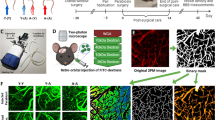
Abstract
Microglia are implicated in the neuropathogenesis of HIV. Tetraspanin 2 (Tspan2) is closely related to CD9 and CD81 proteins, and are expressed on microglia cells. They have been implicated in cell fusion and adhesion and in the immune response, and neuroinflammation. Developing therapeutics that target microglia remains a challenge as these therapeutics must cross the Blood-Brain Barrier (BBB). Our goal was to use microglia derived exosomes as a vehicle to deliver siRNA across the BBB to target human telomerase reverse transcriptase immortalized human microglial cells (HTHU) latently infected by HIV (HTHU-HIV) and to evaluate if the knockdown of Tspan2 gene expression in changes the activation state of microglia cells, thereby modulating the neuroinflammatory response. A blood brain barrier (BBB) model that closely mimics and accurately reflects the characteristics and functional properties of the in vivo BBB was used to examine HTHU microglia exosome effects on BBB permeability, and their ability to migrate across the and delivery small interfering RNA (siRNA) to cells on the CNS side of the BBB model. Exosomes were loaded with Texas-Red control siRNA (20 pmol) or Cy5-Tspan2 siRNA and then placed in the apical side of the BBB model, 24 h after incubation, HTHU-HIV cells microglial cells on the lower chamber were either imaged for siRNA uptake or analyzed for gene expression induced modifications. HTHU exosomes transmigrate from the apical side of the BBB to deliver Texas-Red control siRNA or Cy5-Tspan2 siRNA to HTHU-HIV microglia cells on the CNS side of the BBB model. A dose dependent (5–40 pmol) increase in Cy5-Tspan2 uptake with a corresponding decrease in gene expression for Tspan2 occurred in HTHU-HIV microglia. A decrease in Tspan2 gene expression as a consequence of knockdown with Tspan2 siRNA at both 20 and 40 pmol concentrations resulted in a significant decrease in C-X-C motif chemokine 12 (CXCL12) and C-X-C chemokine receptor type 4 (CXCR4) gene expression in HTHU-HIV microglia. Furthermore, a decrease in the gene expression levels of the Interleukins, IL-13 and IL-10 and an increase in the gene expression levels for the Fc gamma receptor 2A(FCGR2A) and TNF-α occurred in HTHU-HIV microglial cells These data demonstrate that HTHU exosomes cross the BBB and are efficient delivery vehicles to the CNS. Moreover, modifying the expression levels of Tspan2, has downstream consequences that includes alterations in cytokines and microglia biomarkers.

Microglia-derived exosomes loaded with Tspan2 siRNA transmigrate across the BBB and knockdown Tspan2 gene expression in human microglial cells latently infected by HIV. This knockdown increases CXCL12, CXCR4, FCGR2A and TNF-α while decreasing IL-13 and IL-10 gene expression in HTHU-HIV microglial cells. Modulating Tspan2 modulates microglia cytokines and phenotype biomarkers.






Similar content being viewed by others
References
Abbott N (2004) Prediction of blood–brain barrier permeation in drug discovery from in vivo, in vitro and in silico models. Drug Discov Today Technol 1(4):407–416
Abbott NJ (2005) Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol 25(1):5–23
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood M (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29(4):341–345
Andras I, Toborek M (2016) Extracellular vesicles of the blood-brain barrier. Tissue Barriers 4(1):e1131804 -1-6
Aryani A, Denecke B (2016) Exosomes as a Nanodelivery system: a key to the future of Neuromedicine? Mol Neurobiol 53(2):818–834
Bang C, Thum T (2012) Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol 44(11):2060–2064
Chen CC, Liu L, Ma F, Wong CW, Guo XE, Chacko JV et al (2016) Elucidation of exosome migration across the blood-brain barrier model in vitro. Cell Mol Bioeng 9(4):509–529
Cherry J, Olschowka J, O’Banion MK (2014) Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 11:98
El Andaloussi S, Lakhal S, Mager I, Wood MJ (2013) Exosomes for targeted siRNA delivery across biological barriers. Adv Drug Deliv Rev 65(3):391–397
Fitzner D, Schnaars M, Van Rossum D, Krishnamoorthy G, Dibaj P, Bakhit M et al (2011) Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci 124:447–458
Frühbeis C, Fröhlich D, Kuo WP, Krämer-Albers EM (2013) Extracellular vesicles as mediators of neuron-glia communication. Front Cell Neurosci 7:182 1-6
Garcia-Mesa Y, Jay TR, Checkley MA, Luttge B, Dobrowolski C, Valadkhan S et al (2017) Immortalization of primary microglia: a new platform to study HIV regulation in the central nervous system. J Neuro-Oncol 23(1):47–66
Guyon A (2014) CXCL12 chemokine and its receptors as major players in the interactions between immune and nervous systems. Front Cell Neurosci 8(65):1–10
Ha D, Yang N, Nadithe V (2016) Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B 6(4):287–296
Hantak M, Qing E, Earnest JT, Gallagher T (2019) Tetraspanins: architects of viral entry and exit platforms. J Virol 93(9):01429–01417
Hayes G, Woodroofe MN, Cuzner ML (1987) Microglia are the major cell type expressing MHC class II in human white matter. J Neurol Sci 80:25–37
Hickey W, Kimura H (1988) Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 239:290–292
Hogan C, Wilkins E (2011) Neurological complications in HIV. Clinical Medicine 11(6):571–575
Jonavice U, Tunaitis V, Kriauciunaite K, Jarmalaviciute A, Pivoriunas A (2019) Extracellular vesicles can act as a potent immunomodulators of human microglial cells. J Tissue Eng Regen Med 13(2):309–318
Joseph SB, Arrildt KT, Sturdevant CB, Swanstrom R (2015) HIV-1 target cells in the CNS. J Neuro-Oncol 21(3):276–289
Kettenmann H, Hanisch UK, Noda M, Verkhratsky A (2011) Physiology of microglia. Physiol Rev 91:461–553
Krementsov DN, Weng J, Lambele M, Roy NH, Thali M (2009) Tetraspanins regulate cell-to-cell transmission of HIV-1. Retrovirology 6:64
Lai RC, Yeo RW, Tan KH, Lim SK (2013) Exosomes for drug delivery - a novel application for the mesenchymal stem cell. Biotechnol Adv 31(5):543–551
Lampson LA, Hickey WF (1986) Monoclonal antibody analysis of MHC expression in human brain biopsies: tissue ranging from “histologically normal” to that showing different levels of glial tumor involvement. J Immunol 136:4054–4062
Mahajan SD, Roy I, Xu G, Yong KT, Ding H, Aalinkeel R et al (2010) Enhancing the delivery of anti retroviral drug "Saquinavir" across the blood brain barrier using nanoparticles. Curr HIV Res 8(5):396–404
Matsumoto J, Stewart T, Sheng L, Li N, Bullock K, Song N et al (2017) Transmission of alpha-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: another mechanism for initiation and progression of Parkinson's disease? Acta Neuropathol Commun 5(1):71 -1-16
McGeer PL, Itagaki S, McGeer EG (1988) Expression of the histocompatibility glycoprotein HLA-DR in neurological disease. Acta Neurpathol 76:550–555
Paolicelli RC, Bergamini G, Rajendran L (2019) Cell-to-cell communication by extracellular vesicles: focus on microglia. Neuroscience. 405:148–157
Schmittgen TD, Livak K (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Schnell G, Joseph S, Spudich S, Swanstrom R (2001) HIV-1 Replication in the Central Nervous System Occurs in Two Distinct Cell Types. PLoS Pathog 7(10):e1002286 1–13
Serlin Y, Shelef I, Knyazer B, Friedman A (2015) Anatomy and physiology of the blood-brain barrier. Semin Cell Dev Biol 38:2–6
Sillman B, Woldstad C, Mcmillan J, Gendelman HE (2018) Neuropathogenesis of human immunodeficiency virus infection. Handb Clin Neurol 152:21–40
Sims B, Farrow AL, Williams SD, Bansal A, Krendelchtchikov A, Matthews QL (2018) Tetraspanin blockage reduces exosome-mediated HIV-1 entry. Arch Virol 163(6):1683–1689
Sobel RA, Ames MB (1988) Major histocompatibility complex molecule expression in the human central nervous system: immunohistochemical analysis of 40 patients. J Neuropath Exp Neurol 47:19–28
Sobel RA, Blanchette BW, Bhan AK, Colvin RB (1984) The immunopathology of experimental allergic encephalomyelitis. II. Endothelial cell Ia increases prior to inflammatory cell infiltration. J Immunol 132:2402–2407
Tang Y, Weidong L (2016) Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol 53(2):1181–1194
Valcour V, Sithinamsuwan P, Letendre S, Ances B (2011) Pathogenesis of HIV in the central nervous system. Current HIV/AIDS reports 8(1):54–61
van Dommelen SM, Vader P, Lakhal S, Kooijmans SA, van Solinge WW, Wood MJ et al (2012) Microvesicles and exosomes: opportunities for cell-derived membrane vesicles in drug delivery. J Control Release 161(2):635–644
Wang C, Chen L, Huang Y, Li K, Jinye A, Fan T et al (2019a) Exosome-delivered TRPP2 siRNA inhibits the epithelial-mesenchymal transition of FaDu cells. Oncol Lett 17(2):1953–1961
Wang H, Sui H, Zheng Y, Jiang Y, Shi Y, Liang J et al (2019b) Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the tau protein through the AKT/GSK-3beta pathway. Nanoscale. 11(15):7481–7496
Yang Y, Boza-Serrano A, Dunning CJR, Clausen BH, Lambertsen KL, Deierborg T (2018) Inflammation leads to distinct populations of extracellular vesicles from microglia. J Neuroinflammation 15(1):168 1–19
Yaseen IH, Monk PN, Partridge LJ (2017) Tspan2: a tetraspanin protein involved in oligodendrogenesis and cancer metastasis. Biochem Soc Trans 45(2):465–475
Acknowledgements
Research reported in this publication was supported by 1R01AI129649 (NIAID) (JR), 1R01DA047410 (NIDA) (SM) and the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001412 (JR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Reynolds, J.L., Mahajan, S.D. Transmigration of Tetraspanin 2 (Tspan2) siRNA Via Microglia Derived Exosomes across the Blood Brain Barrier Modifies the Production of Immune Mediators by Microglia Cells. J Neuroimmune Pharmacol 15, 554–563 (2020). https://doi.org/10.1007/s11481-019-09895-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-019-09895-6