
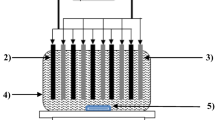
This work is an attempt to remove Cr(VI) from simulated wastewater using electrocoagulation (EC) process where stainless steel (SS) is used as a sacrificial electrode. The central composite design (CCD) of response surface methodology (RSM) is used to optimize different operating parameters including initial pH (pHi: 1.5–9.5), current density (j: 20.75–104.15 A/m2), electrode gap (g: 1.5–2.5 cm), and treatment time (t: 0–30 min), with respect to the removal of Cr(VI) from simulated water. The high coefficient of determination for Cr(VI) (R2 = 0.9922) was found by the analysis of variance (ANOVA) between the experimental data and the predicted data using a second-order regression model. The maximum Cr(VI) removal of 88.9% was achieved at optimum conditions (pH 3.5, j = 83.3 A/m2, g = 1.75 cm, and t = 24 min) as reflected by ANOVA analysis. A foam and residues analysis has also been incorporated.




Similar content being viewed by others
REFERENCES
CPCB. Pollution Control Acts, Rules and Notifications Issued, Delhi: Central Pollution Control Board, 2006.
Golbaz, S., Jafari, A.J., Rafiee, M., and Kalantary, R.R., Separate and simultaneous removal of phenol, chromium, and cyanide from aqueous solution by coagulation/precipitation: Mechanisms and theory, Chem. Eng. J., 2014, vol. 253, pp. 251–257. https://doi.org/10.1016/j.cej.2014.05.074
Khalifa, E.B., Rzig, B., Chakroun, R., Nouagui, H., and Hamrouni, B., Application of response surface methodology for chromium removal by adsorption on low-cost biosorbent, Chemometr. Intell. Lab. Syst., 2019, vol. 189, pp. 18–26. https://doi.org/10.1016/j.chemolab.2019.03.014
Pakzadeh, B. and Batista, J.R., Chromium removal from ion-exchange waste brines with calcium polysulfide, Water Res., 2011, vol. 45, pp. 3055–3064. https://doi.org/10.1016/j.watres.2011.03.006
Kongsricharoern, N. and Polprasert, C., Chromium removal by a bipolar electro-chemical precipitation process, Water Sci. Technol., 1996, vol. 34, pp. 109–116. https://doi.org/10.1016/S0273-1223(96)00793-7
Muthumareeswaran, M., Alhoshan, M., and Agarwal, G., Ultrafiltration membrane for effective removal of chromium ions from potable water, Sci. Rep., 2017, vol. 7, pp. 414–423. https://doi.org/10.1038/srep41423
Chen, G., Electrochemical technologies in wastewater treatment, Sep. Purif. Technol. 2004, vol. 38, pp. 11–41. https://doi.org/10.1016/j.seppur.2003.10.006
Heidmann, I. and Calmano, W., Removal of Cr(VI) from model wastewater by electrocoagulation with Fe electrodes, Sep. Purif. Technol., 2008, vol. 61, pp. 15–21. https://doi.org/10.1016/j.seppur.2007.09.011
Bhatti, M.S., Reddy, A.S., and Thukral, A.K., Electrocoagulation removal of Cr(VI) from simulated wastewater using response surface methodology, J. Hazard. Mater., 2009, vol. 172, pp. 839–846. https://doi.org/10.1016/j.jhazmat.2009.07.072
Aleboyeh, A., Daneshvar, N., and Kasiri, M.B., Optimization of C.I. Acid Red 14 dye removal by electrocoagulation batch process with response surface methodology, Chem. Eng. Process., 2008, vol. 47, pp. 827–832. https://doi.org/10.1016/j.cep.2007.01.033
Guven, G., Perendeci, A., and Tanyolac¸ A., Electrochemical treatment of deproteinated whey wastewater and optimization of treatment conditions with response surface methodology, J. Hazard. Mater., 2008, vol. 157, pp. 69–78. https://doi.org/10.1016/j.jhazmat.2007.12.082
Korbahti, B.K., Aktas, N., and Tanyolac¸ A., Optimization of electrochemical treatment of industrial paint wastewater with response surface methodology, J. Hazard. Mater., 2007, vol. 148, pp. 83–90. https://doi.org/10.1016/j.jhazmat.2007.02.005
Dubey, S., Parmar, N., Rekhate, C., and Prajapati, A.K., Optimization of electrocoagulation process for treatment of rice grain-based biodigester distillery effluent using surface response methodology approach, Int. J. Chem. React. Eng., 2022, vol. 20, no. 12, pp. 1261–1273. https://doi.org/10.1515/ijcre-2021-0253
APHA, AWWA, WEF, Standard Methods for the Examination of Water and Wastewater, Washington, DC: APHA, 1998, 19the ed.
Montgomery, D.C., Design and Analysis of Experiments, New York: Wiley, 1991, 3rd ed.
Gilhotra, V., Das, L., Sharma, A., Kang, T.S., Singh, P., Dhuria, R.S., and Bhatti, M.S., Electrocoagulation technology for high strength arsenic wastewater: Process optimization and mechanistic study, J. Cleaner Prod., 2018, vol. 198, pp. 693–703. https://doi.org/10.1016/j.jclepro.2018.07.023
Dargahi, A., Samarghandi, M.R., Shabanloo, A., Mahmoudi, M.M., and Nasab, H.Z., Statistical modeling of phenolic compounds adsorption onto low-cost adsorbent prepared from Aloe Vera leaves wastes using CCD-RSM optimization: Effect of parameters, isotherm, and kinetic studies, Biomass Convers. Biorefin., 2023, vol. 13, pp. 7859–7873. https://doi.org/10.1007/s13399-021-01601-y
Assadi, A., Fazli, M.M., Emamjomeh, M.M., and Ghasemi, M., Optimization of lead removal by electrocoagulation from aqueous solution using response surface methodology, Desalin. Water Treat., 2016, vol. 57, pp. 9375–9382. https://doi.org/10.1080/19443994.2015.1029529
Yaghmaeian, K., Martinez, S.S., Hoseini, M., and Amiri, H., Optimization of As(III) removal in hard water by electrocoagulation using central composite design with response surface methodology, Desalin. Water Treat., 2016, vol. 57, pp. 27827–27833. https://doi.org/10.1080/19443994.2016.1177735
Acharya, S., Sharma, S.K., Chauhan, G., and Shree, D., Statistical optimization of electrocoagulation process for removal of nitrates using response surface methodology, Indian Chem. Eng., 2018, vol. 60, pp. 269–284. https://doi.org/10.1080/00194506.2017.1365630
Ahmadzadeh, S., Asadipour, A., Pournamdari, M., Behnam, B., Rahimi, H.R., and Dolatabadi, M., Removal of ciprofloxacin from hospital wastewater using electrocoagulation technique by aluminum electrode: Optimization and modelling through response surface methodology, Proc. Saf. Environ. Prot., 2017, vol. 109, pp. 538–547. https://doi.org/10.1016/j.psep.2017.04.026
Un, U.T., Kandemir, A., Erginel, N., and Ocal, S.E., Continuous electrocoagulation of cheese whey wastewater: An application of Response Surface Methodology, J. Environ. Manage., 2014, vol. 146, pp. 245–250. https://doi.org/10.1016/j.jenvman.2014.08.006
Thirugnanasambandham, K. and Shine, K., Investigation on the removal of chromium from wastewater using electrocoagulation, Int. J. Chem. React. Eng., 2018, vol. 16, p. 20170155. https://doi.org/10.1515/ijcre-2017-0155
Sharma, D., Chaudhari, P.K., and Prajapati, A.K., Removal of chromium(VI) and lead from electroplating effluent using electrocoagulation, Sep. Sci. Technol., 2018, vol. 55, pp. 321–331. https://doi.org/10.1080/01496395.2018.1563157
Mahmad, M.K., Rozainy, M.R.M.A.Z., Abustan, I., and Baharun, N., Electrocoagulation process by using aluminum and stainless-steel electrodes to treat total chromium, color and turbidity, Procedia Chem., 2016, vol. 19, pp. 681–686. https://doi.org/10.1016/j.proche.2016.03.070
Lu, J., Wang, Z.R., Liu, Y.L., and Tang, Q., Removal of Cr ions from aqueous solution using batch electrocoagulation: Cr removal mechanism and utilization rate of in-situ generated metal ions, Process Saf. Environ. Prot., 2016, vol. 104, pp. 436–443. https://doi.org/10.1016/j.psep.2016.04.023
Demim, S., Drouiche, N., Aouabed, A., Benayad, T., Couderchet, M., and Semsari. S., Study of heavy metal removal from heavy metal mixture using the CCD method, J. Ind. Eng. Chem., 2014, vol. 20, pp. 512–520. https://doi.org/10.1016/j.jiec.2013.05.010
Li, G., Yang, C., Yao, Y., and Zeng, M., Electrocoagulation of chromium in tannery wastewater by a composite anode modified with titanium: Parametric and kinetic study, Desalin. Water Treat., 2019, vol. 171, pp. 294–301. https://doi.org/10.5004/dwt.2019.24792
ACKNOWLEDGMENTS
The authors are grateful to the department of chemical engineering IPSA, IES Indore, for providing necessary facilities to complete this work.
Funding
The authors received no grant for this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
About this article
Cite this article
Amitesh, Dohare, D., Jyoti, G. et al. Optimization of Electrocoagulation Process for the Removal of Chromium from Simulated Water Using the Response Surface Methodology. J. Water Chem. Technol. 45, 429–439 (2023). https://doi.org/10.3103/S1063455X2305003X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X2305003X