Abstract
Terricolous lichens are abundant in semi-arid areas, where they are exposed to high irradiation. Photoprotection is essential for the algae as the photobiont provides the primer carbon source for both symbionts. The UV-protectant lichen metabolites and different quenching procedures of the alga ensure adequate photoprotection. Since the long-term effect of diminishing UV-protectant lichen metabolites is unknown, a major part of lichen secondary metabolites was removed from Cladonia foliacea thalli by acetone rinsing, and the lichens were then maintained under field conditions to investigate the effect on both symbionts for 3 years. Our aim was to determine if the decreased level of UV-protectant metabolites caused an elevated photoprotection in the algae and to reveal the dynamics of production of the metabolites. Photosynthetic activity and light protection were checked by chlorophyll a fluorescence kinetics measurements every 6 months. The concentrations of fumarprotocetraric and usnic acids were monitored by chromatographic methods. Our results proved that seasonality had a more pronounced effect than that of acetone treatment on the function of lichens over a long-term scale. Even after 3 years, the acetone-treated thalli contained half as much usnic acid as the control thalli, and the level of photoprotection remained unchanged in the algae. However, the amount of available humidity was a more critical limiting environmental factor than the amount of incoming irradiation affecting usnic acid production. The lichenicolous fungus Didymocyrtis cladoniicola became relatively more abundant in the acetone-treated samples than in the control samples, indicating a slight change caused by the treatment.
Similar content being viewed by others
Introduction
Terricolous lichens are characteristic and important members of biological soil crusts, dominating arid and semi-arid ecosystems (Belnap and Lange 2003). These habitats exhibit intense irradiation and UV radiation, temperature extremes, low relative humidity, and precipitation (Borhidi et al. 2012; Dövényi 2010). Terricolous lichens have to cope with these harsh environmental conditions. Furthermore, according to various climate change scenarios, the climate may change in the direction of further extremities. The climate will be drier and warmer in summer and more humid in winter as in the case of Hungary for example (Kocsis et al. 2018). Lichens are extremotolerant organisms, several of which are cosmopolitan and exist under a wide range of environmental conditions; therefore, they are expected to tolerate environmental changes forecasted by the predicted scenarios.
Lichens are a stable symbiosis of at least one mycobiont, a photobiont, and an undetermined number of further micro-organisms (Hawksworth & Grube, 2020), resulting in a ‘self-sustaining miniature ecosystem’ (Farrar 1976) by their interactions. The fungi are responsible for the water holding capacity and provide physical (by structure) and chemical (by lichen secondary metabolites) protection against the external influence of the environment, such as herbivory and UV radiation (Molnár and Farkas 2010). Since the photobiont (green algae or cyanobacteria) provides the primary carbon source for both symbionts, the protection of the photosynthetic system is vitally important (Sadowsky and Ott 2016).
High irradiation is one of the most threatening environmental factors, especially when a lichen thallus is wet, since irreversible damage to the photosystem (PS) may occur (Heber et al. 2000). The excitation energy absorbed by the antenna system may be used for photochemical charge separation (photochemical quenching) in the reaction centres until the electron transport chain is saturated. In a saturated electron transport chain, the excitation energy must be removed and can be dissipated as heat or re-emitted as fluorescence (non-photochemical quenching). The non-photochemical quenching via zeaxanthin (Demmig-Adams and Adams 1992; Färber et al. 1997) and desiccation-induced fluorescence quenching (Heber et al. 2001, 2006; Kopecky et al. 2005) are also exhibited in lichens. In the absence of effective dissipation of excessive light energy, the production of by-products, such as damaging reactive oxygen species, can cause irreversible damage to the PSII (Krieger-Liszkay 2005; Müller et al. 2001). In air dry thalli, PSI is not or only partially inhibited by desiccation; therefore, the protection of PSII is critical since photodamage could also occur (Gauslaa and Solhaug 1999; Heber et al. 2010; Solhaug et al. 2003).
Various mechanisms of the photobiont and mycobiont are described for the protection of the lichen thallus against solar radiation damage (Beckett et al. 2021; Gasulla et al. 2012; Kranner et al. 2005; Nguyen et al. 2013; Sadowsky and Ott 2016). The mycobiont is responsible for the reversible drying out for a short period of time. Dehydration protects poikilohydric organisms from excess light (Veerman et al. 2007). The increased accumulation of solar/UV radiation screening pigments (e.g. BeGora and Fahselt 2001; Singh et al. 2011; Solhaug and Gauslaa 1996; Solhaug et al. 2010) and curling during desiccation (Barták et al. 2006) are also effective defence strategies of the mycobiont under high light exposure. The photobiont can protect its photosynthetic apparatus by aggregation of cells and the change in shape during desiccation (De Los Rios et al., 1999; Scheidegger et al. 1995). The increased dissipation of excess light energy by non-photochemical quenching (ΔpH- and zeaxanthin-dependent and desiccation-induced) or conformational change in the chlorophyll-protein complex can also protect the photosystem in the green algal photobiont (Heber et al. 2001; Heber et al. 2007; Paoli et al. 2010; Vráblíková et al. 2006). In the lichen thallus, the symbiotic partners can regulate the photoprotective system of the other symbiotic component (Kranner et al. 2005; Solhaug and Gauslaa 2004).
Acetone rinsing is a method to extract lichen secondary metabolites (LSMs) without damaging the algae in the dry thallus. Solhaug and Gauslaa (1996, 2001) pointed out that both the mycobiont and photobiont were able to survive acetone soaking treatment. Extraction of the lichen substances from dry thalli by acetone was the least detrimental method compared to other solvents (Solhaug and Gauslaa 2001). The treatment did not influence the pigment composition or lipid peroxidation (Candotto Carniel et al. 2017). The results of preliminary experiments (Farkas et al. 2020) showed that Cladonia foliacea (Huds.) Willd. survived the longest (1024 h long) acetone treatment, and the long-term tolerance of this species is higher than any of the other 12 species collected and investigated earlier from more humid habitats (Solhaug and Gauslaa 2001). For example, moistened Xanthoria parietina re-synthesised part of their parietin content within 3 weeks after acetone rinsing under field conditions (Solhaug et al. 2003). Although several details have been studied in laboratories or short-term experiments, there was no information on the long-term effect of acetone treatment on samples maintained under field conditions for years. The long-term influence of diminishing UV-protectant lichen secondary metabolites from lichen thalli is also unknown. The role of UV-protectant substances in terricolous lichens is more intense because of the high amount of incoming irradiation prevailing over sparse vegetation. Therefore, it was decided to study the long-term effect of usnic acid extraction on the terricolous lichen Cladonia foliacea, a species that exhibits a broad ecological tolerance and is abundant in open, dry, sun-exposed habitats in the Hungarian lowland steppe and low mountain rocky grasslands (Verseghy 1974, 1975, 1994). The species can spread easily by rolling due to the force of the wind (Smith et al. 2009) and has a broad thallus water content range optimal for photosynthesis (Mázsa et al. 2002). The species has great potential in experimental applications (Farkas et al. 2020).
The aim of the present investigation was to answer the following questions. Does the decreased amount of UV-protectant metabolites cause an elevated photoprotection in the algae? What are the dynamics of the production of lichen secondary metabolites after acetone rinsing? Will their levels return to their original level under field conditions during several years of experimentation, and how fast do the photobiont and mycobiont of the transplanted samples from a different habitat acclimate in their photoprotection to the changing environment? It was hypothesised that the usnic acid extraction would not cause any serious damage to the algal cells and their photosystem on a long-term scale. It was assumed that because of the lowered level of usnic acid, the algae would take over the photoprotection role of the fungi and increase the level of non-photochemical quenching mechanisms to avoid photodamage. It was expected that the concentration of usnic acid in the treated and transplanted thalli would return to its original control level and local samples within the experimental period and supposedly within 18–20 months (estimation based on Solhaug et al. 2003 and Veres et al. 2022).
Materials and methods
Site
Lichen thalli were collected from lowland and mountain sites at the end of summer 2017 in open perennial grassland (Festucetum vaginatae Rapaics ex Soó 1929 em. Borhidi 1996) and open dolomite rocky grassland (Seselio leucospermi-Festucetum pallentis Zólyomi (1936) 1958) (Borhidi et al. 2012). The two collection sites are in the lowland area of Vácrátót (47.702422 °N, 19.223910 °E) and montane dolomite grassland in the Bakony Mts (Sóly 47.141324 °N, 18.051639 °E), Hungary. Both sites are characterised by a continental climate (mean annual temperature Vácrátót: 11.7 °C, Sóly: 11.17 °C; mean annual precipitation Vácrátót: 442 mm, Sóly: 532 mm) (Hungarian Meteorological Services 2022).
The research object
Cladonia foliacea (Fig. 1a, b) is a relatively abundant, terricolous lichen species in Hungary (Verseghy 1974, 1975, 1994) in open, dry, and sun-exposed habitats in lowland steppe and mountain grassland communities. It is widely distributed over Europe and also found in the northern hemisphere temperate region of North America (Smith et al. 2009; Wirth et al. 2013).
a–e Dry (a) and rehydrated (b) thalli of the study object Cladonia foliacea; its photobiont Asterochloris sp. in the photosynthetic layer (c); crystals of usnic acid in the cortex (c) and fumarprotocetraric acid in the medulla (d) are indicated by arrows. Transplantation (e) to the experimental field in National Botanical Garden (Vácrátót, Hungary). Scale bars: c–d = 10 μm
The thallus exhibits a thickened upper cortex (Osyczka and Rola 2013) containing usnic acid (Fig. 1c) as a UV-protectant compound. Fumarprotocetraric acid (Fig. 1d) is also detected in the medullary and photosynthetic layer (Farkas et al. 2020b; Hillmann and Grummann 1957; Honegger 1986, 1997, 2012). No lower cortical layer is developed and the photobiont is Asterochloris sp., Trebouxiaceae (cf. Škaloud and Peksa 2008).
Usnic acid plays a significant role in protecting the species against the harmful effect of intense radiation and also has other effects such as antibiotics or insecticides (e.g. Cocchietto et al. 2002; Muhoro and Farkas 2021; Nimis and Skert 2006; Seaward 1988; Yilmaz et al. 2004). Usnic acid has a significant role when the species is rehydrated and hence unfolded (Fig. 1b) since the thalli have only a dense, upper cortex; otherwise, the thick, whitish medulla covers them, reflecting the strong solar irradiation (Verseghy 1971). Usnic acid absorbs light and functions as a very effective solar and UV radiation screening pigment (McEvoy et al. 2007; Rancan et al. 2002). The morphology and anatomy were studied by using a Nikon Eclipse/NiU compound microscope (Nikon Corporation, Tokyo, Japan) and a Nikon SMZ18 stereo microscope (Nikon Corporation, Tokyo, Japan). Micrographs were prepared using a Nikon Fi1c camera with NIS-Elements BR ML software (Nikon Corporation, Tokyo, Japan). Voucher specimens were deposited in Lichen Herbarium VBI, Hungary.
Treatment, field experiment, and collection
After the first collection, the samples were cleaned from plant and moss particles and randomised within each locality (lowland and mountain samples). Then, acetone rinsing was carried out two times for half an hour (Farkas et al. 2020b). After the treatment, the acetone soaked and control samples were placed in the experimental area in National Botanical Garden (Vácrátót, Hungary, 47.705821 °N, 19.229580 °E) for a 3-year experiment (Fig. 1e). An experimental area was chosen where the microenvironmental conditions were quite similar. The thalli were arranged into rows, and every row represented a different treatment. There were no differences between the rows in the shade conditions, vegetation, micro-terrain, or micro-topography. Since the experimental area is situated at a 0.5 km (air distance) from the collection site of the lowland samples, it is considered that these samples are practically placed back in their natural habitat under the same environmental conditions. At the same time, the samples collected at the mountain site were transplanted to a different lowland habitat. Therefore, the lowland samples can be considered as control samples for the mountain ones. This experimental arrangement allows comparisons to be made between the treated and control samples and also between the mountain and lowland samples in each pair of treatments at each time period. Values at time zero were measured in thalli before treatment. Samples were recollected every half a year in spring and autumn to take seasonal advantage of the favourable light and humidity conditions for active metabolism (e.g. Lange 2003; Verseghy 1976). In addition, the high and low temperatures did not limit the photosynthetic activity in these seasons. Thalli of every treatment (treated and control samples: control lowland, treated lowland, control mountain, treated mountain) were recollected every half a year and dried in the laboratory. Photographs were taken regularly for documentation in the field.
Data of the Hungarian Meteorological Services, 2022 measured at stations near the experimental area (6.5 km from Tece and 7.5 km from Sóly) were analysed.
Analysis of LSMs
Collected samples were checked for the presence of usnic acid and fumarprotocetraric acid by high-performance thin layer chromatography (HPTLC) according to standard methods for analysing lichen samples described by Arup et al. (1993) and Molnár and Farkas (2011). CAMAG horizontal chamber of 10 cm × 10 cm (DONAU LAB Kft., Budapest, Hungary), CAMAG TLC Plate Heater III (DONAU LAB Kft., Budapest, Hungary), and 10 cm × 10 cm thin-layer chromatographic plates (Merck, Kieselgel 60 F254) were used. Solvent system C (toluene: acetic acid, 20 : 3) was applied.
The amount of usnic acid and fumarprotocetraric acid was measured by high-performance liquid chromatography (HPLC, Alliance e2695, Waters Corporation, Milford, MA, USA) system, including a photodiode array detector (2998, Waters Corporation, Milford, MA, USA). Fifteen thalli were chosen from the randomised material of each treatment type in every sampling period. Thalli were c. 4–5 cm in diameter. The homogenised material (15 mg) was dissolved in 10 ml pure acetone and placed into an ultrasonic water bath for 10 min. The samples were then centrifuged for 20 min, and the supernatant was filtered through a Cronus Ø 25 mm PTFE syringe filter (0.22 μm). Standard stock solutions (1 mg ml−1) were made from reference standards for usnic acid (Sigma Aldrich Kft., Budapest, Hungary) and for fumarprotocetraric acid (Phytolab GmbH & Co. KG, Vestenbergsgreuth, Germany) dissolved in acetone for calibration purposes. The lichen metabolites were quantified according to a five-point (5, 10, 20, 50, 100 μg ml−1) calibration. The chromatographic method based on Ji and Khan (2005) was used. For chromatographic separation, a Phenomenex Luna 5 μm C18, 150 × 4.6 mm column was used, and 10 μl sample volume was injected. There was 40 °C in the column oven and 5 °C in the sample cooler. For the baseline separation of LSMs, a gradient elution program was used. Solvent A consisted of ortho-phosphoric acid and deionised (Milli-Q ultrapure) water (0.5 : 99.5), and solvent B contained ortho-phosphoric acid and acetonitrile (0.5 : 99.5). All the chemicals used were HPLC grade. The linear gradient started with a 60% A solvent after the volume decreased to 10% within 20 min and then to 0.5% in 30 s after which the volume remained constant for 9.5 min. The volume of solvent A was changed back to 60% within 1 min. The flow rate of solvents was 1 ml min−1. Lichen metabolites were detected at 280 nm (usnic acid) and 240 nm (fumarprotocetraric acid).
Measurement of photosynthetic activity/vitality
Further thalli (n = 15) were chosen randomly from the recollected material. After cleaning, thalli were rehydrated by spraying with distilled water. Thalli were kept under low light (c. 10 μmol m−2 s−1) at seasonal ambient temperature for 1–2 days until the photosynthetic system regenerated (i.e. until Fv/Fm became constant). After 30 min of dark adaptation, chlorophyll a fluorescence kinetics were measured (described by Jensen 2002) on fully water-saturated lichen thalli with a portable pulse amplitude modulated fluorometer (FMS 2 Hansatech Instruments Ltd. UK; Modfluor software) in the laboratory. After 30 min of dark adaptation, the minimum fluorescence yield (Fo) was measured using a weak measuring beam for 3 s. The maximum fluorescence yield of the dark-adapted sample (Fm) was obtained with a saturation pulse of 7,500 μmol m−2 s−1 light intensity for 800 ms. From the measured parameters, maximum variable chlorophyll fluorescence yield in dark-adapted state (Fv = Fm − Fo) and maximum quantum yield of PSII photochemistry (Fv/Fm = (Fm − Fo) / Fm; Kitajima and Butler 1975) were calculated. After two additional saturating pulses were added for 800 ms, the maximum (Fm’) and the steady-state (Ft) fluorescence yields were determined. The Stern-Volmer non-photochemical quenching (NPQ), the yield of photochemical electron transport (φPSII), non-photochemical quenching (φNPQ), and the yield of non-regulated excitation dissipation (φNO) were calculated (Bilger and Björkman 1990; Kitajima and Butler 1975; Klughammer and Schreiber 2008). The last Ft and Fm’ values were used for calculations.
Fv/Fm and NPQ are the most frequently used chlorophyll fluorescence variables in ecological investigations. The values of Fv/Fm show the condition of the photosynthetic systems within the thalli and the efficiency of the photochemical reaction. The photobiont algae are self-protected from the harmful effects of high light intensity which cannot be used for photosynthesis (Demmig-Adams et al. 1989). The non-photochemical quenching represents the degree of these protective mechanisms (photoprotection) while photosynthesis is maintained. The values of φPSII give an insight into the effective photochemical quantum yield of PSII and describe the proportion of excitation energy used for charge separation. The φNPQ parameter represents the quantum yield of light-induced (ΔpH- and zeaxanthin-dependent) non-photochemical fluorescence quenching. The φNO parameter shows the combined pathways of radiative and non-radiative deexcitation reactions that do not lead to photochemical energy conversion and are not involved in the NPQ mechanisms (Klughammer and Schreiber 2008). The sum of these complementary processes equals 1 (Kramer et al. 2004).
Usually, the chlorophyll fluorescence and HPLC measurements were started a few days after collection, except in the case of samples collected in spring 2020 because of the COVID-19 situation. The investigations had to be delayed, and thalli deteriorated during that time.
Statistical analysis
The effect of seasons (autumn and spring), locality (lowland, mountain), and treatment (acetone rinsed, control) on usnic acid, fumarprotocetraric acid, and the values of Fv/Fm and NPQ were statistically evaluated. The statistical analyses were carried out with the R software version 3.6.3 (R Core Team 2020). The effect of long-term (site) and short-term (season) environmental changes and treatments on LSMs concentration was tested by a two-way ANOVA followed by a Tukey HSD test. The normality of data distribution was checked visually with a Q-Q plot (quantiles of the residuals are plotted against the quantiles of the normal distribution with a 45° degree reference line) and by Shapiro-Wilk normality test. The homogeneity of variances was tested by Levene’s test. A level of p < 0.05 was considered a significant difference. Graphs were prepared in MS Excel (Fig. 2) and R environment (Figs. 3, 4 and Supplementary Figure S1).
a–b The concentration of fumarprotocetraric acid (a) and usnic acid (b) in the acetone rinsed and control samples of Cladonia foliacea deriving from the lowland and mountain habitats during a 3-year field experiment. Significant differences between the treated and control thalli are marked with asterisks. Sample size: mountain treated = 105, mountain control = 105, lowland treated = 105, lowland control = 105. The timepoint zero refers to the state when every thallus was still before treatment
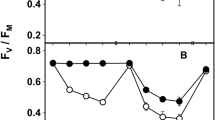
a–b The photosynthetic activity (Fv/Fm) of the acetone rinsed (= treated) and control samples of Cladonia foliacea deriving from the lowland (a) and mountain (b) habitats during a 3-year field experiment. Treatments with the same letter are not significantly different at 95% confidence. Sample size: mountain treated = 88, mountain control = 96, lowland treated = 105, lowland control = 97. The timepoint zero refers to the state when every thallus was still before treatment
a–b The non-photochemical quenching (NPQ) values of the acetone rinsed (=treated) and control samples of Cladonia foliacea deriving from the lowland (a) and mountain (b) habitats during a 3-year field experiment. Treatments with the same letter are not significantly different at 95% confidence. Sample size: mountain treated = 88, mountain control = 96, lowland treated = 105, lowland control = 97. The timepoint zero refers to the state when every thallus was still before treatment
Results
The concentrations of usnic acid and fumarprotocetraric acid
Difference between the lowland and mountain thalli
During the whole investigation period, there was a highly significant difference in fumarprotocetraric acid and usnic acid concentrations between the lowland and mountain thalli, both in the control and treated samples (Supplementary Table S1). The fumarprotocetraric acid concentration was higher in the mountain than in the lowland samples, whereas the usnic acid concentration was significantly higher than in the mountain thalli (Fig. 2).
Difference between the treated and control samples
The control samples showed significantly higher fumarprotocetraric acid (p < 0.001 for mountain and lowland, respectively) (Fig. 2a) and usnic acid concentrations (p < 0.001 for mountain and lowland, respectively, Fig. 2b) than the treated samples deriving from both sites (Supplementary Table S2). The ranges of fumarprotocetraric acid concentrations were 2.07–4.22 mg g−1 in the treated mountain thalli (n = 105) and 1.08–2.17 mg g−1 in the treated lowland (n = 105) thalli; the control samples showed values of 3.06–5.85 mg g−1 in the mountain thalli (n = 105) and 0.86–4.33 mg g−1 in the lowland samples (n = 105). Usnic acid concentrations were 0.81–6.66 mg g−1 in the treated mountain thalli (n = 105) and 1.24–6.79 mg g−1 in the treated lowland (n = 105) thalli; control values were 9.1–12.97 mg g−1 in the mountain thalli and 8.7–16.89 mg g−1 in the lowland thalli. After half a year, the concentration of usnic acid achieved a saturation value when its amount did not rise significantly in the treated samples during the following years (Fig. 2b), being only half of the values measured in the control thalli. The trends in the production of fumarprotocetraric acid are not so clear: the treated lowland samples from 1.5 to 2 years did not show the seasonal trends characterising the other samples (Fig. 2a).
The effect of seasonality on LSM concentration
There was a seasonal fluctuation of the LSMs in the control and treated samples (Fig. 2). The fumarprotocetraric acid concentrations were significantly higher (p < 0.0001) in spring than in autumn in the mountain, unlike in the lowland (p = 0.415) samples (Supplementary Table S2). A significantly higher level of usnic acid was measured in spring than in autumn collected samples (p < 0.0001 for the lowland and mountain thalli, Supplementary Table S2).
Chlorophyll fluorescence parameters
The lowland and mountain control thalli did not differ significantly (p = 1.00, Supplementary Table S1) in Fv/Fm at the start of the experiment. Control and treated samples did not show significant differences in Fv/Fm in the lowland (p = 0.675, ncontrol = 97, ntreated = 105) or mountain (p = 0.665, ncontrol = 96, ntreated = 88) thalli (Fig. 3, Supplementary Table S3). The values showed a seasonal fluctuation in the mountain (p < 0.001) and lowland (p < 0.001) samples (Fig. 3, Supplementary Table S3). The Fv/Fm was higher in autumn than in spring samples (Fig. 3). There were no significant differences in Fv/Fm between the lowland and mountain samples, except for the control thalli of the second spring (p = 0.05) (Supplementary Table S1).
There was no significant difference in NPQ between the lowland and mountain control thalli at the start of the experiment (p = 0.99, Supplementary Table S1). The NPQ did not differ significantly between the control and treated thalli during the investigation period (mountain: p = 0.769; lowland: p = 0.064) (Fig. 4, Supplementary Table S3). The seasons affected the level of NPQ in the lowland samples (p = 0.0027), unlike those in the mountain (p = 0.422) samples (Fig. 4, Supplementary Table S3). The spring samples showed higher values than the autumn samples (Fig. 4). Lowland and mountain samples did not differ significantly in NPQ, except for the thalli of the second spring (p < 0.0001) (Supplementary Table S1).
There was no significant difference in φPSII between the lowland and mountain control thalli at the start of the experiment (p = 0.098, Supplementary Table S1). Control and treated samples did not show significant differences in φPSII in the lowland (p = 0.64) or mountain (p = 0.97) thalli (Supplementary Table S3). The values differed significantly between autumn and spring in the mountain (p = 0.003) and lowland (p = 0.04) samples (Supplementary Table S3). There were no significant differences in φPSII between the lowland and mountain samples during the investigation period, except for the thalli of the second spring (p < 0.0001) (Supplementary Table S1).
There was no significant difference in φNPQ between the lowland and mountain control thalli at the start of the experiment (p = 0.45, Supplementary Table S1). Control and treated samples did not show significant differences in φNPQ in the lowland (p = 0.16) or mountain (p = 0.85) thalli (Supplementary Table S3). The values differed significantly between autumn and spring in the mountain samples (p = 0.0003, autumn > spring) and lowland samples (p = 0.025, autumn < spring) (Supplementary Table S3). The φNPQ was usually higher in autumn than in spring collected thalli (Table 1). There were no significant differences in φNPQ between the lowland and mountain samples, except for the thalli of the second spring (p < 0.0001 for control and p = 0.04 for treated thalli) (Supplementary Table S1).
There was no significant difference in φNO between the lowland and mountain control thalli at the start of the experiment (p = 1.00, Supplementary Table S1). Control and treated samples did not show significant differences in φNO in the lowland (p = 0.22) or mountain (p = 0.88) thalli (Supplementary Table S3). The values differed significantly between autumn and spring in the mountain samples (p = 0.0001), unlike those in the lowland (p = 0.89) samples. There were no significant differences in φNO between the lowland and mountain samples, except for the thalli of the second spring (p < 0.0001) (Supplementary Table S1).
The appearance of lichenicolous fungi
After half a year of transplantation, black necrotic lines and necrotic patches of various sizes were observed on several acetone rinsed transplanted thalli originating from the lowland site (Fig. 5), either at lobe ends (c. 0.5 cm2) (Fig. 5a) or later as larger continuous spots (Fig. 5b) laminally, due to infection by lichenicolous fungi. They grew to colonise entire thalli over time. Investigating the non-transplanted control samples, it was found that the lichens were already infected by the fungi upon collection, although it was less obvious due to the small size of the necrotic patches (less than c. 0.5 cm2) and the low abundance (most of the thalli did not appear to be infected). The lichenicolous fungus found on both the control and acetone-treated thalli originating either from lowland or mountain habitats was identified as Didymocyrtis cladoniicola (Diederich, Kocourk. & Etayo) Ertz & Diederich (Diederich et al. 2007, Ertz et al. 2015). The lichenicolous Syspastospora cladoniae Etayo (Etayo 2008) was only found on one of the lowland control thalli and not observed on the transplanted samples.
Discussion
The long-term effect of acetone rinsing
Our results highlight that lower levels of usnic acid are present in the acetone treated than in the control thalli even after 3 years under field conditions in the lowland and mountain samples. The results suggested that the levels of usnic acid did not rise further after half a year, only fluctuating around a saturation value between the seasons. However, the difference in levels between the control and treated samples showed a slight decrease. After 3 years, the control samples contained about two times more usnic acid than the treated thalli indicating that usnic acid production needs a more extended period for regeneration after acetone treatment. At the same time, the amount of usnic acid proved to be enough to protect the thalli from damage to the photosynthetic system, as noted, for example, by Solhaug et al. (2003).
Our results also revealed that the control and treated samples did not show significant differences in Fv/Fm, NPQ, φPSII, φNPQ, and φNO, indicating a similarly good condition of all the samples. The photosynthetic systems worked on a high level and could safely dissipate the excessive light energy mainly in a regulated way (φNPQ > φNO), except in the first and second spring, which were extremely dry seasons (Supplementary Figure S1). Therefore, the thalli of Cladonia foliacea can survive for years after acetone treatment. Veres et al. (2022) pointed out that C. foliacea contained approximately four times more cortical pigment (usnic acid) compared to, for example, C. furcata or C. magyarica containing atranorin in their cortex derived from the same microhabitat collected at the same time. Probably the usnic acid can significantly contribute to the survival of C. foliacea in habitats with extreme irradiation and drought, helping the thalli against photodamage. Furthermore, the species can avoid photoinhibition by curling during desiccation (Barták et al. 2006), providing an advantage over other species. In the desiccated state, the white medulla and the fumarprotocetraric acid crystals on the hyphae can reflect radiation, thereby protecting algae against photodamage.
The lichenicolous fungus Didymocyrtis cladoniicola was relatively more abundant on acetone rinsed than on control thalli; therefore, it is concluded that the acetone treatment could have an effect on the relation of the lichens and the lichenicolous fungus, as also mentioned by Bergmann and Werth (2017). Since the fungus could spread more easily on thalli with lowered levels of usnic acid in the cortex and fumarprotocetraric acid in the medulla due to acetone treatment, the antifungal role of usnic acid and fumarprotocetraric acid can be hypothesised. It corresponds well with former results on influencing lichen fitness by lichenicolous fungi via reducing their growth rates (Asplund et al. 2018, Lawrey 1993, Merinero and Gauslaa 2018). Lawrey et al. (1994) and Lawrey (2000) explain easier colonisation on the lichen thallus by enzymatic degradation of lichen secondary metabolites from the lichenicolous fungi.
The effect of transplantation
Usnic acid showed higher concentrations in the lowland than in the mountain samples during the whole investigation period. The lowland samples were derived from more open vegetation than the mountain samples, where less irradiation could reach lichen thalli, proved by the lower level of usnic acid at the start of the experiment in the control samples. Probably the level of photoprotection could be better explained by a long-term adaptation than a short-term acclimation. A higher amount of UV-protectant lichen metabolite indicates a higher level of incoming irradiation in thalli growing under humid conditions (Nybakken and Julkunen-Tiitto, 2006; Solhaug et al. 2003) and thus an elevated level of need in photoprotection (Heber et al. 2006). The LSMs can be produced only by rehydrated thalli (Solhaug et al. 2003) and need enough photosynthates for production (Solhaug and Gauslaa 2004). There were no significant differences in Fv/Fm and φPSII between the lowland and mountain control thalli at the start and during the experiment, indicating that the photosynthetic activity did not differ and the amount of photosynthates needed for LSM production could also reach the same level. Since the thalli of various treatments were only within a c. 0.5 m distance in the experimental area, the very similar microenvironmental conditions could not cause a significant difference in the function of PSII. The difference in the LSM production between the lowland and mountain thalli can be explained by a long-term adaptation of the fungi (on a genetic, anatomical, or structural level) that did not change with transplantation. NPQ and φNPQ also showed similar levels, suggesting that the amount of light reaching algal cells must be the same, triggering the same level of response in the algal photoprotection. Therefore, a thicker cortical layer was probably exhibited in the lowland samples containing more cortical pigment (but needs further investigation). We also detected the sudden increase of significant differences between the lowland and mountain samples (collected/measured) after 1.5 years. We did not find any differences in the soil microenvironment, shade conditions, or the conditions of the thalli compared to other thalli collected earlier or remaining on the field.
The lichenicolous fungus Didymocyrtis cladoniicola found both in the control and treated thalli originating from the lowland and mountain habitats was more and more obvious over time. It was hardly visible at the time of transplantation and reached a detectable size after 6 months in the lowland and 12 months in the mountain thalli. However, the transplantation probably caused a slight change that was advantageous for the growth of the fungus, and thus, their appearance became more obvious (reaching more than 50 cm2 area of the thalli — at various treatments) during the experimental period. Therefore, the increased growth of lichenicolous fungi is a possible effect of the disturbance caused by the transplantation itself.
The effect of seasonality on the production of LSMs
There was a seasonal fluctuation of the LSMs in the control and treated mountain and lowland lichens. A significantly higher level of usnic acid was measured in spring than in autumn collected samples. The seasonal change in usnic acid concentration was compared with meteorological data (the means of 3 months before sampling) (Supplementary Figure S1). It was revealed that the usnic acid level, especially in mountain samples, mainly reflected the changes in relative humidity (spring > autumn), unlike global irradiation (spring < autumn). Moistened Xanthoria parietina re-synthesised 15–25% of their parietin content within 3 weeks after acetone rinsing between field conditions, and thalli kept under dry conditions did not produce parietin regardless of the amount of incoming irradiation (Solhaug et al. 2003). Our results also showed that the amount of available humidity largely affected the production of cortical usnic acid concentration in the investigated semi-arid region on a long-term scale. Between more balanced humidity conditions, the available global irradiation possibly limits the production of cortical UV protective pigments (e.g. Nybakken and Julkunen-Tiitto 2006). Probably the thinner cortical layer of the mountain thalli could cause them to reflect more sensitively the seasonal changes of the environment (Veres et al. 2022), but this needs further evidence.
The seasons also affected the values of Fv/Fm, NPQ, and the proportion of incoming light energy between the quenching mechanisms. In spring, lower Fv/Fm and higher NPQ and φNO were measured than in the thalli collected in autumn. These phenomena indicated a higher need for photoprotection and a lowered photosynthetic activity during spring, probably caused by a higher level of incoming irradiation reaching algal cells. The higher relative humidity during spring (Supplementary Figure S1) may result in the longer time spent in unfolded state and an elevated cortex transmittance (Dietz et al. 2000). The φNO values were higher in spring than in autumn, suggesting that the excessive light energy was dissipated mainly in a non-regulated way (φNO > φNPQ). In semi-arid grasslands, a lichen shows its highest photosynthetic activity (Farkas et al. 2020, Veres et al. 2020) and biomass production (Verseghy 1976) during autumn in Hungary, in agreement with our current results. It was assumed that the higher photosynthetic activity and biomass production might allow an elevated level of photosynthates used for LSM production. However, Gauslaa et al. (2013) demonstrated that growth and defence by LSMs (e.g. against herbivory) are such investments that did not compete for photosynthates.
It can be concluded that the algae were more sensitive to the seasonally changing environment than the acetone treatment. The treatment mostly affected the mycobiont partner and the algae did not elevate their photoprotective role (φNO and φNPQ did not differ in the control and treated thalli), as Veres et al. (2022) pointed out in Cladonia furcata. Probably the initial amount of UV-protectant lichen metabolite originated from a high level of constitutive photoprotection (Asplund et al. 2009), and the system was over-secured. After the treatment, a lower amount of usnic acid would be enough for the necessary level of UV defence. In every measured chlorophyll fluorescence parameter, the seasonal fluctuation was more pronounced in the mountain than in the lowland samples. The mountain thalli reflected the environmental changes more intensely than the lowland samples, probably because of their sensitivity deriving from a thinner cortex (needs further evidence) impacting water holding capacity (Verseghy 1971).
The monitoring of the usnic acid production within a half year would reveal further details on/about the intensity of reproduction of this metabolite under semi-arid field conditions and from the climate change point of view. The scenario that the severity and frequency of drought events will be increasing in some regions (Bartholy et al. 2009; Ferrenberg et al. 2015) raises the question of how terricolous lichen will or could acclimate and adapt to the changing environment. The reduced rehydration could significantly decrease the vitality of terricolous lichens (Morillas et al. 2021) and thereby create less chance for UV-protectant metabolite production. The investigation of the long-term effect of acetone rinsing on other species containing various UV-protectant metabolites or living in different habitats would expand our knowledge on this topic.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request. Lichen specimens are deposited in the Lichen Herbarium VBI (Vácrátót, Hungary).
References
Arup U, Ekman S, Lindblom L, Mattsson J-E (1993) High performance thin layer chromatography (HPTLC), an improved technique for screening lichen substances. Lichenologist 25:61–71
Asplund J, Solhaug KA, Gauslaa Y (2009) Fungal depsidones — an inducible or constitutive defence against herbivores in the lichen Lobaria pulmonaria? Basic Appl Ecol 10:273–278
Asplund J, Gauslaa Y, Merinero S (2018[2017]) Low synthesis of secondary compounds in the lichen Lobaria pulmonaria infected by the lichenicolous fungus Plectocarpon lichenum. New Phytol 217(4):1397–1400. https://doi.org/10.1111/nph.14978
Barták M, Solhaug KA, Vráblíková H, Gauslaa Y (2006) Curling during desiccation protects the foliose lichen Lobaria pulmonaria against photoinhibition. Oecologia 149(4):553–560. https://doi.org/10.1007/s00442-006-0476-2
Bartholy J, Pongrácz R, Cs Torma, Pieczka I, Kardos P, Hunyady A (2009) Analysis of regional climate change modelling experiments for the Carpathian Basin. Int J Glob 1(1–3):238–252
Beckett R, Minibayeva F, Solhaug KA, Roach T (2021) Photoprotection in lichens: adaptations of photobionts to high light. Lichenologist 53(1):21–33. https://doi.org/10.1017/S0024282920000535
Begora M, Fahselt D (2001) Usnic acid and atranorin concentrations in lichens in relation to bands of UV irradiance. Bryologist 104(1):134–140
Belnap J, Lange OL (2003) Biological soil crusts: structure, function, and management. Heidelberg, Springer-Verlag, Berlin
Bergmann TC, Werth S (2017) Intrathalline distribution of two lichenicolous fungi on Lobaria hosts — an analysis based on quantitative real-time PCR [Intrathallines Vorkommen zweier lichenikoler Pilze auf Wirtsflechten der Gattung Lobaria — eine Analyse basierend auf quantitativer Real-Time PCR]. Herzogia 30(1):253–271. https://doi.org/10.13158/heia.30.1.2017.253
Bilger W, Björkman O (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res 25:173–185. https://doi.org/10.1007/BF00033159
Borhidi A, Kevey B, Lendvai G (2012) Plant communities of Hungary. Akadémiai Kiadó, Budapest
Candotto Carniel F, Pellegrini E, Bove F, Crosera M, Adami G, Nali C, Lorenzini G, Tretiach M (2017) Acetone washing for the removal of lichen substances affects membrane permeability. Lichenologist 49(4):387–395. https://doi.org/10.1017/S0024282917000263
Cocchietto M, Skert N, Nimis PL, Sava G (2002) A review on usnic acid, an interesting natural compound. Die Naturwissenschaften 89:137–146. https://doi.org/10.1007/s00114-002-0305-3
De Los Rios A, Wierzchos J, Ascaso C (1999) Study of lichens with different state of hydration by the combination of low temperature scanning electron and confocal laser scanning microscopies. Int Microbiol 2(4):251–257
Demmig-Adams B, Winter K, Krüger A, Czygan FC (1989) Light stress and photoprotection related to the carotenoid zeaxanthin in higher plants. In: Briggs WR (ed) Photosynthesis. Allen R Liss, New York, pp 375–391
Demmig-Adams B, Adams W (1992) Carotenoid composition in sun and shade leaves of plants with different life forms. Plant Cell Environ 15:411–419
Diederich P, Kocourková J, Etayo J, Zhurbenko M (2007) The lichenicolous Phoma species (coelomycetes) on Cladonia. Lichenologist 39(2):153–163. https://doi.org/10.1017/S0024282907006044
Dietz S, Büdel B, Lange OL, Bilger W (2000) Transmittance of light through the cortex of lichens from contrasting habitats. In: Schroeter B, Schlensog M, TGA G (eds) New aspects in cryptogamic research: contributions in honour of Ludger Kappen (Bibliotheca Lichenologica). J. Cramer, Berlin, pp 171–182
Dövényi Z (ed) (2010) Magyarország kistájainak katasztere. [Cadastre of the small regions of Hungary (in Hungarian)]. MTA Földrajztudományi Kutatóintézet, Budapest
Ertz D, Diederich P, Lawrey JD, Berger F, Freebury CE, Coppins B, Gardiennet A, Hafellner J (2015) Phylogenetic insights resolve Dacampiaceae (Pleosporales) as polyphyletic: Didymocyrtis (Pleosporales, Phaeosphaeriaceae) with Phoma-like anamorphs resurrected and segregated from Polycoccum (Trypetheliales, Polycoccaceae fam. nov.). Fungal Divers 74(1):53–89. https://doi.org/10.1007/s13225-015-0345-6
Etayo J (2008) Líquenes y hongos liquenícolas del LIC de Ablitas (S. Navarra, España). [Lichens and lichenicolous fungi of the LIC de Ablitas (S. Navarra, España) (in Spanish)]. Cryptogam Mycol 29(1):63–94
Farkas E, Biró B, Csintalan Z, Veres K (2020) Acetone rinsing tolerance of the lichen species Cladonia foliacea is considerable. Lichenologist 52(4):325–327. https://doi.org/10.1017/S0024282920000237
Farkas E, Biró B, Szabó K, Veres K, Csintalan Z, Engel R (2020b) The amount of lichen secondary metabolites in Cladonia foliacea (Cladoniaceae, lichenised Ascomycota). Acta Bot Hung 62(1–2):33–48. https://doi.org/10.1556/034.62.2020.1-2.4
Farrar JF (1976) The lichen as an ecosystem: observation and experiment. In: Brown DH, Hawksworth DL, Bailey RH (eds) Lichenology: progress and problems. UK: Academic Press, London, pp 385–406
Färber A, Young AJ, Ruban AV, Horton P, Jahns P (1997) Dynamics of the xanthophyll cycle in different antenna sub-complexes in the photosynthetic membranes of higher plants. Plant Physiol 115:1609–1618. https://doi.org/10.1104/pp.115.4.1609
Ferrenberg S, Reed SC, Belnap J (2015) Climate change and physical disturbance cause similar community shifts in biological soil crusts. PNAS 112:12116–12121
Gasulla F, Herrero J, Esteban-Carrasco A, Ros-Barceló A, Barre-No E, Zapata JM, Guéra A (2012) Photosynthesis in lichen: light reactions and protective mechanisms. In: Najafpour M (ed) Advances in photosynthesis — fundamental aspects. IntechOpen, pp 149–174. https://doi.org/10.5772/26204 Available from: http://www.intechopen.com/books/advances-in-photosynthesisfundamental-aspects/photosynthesis-in-lichen-light-reactions-and-protective-mechanisms
Gauslaa Y, Solhaug KA (1999) High-light damage in air-dry thalli of the old forest lichen Lobaria pulmonaria — interactions of irradiance, exposure duration and high temperature. J Exp Bot 50(334):697–705. https://doi.org/10.1093/jxb/50.334.697
Gauslaa Y, Bidussi M, Solhaug KA, Asplund J, Larsson P (2013) Seasonal and spatial variation in carbon based secondary compounds in green algal and cyanobacterial members of the epiphytic lichen genus Lobaria. Phytochemistry 94:91–98. https://doi.org/10.1016/j.phytochem.2013.04.003
Hawksworth DL, Grube M (2020) Lichens redefined as complex ecosystems. New Phytol 227:1281–1283. https://doi.org/10.1111/nph.16630
Heber U, Bilger W, Bligny R, Lange OL (2000) Phototolerance of lichens, mosses and higher plants in an alpine environment: analysis of photoreactions. Planta 211(6):770–780. https://doi.org/10.1007/s004250000356
Heber U, Bukhov NG, Shuvalov VA, Kobayashi Y, Lange OL (2001) Protection of the photosynthetic apparatus against damage by excessive illumination in homoiohydric leaves and poikilohydric mosses and lichens. J Exp Bot 52:1999–2006. https://doi.org/10.1093/jexbot/52.363.1999
Heber U, Lange OL, Shuvalov VA (2006) Conservation and dissipation of lichen energy as complementary processes: homiohydric and poikilohydric autotrophs. J Exp Bot 57(6):1211–1223. https://doi.org/10.1093/jxb/erj104
Heber U, Azarkovich M, Shuvalov V (2007) Activation of mechanisms of photoprotection by desiccation and by light: poikilohydric photoautotrophs. J Exp Bot 58(11):2745–2759. https://doi.org/10.1093/jxb/erm139
Heber U, Bilger W, Türk R, Lange OL (2010) Photoprotection of reaction centres in photosynthetic organisms: mechanisms of thermal energy dissipation in desiccated thalli of the lichen Lobaria pulmonaria. New Phytol 185:459–470. https://doi.org/10.1111/j.1469-8137.2009.03064.x
Hillmann J, Grummann V (1957) Kryptogamenflora der Mark Brandenburg und angrenzender Gebiete. Band VIII: Flechten. [The cryptogamic flora of the March of Brandenburg and adjacent areas. Volume VIII: Lichens (in German)]. Gebrüder Borntraeger, Berlin-Nikolassee
Honegger R (1986) Ultrastructural studies in lichens. II. Mycobiont and photobiont cell wall surface layers and adhering crystalline lichen products in four Parmeliaceae. New Phytol 103:797–808. https://doi.org/10.1111/j.1469-8137.1986.tb00854.x
Honegger R (1997) Metabolic interactions at the mycobiont–photobiont interface in lichens. In: Carroll GC, Tudzynski P (eds) The mycota V. plant relationships. Springer, Berlin, Heidelberg, pp 209–221
Honegger R (2012) The symbiotic phenotype of lichen-forming ascomycetes and their endo- and epibionts. In: Hock B (ed) The mycota IX. fungal associations. Springer, Berlin, Heidelberg, pp 287–339
Hungarian Meteorological Services (2022) Meteorológiai adattár [Meteorological database]. Országos Meteorológiai Szolgálat. https://odp.met.hu/climate/observations_hungary/monthly/. Accessed 16 Mar 2022
Jensen M (2002) Measurement of chlorophyll fluorescence in lichens. In: Kranner I, Beckett RP, Varma AK (eds) Protocols in lichenology. Culturing, biochemistry, ecophysiology and use in biomonitoring. Springer-Verlag, Berlin, Heidelberg, pp 135–151. https://doi.org/10.1007/978-3-642-56359-1_9
Ji X, Khan IA (2005) Quantitative determination of usnic acid in Usnea lichen and its products by reversed-phase liquid chromatography with photodiode array detector. J AOAC Int 88(5):1265–1268. https://doi.org/10.1093/jaoac/88.5.1265
Kitajima ML, Butler W (1975) Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim Biophys Acta 376(1):105–115. https://doi.org/10.1016/0005-2728(75)90209-1
Klughammer C, Schreiber U (2008) Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the saturation pulse method. PAM Applied Notes 1:27–35
Kocsis K, Gercsák G, Horváth G, Keresztesi Z, Nemerkényi ZS (eds) (2018) National atlas of Hungary: volume 2. Natural environment. Geographical Institute, Research Centre for Astronomy and Earth Sciences, Budapest, pp 1–183
Kopecky J, Azarkovich M, Pfundel EE, Shuvalov VA, Heber U (2005) Thermal dissipation of light energy is regulated differently and by different mechanisms in lichens and higher plants. Plant Biol 7(2):156–167. https://doi.org/10.1055/s-2005-837471
Kramer DM, Johnson G, Kiirats O, Edwards GE (2004) New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth Res 79(2):209–218. https://doi.org/10.1023/B:PRES.0000015391.99477.0d
Kranner I, Cram WJ, Zorn M, Wornik S, Yoshimura I, Stabenthei-Ner E, Pfeifhofer HW (2005) Antioxidants and photoprotection in a lichen as compared with its isolated symbiotic partners. Proc Natl Acad Sci U S A. 102(8):3141–3146. https://doi.org/10.1073/pnas.0407716102
Krieger-Liszkay A (2005) Singlet oxygen production in photosynthesis. J Exp Bot 56(411):337–346. https://doi.org/10.1093/jxb/erh237
Lange OL (2003) Photosynthetic productivity of the epilithic lichen Lecanora muralis: long-term field monitoring of CO2 exchange and its physiological interpretation II. Diel and seasonal patterns of net photosynthesis and respiration. Flora 198:55–70
Lawrey JD (1993) Chemical ecology of Hobsonia christiansenii, a lichenicolous hyphomycete. Am J Bot 80:1109–1113
Lawrey JD, Rossman AZ, Lowen R (1994) Inhibition of selected hypocrealean fungi by lichen secondary metabolites. Mycologia 86(4):502–506
Lawrey JD (2000) Chemical interactions between two lichen-degrading fungi. J Chem Ecol 26:1821–1831
Mázsa K, Kalapos T, Draskovits R (2002) Fotoszintézis válaszreakciók és élőhely mozaik preferencia összefüggése talajlakó xeroterm zuzmóknál és moháknál. [Correlation between photosynthetic responses and habitat mosaic preference in soil-dwelling xerothermic lichens and bryophytes (in Hungarian)]. Szupraindividuális biológiai kutatások. MTA Ökológiai és Botanikai Kutatóintézete, Vácrátót, pp 41–46
McEvoy M, Solhaug KA, Gauslaa Y (2007) Solar radiation screening in usnic acid containing cortices of the lichen Nephroma arcticum. Symbiosis 43:143–150
Merinero S, Gauslaa Y (2018[2017]) Specialized fungal parasites reduce fitness of their lichen hosts. Ann Bot 121(1):175–182. https://doi.org/10.1093/aob/mcx124
Molnár K, Farkas E (2010) Current results on biological activities of lichen secondary metabolites: a review. Z Naturforsch 65C:157–173. https://doi.org/10.1515/znc-2010-3-401
Molnár K, Farkas E (2011) Depsides and depsidones in populations of the lichen Hypogymnia physodes and its genetic diversity. Ann Bot Fenn 48:473–482. https://doi.org/10.5735/085.048.0605
Morillas L, Roales J, Cruz C, Munzi S (2021) Resilience of epiphytic lichens to combined effects of increasing nitrogen and solar radiation. J Fungi 7(5):333. https://doi.org/10.3390/jof7050333
Muhoro AM, Farkas EÉ (2021) Insecticidal and antiprotozoal properties of lichen secondary metabolites on insect vectors and their transmitted protozoal diseases to humans. Diversity 13(8):342. https://doi.org/10.3390/d13080342
Müller P, Xiao-Ping L, Krishna NK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125(4):1558–1566. https://doi.org/10.1104/pp.125.4.1558
Nguyen K-H, Chollet-Krugler M, Gouault N, Tomasi S (2013) UV-protectant metabolites from lichens and their symbiotic partners. Nat Prod Rep 30(12):1490–1508. https://doi.org/10.1039/c3np70064j
Nimis PL, Skert N (2006) Lichen chemistry and selective grazing by the Coleopteran Lasioderma serricorne. Environ Exp Bot 55(1-2):175–182. https://doi.org/10.1016/j.envexpbot.2004.10.011
Nybakken L, Julkunen-Tiitto R (2006) UV-B induces usnic acid in reindeer lichens. Lichenologist 38(5):477–485. https://doi.org/10.1017/S0024282906005883
Osyczka P, Rola K (2013) Phenotypic plasticity of primary thallus in selected Cladonia species (lichenized Ascomycota: Cladoniaceae). Biologia 68(3):365–372. https://doi.org/10.2478/s11756-013-0169-3
Paoli L, Pisani T, Munzi S, Gaggi C, Loppi S (2010) Influence of sun irradiance and water availability on lichen photosynthetic pigments during a Mediterranean summer. Biologia 65(5):776–783. https://doi.org/10.2478/s11756-010-0087-6
Rancan F, Rosan S, Boehm K, Fernández E, Hidalgo ME, Quilhot W, Rubio C, Boehm F, Piazena H, Oltmanns U (2002) Protection against UVB irradiation by natural filters extracted from lichens. Photochem Photobiol B 68:133–139. https://doi.org/10.1016/S1011-1344(02)00362-7
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 10 Feb 2022
Sadowsky A, Ott S (2016) Symbiosis as a successful strategy in continental Antarctica: performance and protection of Trebouxia photosystem II in relation to lichen pigmentation. Polar Biol 39(1):139–151. https://doi.org/10.1007/s00300-015-1677-0
Scheidegger C, Schroeter B, Frey B (1995) Structural and functional processes during water-vapor uptake and desiccation in selected lichens with green algal photobionts. Planta 197:399–409. https://doi.org/10.1007/BF00202663
Seaward MRD (1988) Contribution of lichens to ecosystems. In: Galun M (ed) CRC handbook of lichenology, vol II. CRC Press, Inc, Boca Raton, pp 107–129
Singh J, Dubey AK, Singh RP (2011) Antarctic terrestrial ecosystem and role of pigments in enhanced UV-B radiations. Rev Environ Sci Biotechnol 10:63–77. https://doi.org/10.1007/s11157-010-9226-3
Škaloud P, Peksa O (2008) Comparative study of chloroplast morphology and ontogeny in Asterochloris (Trebouxiophyceae, Chlorophyta). Biologia 63(6):873–880. https://doi.org/10.2478/s11756-008-0115-y
Smith CW, Aptroot A, Coppins BJ, Fletcher A, Gilbert OL, James PW, Wolseley PA (eds) (2009) The lichens of Great Britain and Ireland. British Lichen Society, London
Solhaug KA, Gauslaa Y (1996) Parietin, a photoprotective secondary product of the lichen Xanthoria parietina. Oecologia 108:412–418. https://doi.org/10.1007/BF00333715
Solhaug KA, Gauslaa Y (2001) Acetone rinsing — a method for testing ecological and physiological roles of secondary compounds in living lichens. Symbiosis 30(4):301–315
Solhaug KA, Gauslaa Y, Nybakken L, Bilger W (2003) UV-induction of sun-screening pigments in lichens. New Phytol 158(1):91–100. https://doi.org/10.1046/j.1469-8137.2003.00708.x
Solhaug KA, Gauslaa Y (2004) Photosynthates stimulate the UV-B induced fungal anthraquinone synthesis in the foliose lichen Xanthoria parietina. Plant Cell Environ 27:167–176. https://doi.org/10.1111/j.1365-3040.2003.01129.x
Solhaug KA, Larsson P, Gauslaa Y (2010) Light screening in lichen cortices can be quantified by chlorophyll fluorescence techniques for both reflecting and absorbing pigments. Planta 231(5):1003–1011. https://doi.org/10.1007/s00425-010-1103-3
Veerman J, Vasil’ev S, Paton GD, Ramanauskas J, Bruce D (2007) Photoprotection in the lichen Parmelia sulcata: the origins of desiccation-induced fluorescence quenching. Plant Physiol 145:997–1005. https://doi.org/10.1104/pp.107.106872
Veres K, Farkas E, Csintalan Z (2020) The bright and shaded side of duneland life: the photosynthetic response of lichens to seasonal changes is species-specific. Mycol Prog 19(6):629–641. https://doi.org/10.1007/s11557-020-01584-6
Veres K, Csintalan Z, Laufer Z, Engel R, Szabó K, Farkas E (2022) Photoprotection and high-light acclimation in semi-arid grassland lichens — a cooperation between algal and fungal partners. Symbiosis 86:33–48. https://doi.org/10.1007/s13199-021-00823-y
Verseghy K (1971) Angaben über den Wasserhaushalt einiger Xerotherm-Erdflechten. [Data on the water balance of some xerothermic terricolous lichens (in German)]. Ann Hist-Nat Mus Natl Hungarici 63:83–97
Verseghy K (1974) Talajlakó xerofiton zuzmófajok ökológiája és elterjedése Magyarországon. I. (Ökologie und Verbreitung der bodenbewohnenden xerophytischen Flechtenarten in Ungarn). [Ecology and distribution of terricolous xerophytic lichens in Hungary I. (in Hungarian with German summary)]. Stud Bot Hung 9:31–42
Verseghy K (1975) Talajlakó xerofiton zuzmófajok ökológiája és elterjedése Magyarországon (II.) s néhány taxon revíziója. (Ökologie und Verbreitung der bodenbewohnenden xerophytischen Flechtenarten in Ungarn (II), und Revision einiger Taxonen). [Ecology and distribution of terricolous xerophytic lichens in Hungary (II.), and revision of some taxa (in Hungarian with German summary)]. Stud Bot Hung 10:41–61
Verseghy K (1976) Quantitative investigation of xerothermophilous lichens of sandy soil. Ann hist-nat Mus Natl Hung 68:59–63
Verseghy K (1994) Magyarország Zuzmóflórájának Kézikönyve [The handbook of the Hungarian lichen flora (in Hungarian)]. Magyar Természettudományi Múzeum, Budapest, Hungary, pp 1–415
Vráblíková H, Mcevoy M, Solhaug KA, Barták M, Gauslaa Y (2006) Annual variation in photoacclimation and photoprotection of the photobiont in the foliose lichen Xanthoria parietina. J Photochem Photobiol B 83(2):151–162. https://doi.org/10.1016/j.jphotobiol.2005.12.019
Wirth V, Hauck M, Schultz M (2013) Die Flechten Deutschlands. [Lichens of Germany (in German)]. Ulmer, Stuttgart
Yilmaz M, Turk AO, Tay T, Kıvanc M (2004) The antimicrobial activity of extracts of the lichen Cladonia foliacea and its (-)-usnic acid, atranorin, and fumarprotocetraric acid constituents. Z Naturforsch Sect C 59:249–254
Acknowledgements
The authors are grateful to Mrs Bernadett Kolonics, Mrs Sándorné Vadkerti, and Ms Dóra Smahajcsik for technical help in collecting and transplanting specimens, Dr Csaba Locsmándi who kindly provided help with the lyophilisation of samples, Prof Mark Seaward who corrected the English text, and Dr László Lőkös for reading the manuscript.
Funding
Open access funding provided by ELKH Centre for Ecological Research. This work was supported by the project financed by the National Research Development and Innovation Fund (NKFI K 124341–PI: EF).
Author information
Authors and Affiliations
Contributions
EF and KV conceived and designed the research. Material collection and preparation were performed by EF, KV, NV, and MS. KS and KV conducted measurements. NV identified lichenicolous fungi. Data were analysed by KV, EF, and NV. The first draft of the manuscript was written by KV and EF, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
We have no conflicts of interest to disclose.
Competing interests
The authors declare no competing interests.
Additional information
Section Editor: Gerhard Rambold
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 429 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Veres, K., Sinigla, M., Szabó, K. et al. The long-term effect of removing the UV-protectant usnic acid from the thalli of the lichen Cladonia foliacea. Mycol Progress 21, 83 (2022). https://doi.org/10.1007/s11557-022-01831-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-022-01831-y