Abstract
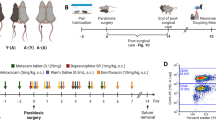
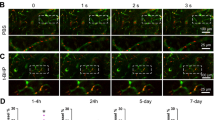
Age-related cerebromicrovascular changes, including blood-brain barrier (BBB) disruption and microvascular rarefaction, play a significant role in the development of vascular cognitive impairment (VCI) and neurodegenerative diseases. Utilizing the unique model of heterochronic parabiosis, which involves surgically joining young and old animals, we investigated the influence of systemic factors on these vascular changes. Our study employed heterochronic parabiosis to explore the effects of young and aged systemic environments on cerebromicrovascular aging in mice. We evaluated microvascular density and BBB integrity in parabiotic pairs equipped with chronic cranial windows, using intravital two-photon imaging techniques. Our results indicate that short-term exposure to young systemic factors leads to both functional and structural rejuvenation of cerebral microcirculation. Notably, we observed a marked decrease in capillary density and an increase in BBB permeability to fluorescent tracers in the cortices of aged mice undergoing isochronic parabiosis (20-month-old C57BL/6 mice [A-(A)]; 6 weeks of parabiosis), compared to young isochronic parabionts (6-month-old, [Y-(Y)]). However, aged heterochronic parabionts (A-(Y)) exposed to young blood exhibited a significant increase in cortical capillary density and restoration of BBB integrity. In contrast, young mice exposed to old blood from aged parabionts (Y-(A)) rapidly developed cerebromicrovascular aging traits, evidenced by reduced capillary density and increased BBB permeability. These findings underscore the profound impact of systemic factors in regulating cerebromicrovascular aging. The rejuvenation observed in the endothelium, following exposure to young blood, suggests the existence of anti-geronic elements that counteract microvascular aging. Conversely, pro-geronic factors in aged blood appear to accelerate cerebromicrovascular aging. Further research is needed to assess whether the rejuvenating effects of young blood factors could extend to other age-related cerebromicrovascular pathologies, such as microvascular amyloid deposition and increased microvascular fragility.




Similar content being viewed by others
References
Johnson AC. Hippocampal vascular supply and its role in vascular cognitive impairment. Stroke. 2023;54:673–85. https://doi.org/10.1161/STROKEAHA.122.038263.
Iadecola C, Duering M, Hachinski V, Joutel A, Pendlebury ST, Schneider JA, Dichgans M. Vascular cognitive impairment and dementia: JACC scientific expert panel. J Am Coll Cardiol. 2019;73:3326–44. https://doi.org/10.1016/j.jacc.2019.04.034.
Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312:H1–20. https://doi.org/10.1152/ajpheart.00581.2016.
Gorelick PB, Counts SE, Nyenhuis D. Vascular cognitive impairment and dementia. Biochim Biophys Acta. 2016;1862:860–8. https://doi.org/10.1016/j.bbadis.2015.12.015.
Mahinrad S, Sorond F, Gorelick PB. The role of vascular risk factors in cognitive impairment and dementia and prospects for prevention. Clin Geriatr Med. 2023;39:123–34. https://doi.org/10.1016/j.cger.2022.07.007.
Wolters FJ, Ikram MA. Epidemiology of vascular dementia. Arterioscler Thromb Vasc Biol. 2019;39:1542–9. https://doi.org/10.1161/ATVBAHA.119.311908.
Rizzi L, Rosset I, Roriz-Cruz M. Global epidemiology of dementia: Alzheimer’s and vascular types. Biomed Res Int. 2014;2014:908915. https://doi.org/10.1155/2014/908915.
Rocca WA, Hofman A, Brayne C, Breteler MMB, Clarke M, Copeland JRM, Dartiques J-F, Engedal K, Hagnell O, Heeren TJ, et al. The prevalence of vascular dementia in Europe: facts and fragments from 1980–1990 studies. Ann Neurol. 1991;30:817–24. https://doi.org/10.1002/ana.410300611.
Hébert R, Brayne C. Epidemiology of vascular dementia. Neuroepidemiology. 1995;14:240–57. https://doi.org/10.1159/000109800.
Tong X, Yang Q, Ritchey MD, George MG, Jackson SL, Gillespie C, Merritt RK. The burden of cerebrovascular disease in the United States. Prev Chronic Dis. 2019;16:E52. https://doi.org/10.5888/pcd16.180411.
Eurostat: aging Europe. https://ec.europa.eu/eurostat/cache/digpub/ageing/. Accessed on 11 April 2022
World Health Organization. Health data overview for Japan. https://data.who.int/countries/392. Accessed on 10 Feb 2023
“Aging in the United States”. Population Reference Bureau, 2021, https://www.prb.org/aging-unitedstates-fact-sheet/. Accessed on 05 Sept 2023
United States Census Bureau. 2020 Census: 1 in 6 People in the United States Were 65 and Over. https://www.census.gov/library/stories/2023/05/2020-census-united-states-older-population-grew.html. Accessed on 10 Feb 2023
Nichols E, Steinmetz JD, Vollset SE, Fukutaki K, Chalek J, Abd-Allah F, Abdoli A, Abualhasan A, Abu-Gharbieh E, Akram TT, et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. The Lancet Public Health. 2022;7:e105–25. https://doi.org/10.1016/S2468-2667(21)00249-8.
Nandi A, Counts N, Chen S, Seligman B, Tortorice D, Vigo D, Bloom DE. Global and regional projections of the economic burden of Alzheimer’s disease and related dementias from 2019 to 2050: a value of statistical life approach. EClinicalMedicine. 2022;51:101580. https://doi.org/10.1016/j.eclinm.2022.101580.
Han EJ, Lee J, Cho E, Kim H. Socioeconomic costs of dementia based on utilization of health care and long-term-care services: a retrospective cohort study. Int J Environ Res Public Health. 2021;18. https://doi.org/10.3390/ijerph18020376
Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: from physiology to disease and back. Physiol Rev. 2019;99:21–78. https://doi.org/10.1152/physrev.00050.2017.
Nyul-Toth A, Tarantini S, DelFavero J, Yan F, Balasubramanian P, Yabluchanskiy A, Ahire C, Kiss T, Csipo T, Lipecz A, et al. Demonstration of age-related blood-brain barrier disruption and cerebromicrovascular rarefaction in mice by longitudinal intravital two-photon microscopy and optical coherence tomography. Am J Physiol Heart Circ Physiol. 2021;320:H1370–92. https://doi.org/10.1152/ajpheart.00709.2020.
Fulop GA, Ahire C, Csipo T, Tarantini S, Kiss T, Balasubramanian P, Yabluchanskiy A, Farkas E, Toth A, Nyul-Toth A, et al. Cerebral venous congestion promotes blood-brain barrier disruption and neuroinflammation, impairing cognitive function in mice. Geroscience. 2019;41:575–89. https://doi.org/10.1007/s11357-019-00110-1.
Tucsek Z, Toth P, Sosnowsk D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z, Csiszar A. Obesity in aging exacerbates blood brain barrier disruption, neuroinflammation and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2014;69:1212–26.
Van Skike CE, Jahrling JB, Olson AB, Sayre NL, Hussong SA, Ungvari Z, Lechleiter JD, Galvan V. Inhibition of mTOR protects the blood-brain barrier in models of Alzheimer’s disease and vascular cognitive impairment. Am J Physiol Heart Circ Physiol. 2018;314:H693–703. https://doi.org/10.1152/ajpheart.00570.2017.
Miller LR, Tarantini S, Nyul-Toth A, Johnston MP, Martin T, Bullen EC, Bickel MA, Sonntag WE, Yabluchanskiy A, Csiszar A, et al. Increased susceptibility to cerebral microhemorrhages is associated with imaging signs of microvascular degeneration in the retina in an insulin-like growth factor 1 deficient mouse model of accelerated aging. Front Aging Neurosci. 2022;14:788296. https://doi.org/10.3389/fnagi.2022.788296.
Nyul-Toth A, Fulop GA, Tarantini S, Kiss T, Ahire C, Faakye JA, Ungvari A, Toth P, Toth A, Csiszar A, Ungvari Z. Cerebral venous congestion exacerbates cerebral microhemorrhages in mice. Geroscience. 2022;44:805–16. https://doi.org/10.1007/s11357-021-00504-0.
Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Springo Z, Fulop GA, Ashpole N, Gautam T, Giles CB, Wren JD, Sonntag WE, et al. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell. 2017;16:469–79. https://doi.org/10.1111/acel.12583.
Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell. 2015;14:400–8. https://doi.org/10.1111/acel.12315.
Ungvari Z, Tarantini S, Kirkpatrick AC, Csiszar A, Prodan CI. Cerebral microhemorrhages: mechanisms, consequences, and prevention. Am J Physiol Heart Circ Physiol. 2017;312:H1128–43. https://doi.org/10.1152/ajpheart.00780.2016.
Riddle DR, Sonntag WE, Lichtenwalner RJ. Microvascular plasticity in aging. Ageing Res Rev. 2003;2:149–68.
Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–20.
Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, et al. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69:1339–52. https://doi.org/10.1093/gerona/glu080.
Balasubramanian P, Kiss T, Tarantini S, Nyul-Toth A, Ahire C, Yabluchanskiy A, Csipo T, Lipecz A, Tabak A, Institoris A, et al. Obesity-induced cognitive impairment in older adults: a microvascular perspective. Am J Physiol Heart Circ Physiol. 2021;320:H740–61. https://doi.org/10.1152/ajpheart.00736.2020.
Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab. 2013;33:1732–42. https://doi.org/10.1038/jcbfm.2013.143.
Ungvari Z, Toth P, Tarantini S, Prodan CI, Sorond F, Merkely B, Csiszar A. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat Rev Nephrol. 2021;17:639–54. https://doi.org/10.1038/s41581-021-00430-6.
Elahy M, Jackaman C, Mamo JC, Lam V, Dhaliwal SS, Giles C, Nelson D, Takechi R. Blood-brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immun Ageing. 2015;12:2. https://doi.org/10.1186/s12979-015-0029-9.
Kerkhofs D, Wong SM, Zhang E, Uiterwijk R, Hoff EI, Jansen JFA, Staals J, Backes WH, van Oostenbrugge RJ. Blood-brain barrier leakage at baseline and cognitive decline in cerebral small vessel disease: a 2-year follow-up study. Geroscience. 2021;43:1643–52. https://doi.org/10.1007/s11357-021-00399-x.
Starr JM, Farrall AJ, Armitage P, McGurn B, Wardlaw J. Blood-brain barrier permeability in Alzheimer’s disease: a case-control MRI study. Psychiatry Res. 2009;171:232–41. https://doi.org/10.1016/j.pscychresns.2008.04.003.
Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease–systematic review and meta-analysis. Neurobiol Aging. 2009;30:337–52. https://doi.org/10.1016/j.neurobiolaging.2007.07.015.
Banks WA, Reed MJ, Logsdon AF, Rhea EM, Erickson MA. Healthy aging and the blood-brain barrier. Nat Aging. 2021;1:243–54. https://doi.org/10.1038/s43587-021-00043-5.
Li Y, Li M, Zhang X, Shi Q, Yang S, Fan H, Qin W, Yang L, Yuan J, Jiang T, Hu W. Higher blood-brain barrier permeability is associated with higher white matter hyperintensities burden. J Neurol. 2017;264:1474–81. https://doi.org/10.1007/s00415-017-8550-8.
Verheggen ICM, de Jong JJA, van Boxtel MPJ, Postma AA, Jansen JFA, Verhey FRJ, Backes WH. Imaging the role of blood-brain barrier disruption in normal cognitive ageing. Geroscience. 2020. https://doi.org/10.1007/s11357-020-00282-1.
Verheggen ICM, de Jong JJA, van Boxtel MPJ, Gronenschild E, Palm WM, Postma AA, Jansen JFA, Verhey FRJ, Backes WH. Increase in blood-brain barrier leakage in healthy, older adults. Geroscience. 2020;42:1183–93. https://doi.org/10.1007/s11357-020-00211-2.
Wardlaw JM, Doubal F, Armitage P, Chappell F, Carpenter T, Munoz Maniega S, Farrall A, Sudlow C, Dennis M, Dhillon B. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann Neurol. 2009;65:194–202. https://doi.org/10.1002/ana.21549.
Freeze WM, Jacobs HIL, de Jong JJ, Verheggen ICM, Gronenschild E, Palm WM, Hoff EI, Wardlaw JM, Jansen JFA, Verhey FR, Backes WH. White matter hyperintensities mediate the association between blood-brain barrier leakage and information processing speed. Neurobiol Aging. 2020;85:113–22. https://doi.org/10.1016/j.neurobiolaging.2019.09.017.
Lowerison MR, Sekaran NVC, Zhang W, Dong Z, Chen X, Llano DA, Song P. Aging-related cerebral microvascular changes visualized using ultrasound localization microscopy in the living mouse. Sci Rep. 2022;12:619. https://doi.org/10.1038/s41598-021-04712-8.
Cai C, Zambach SA, Grubb S, Tao L, He C, Lind BL, Thomsen KJ, Zhang X, Hald BO, Nielsen RM, et al. Impaired dynamics of precapillary sphincters and pericytes at first-order capillaries predict reduced neurovascular function in the aging mouse brain. Nat Aging. 2023;3:173–84. https://doi.org/10.1038/s43587-022-00354-1.
Zimmerman B, Rypma B, Gratton G, Fabiani M. Age-related changes in cerebrovascular health and their effects on neural function and cognition: a comprehensive review. Psychophysiology. 2021;58:e13796. https://doi.org/10.1111/psyp.13796.
Leeuwis AE, Smith LA, Melbourne A, Hughes AD, Richards M, Prins ND, Sokolska M, Atkinson D, Tillin T, Jäger HR, et al. Cerebral blood flow and cognitive functioning in a community-based, multi-ethnic cohort: the SABRE study. Front Aging Neurosci. 2018;10. https://doi.org/10.3389/fnagi.2018.00279
van Dinther M, Voorter PH, Jansen JF, Jones EA, van Oostenbrugge RJ, Staals J, Backes WH. Assessment of microvascular rarefaction in human brain disorders using physiological magnetic resonance imaging. J Cereb Blood Flow Metab. 2022;42:718–37. https://doi.org/10.1177/0271678x221076557.
De Silva TM, Faraci FM. Microvascular dysfunction and cognitive impairment. Cell Mol Neurobiol. 2016;36:241–58. https://doi.org/10.1007/s10571-015-0308-1.
Nyúl-Tóth Á, Tarantini S, DelFavero J, Yan F, Balasubramanian P, Yabluchanskiy A, Ahire C, Kiss T, Csipo T, Lipecz A, et al. Demonstration of age-related blood-brain barrier disruption and cerebromicrovascular rarefaction in mice by longitudinal intravital two-photon microscopy and optical coherence tomography. Am J Physiol Heart Circ Physiol. 2021;320:H1370-h1392. https://doi.org/10.1152/ajpheart.00709.2020.
Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14:133–50. https://doi.org/10.1038/nrneurol.2017.188.
Gulej R, Nyul-Toth A, Ahire C, DelFavero J, Balasubramanian P, Kiss T, Tarantini S, Benyo Z, Pacher P, Csik B, et al. Elimination of senescent cells by treatment with Navitoclax/ABT263 reverses whole brain irradiation-induced blood-brain barrier disruption in the mouse brain. Geroscience. 2023;45:2983–3002. https://doi.org/10.1007/s11357-023-00870-x.
Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302.
Barisano G, Montagne A, Kisler K, Schneider JA, Wardlaw JM, Zlokovic BV. Blood–brain barrier link to human cognitive impairment and Alzheimer’s disease. Nat Cardiovasc Res. 2022;1:108–15. https://doi.org/10.1038/s44161-021-00014-4.
Dickie BR, Boutin H, Parker GJM, Parkes LM. Alzheimer’s disease pathology is associated with earlier alterations to blood–brain barrier water permeability compared with healthy ageing in TgF344-AD rats. NMR Biomed. 2021;34:e4510. https://doi.org/10.1002/nbm.4510.
Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25:270–6. https://doi.org/10.1038/s41591-018-0297-y.
Haar HJvd, Burgmans S, Jansen JFA, Osch MJPv, Buchem MAv, Muller M, Hofman PAM, Verhey FRJ, Backes WH. Blood-brain barrier leakage in patients with early Alzheimer disease. Radiology. 2016; 281:527-535 https://doi.org/10.1148/radiol.2016152244
Skoog I, Wallin A, Fredman P, Hesse C, Aevarsson O, Karlsson I, Gottfries CG, Blennow K. A population study on blood-brain barrier function in 85-year-olds: relation to Alzheimer’s disease and vascular dementia. Neurology. 1998;50:966–71. https://doi.org/10.1212/wnl.50.4.966.
Li M, Li Y, Zuo L, Hu W, Jiang T. Increase of blood-brain barrier leakage is related to cognitive decline in vascular mild cognitive impairment. BMC Neurol. 2021;21:159. https://doi.org/10.1186/s12883-021-02189-6.
Lee E-S, Yoon J-H, Choi J, Andika FR, Lee T, Jeong Y. A mouse model of subcortical vascular dementia reflecting degeneration of cerebral white matter and microcirculation. J Cereb Blood Flow Metab. 2019;39:44–57.
Yang L, Song J, Nan D, Wan Y, Guo H. Cognitive impairments and blood-brain barrier damage in a mouse model of chronic cerebral hypoperfusion. Neurochem Res. 2022;47:3817–28. https://doi.org/10.1007/s11064-022-03799-3.
Takechi R, Lam V, Brook E, Giles C, Fimognari N, Mooranian A, Al-Salami H, Coulson SH, Nesbit M, Mamo JCL. Blood-brain barrier dysfunction precedes cognitive decline and neurodegeneration in diabetic insulin resistant mouse model: an implication for causal link. Front Aging Neurosci. 2017;9. https://doi.org/10.3389/fnagi.2017.00399
Geng J, Wang L, Zhang L, Qin C, Song Y, Ma Y, Chen Y, Chen S, Wang Y, Zhang Z, Yang G-Y. Blood-brain barrier disruption induced cognitive impairment is associated with increase of inflammatory cytokine. Front Aging Neurosci. 2018;10. https://doi.org/10.3389/fnagi.2018.00129
Ni P, Dong H, Wang Y, Zhou Q, Xu M, Qian Y, Sun J. IL-17A contributes to perioperative neurocognitive disorders through blood-brain barrier disruption in aged mice. Journal of Neuroinflammation. 2018;15:332. https://doi.org/10.1186/s12974-018-1374-3.
Stranahan AM, Hao S, Dey A, Yu X, Baban B. Blood–brain barrier breakdown promotes macrophage infiltration and cognitive impairment in leptin receptor-deficient mice. J Cereb Blood Flow Metab. 2016;36:2108–21. https://doi.org/10.1177/0271678x16642233.
Wallin A, Sjögren M, Edman A, Blennow K, Regland B. Symptoms, vascular risk factors and blood-brain barrier function in relation to CT white-matter changes in dementia. Eur Neurol. 2000;44:229–35. https://doi.org/10.1159/000008242.
Merlini M, Rafalski VA, Rios Coronado PE, Gill TM, Ellisman M, Muthukumar G, Subramanian KS, Ryu JK, Syme CA, Davalos D, et al. Fibrinogen induces microglia-mediated spine elimination and cognitive impairment in an Alzheimer’s disease model. Neuron. 2019;101:1099-1108.e1096. https://doi.org/10.1016/j.neuron.2019.01.014.
Senatorov VV, Friedman AR, Milikovsky DZ, Ofer J, Saar-Ashkenazy R, Charbash A, Jahan N, Chin G, Mihaly E, Lin JM, et al. Blood-brain barrier dysfunction in aging induces hyperactivation of TGFβ signaling and chronic yet reversible neural dysfunction. Sci Transl Med. 2019; 11:eaaw8283 https://doi.org/10.1126/scitranslmed.aaw8283
Kim S, Sharma C, Jung UJ, Kim SR. Pathophysiological role of microglial activation induced by blood-borne proteins in Alzheimer’s disease. Biomedicines. 2023;11. https://doi.org/10.3390/biomedicines11051383
Ju F, Ran Y, Zhu L, Cheng X, Gao H, Xi X, Yang Z, Zhang S. Increased BBB Permeability Enhances Activation of Microglia and Exacerbates Loss of Dendritic Spines After Transient Global Cerebral Ischemia. Front Cell Neurosci. 2018;12. https://doi.org/10.3389/fncel.2018.00236
Davalos D, Ryu JK, Merlini M, Baeten KM, Le Moan N, Petersen MA, Deerinck TJ, Smirnoff DS, Bedard C, Hakozaki H, et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun. 2012;3:1227. https://doi.org/10.1038/ncomms2230.
Ungvari Z, Tarantini S, Kiss T, Wren JD, Giles CB, Griffin CT, Murfee WL, Pacher P, Csiszar A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol. 2018;15:555–65. https://doi.org/10.1038/s41569-018-0030-z.
Ungvari Z, Tarantini S, Sorond F, Merkely B, Csiszar A. Mechanisms of vascular aging, a geroscience perspective: JACC focus seminar. J Am Coll Cardiol. 2020;75:931–41. https://doi.org/10.1016/j.jacc.2019.11.061.
Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123:849–67. https://doi.org/10.1161/circresaha.118.311378.
Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, et al. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018;17. https://doi.org/10.1111/acel.12731
Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol. 2014;592:2549–61. https://doi.org/10.1113/jphysiol.2013.268680.
Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, et al. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol. 2014;307:H292-306. https://doi.org/10.1152/ajpheart.00307.2014.
Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–27. https://doi.org/10.1016/j.mad.2009.06.004.
Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37-47.
Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–66.
Labinskyy N, Csiszar A, Orosz Z, Smith K, Rivera A, Buffenstein R, Ungvari Z. Comparison of endothelial function, O2.- and H2O2 production, and vascular oxidative stress resistance between the longest-living rodent, the naked mole rat, and mice. Am J Physiol. 2006;291:H2698-2704.
Csiszar A, Labinskyy N, Orosz Z, Xiangmin Z, Buffenstein R, Ungvari Z. Vascular aging in the longest-living rodent, the naked mole rat. Am J Physiol. 2007;293:H919-927.
Csiszar A, Labinskyy N, Zhao X, Hu F, Serpillon S, Huang Z, Ballabh P, Levy RJ, Hintze TH, Wolin MS, et al. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell. 2007;6:783–97.
Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci. 2012;67:811–20. https://doi.org/10.1093/gerona/glr228.
Fulop GA, Kiss T, Tarantini S, Balasubramanian P, Yabluchanskiy A, Farkas E, Bari F, Ungvari Z, Csiszar A. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience. 2018;40:513–21. https://doi.org/10.1007/s11357-018-0047-6.
Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE, Csiszar A. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of Nrf2-mediated antioxidant response Am J Physiol Heart Circ Physiol. 2011; 301:H363-372
Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Sule Z, et al. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24:101192. https://doi.org/10.1016/j.redox.2019.101192.
Kiss T, Balasubramanian P, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Csipo T, Lipecz A, Reglodi D, Zhang XA, Bari F, et al. Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for prevention of vascular cognitive impairment. GeroScience. 2019; 41:619-630
Kiss T, Giles CB, Tarantini S, Yabluchanskiy A, Balasubramanian P, Gautam T, Csipo T, Nyul-Toth A, Lipecz A, Szabo C, et al. Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects. Geroscience. 2019;41:419–39. https://doi.org/10.1007/s11357-019-00095-x.
Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, Kim LJ, Osborne B, Joshi S, Lu Y, et al. Impairment of an endothelial NAD(+)-H2S signaling network is a reversible cause of vascular aging. Cell. 2018;173:74-89.e20. https://doi.org/10.1016/j.cell.2018.02.008.
Mattison JA, Wang M, Bernier M, Zhang J, Park SS, Maudsley S, An SS, Santhanam L, Martin B, Faulkner S, et al. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab. 2014;20:183–90. https://doi.org/10.1016/j.cmet.2014.04.018.
Bickel MA, Csik B, Gulej R, Ungvari A, Nyul-Toth A, Conley SM. Cell non-autonomous regulation of cerebrovascular aging processes by the somatotropic axis. Front Endocrinol (Lausanne). 2023;14:1087053. https://doi.org/10.3389/fendo.2023.1087053.
Kiss T, Nyul-Toth A, Gulej R, Tarantini S, Csipo T, Mukli P, Ungvari A, Balasubramanian P, Yabluchanskiy A, Benyo Z, et al. Old blood from heterochronic parabionts accelerates vascular aging in young mice: transcriptomic signature of pathologic smooth muscle remodeling. Geroscience. 2022;44:953–81. https://doi.org/10.1007/s11357-022-00519-1.
Kiss T, Tarantini S, Csipo T, Balasubramanian P, Nyul-Toth A, Yabluchanskiy A, Wren JD, Garman L, Huffman DM, Csiszar A, Ungvari Z. Circulating anti-geronic factors from heterochonic parabionts promote vascular rejuvenation in aged mice: transcriptional footprint of mitochondrial protection, attenuation of oxidative stress, and rescue of endothelial function by young blood. Geroscience. 2020;42:727–48. https://doi.org/10.1007/s11357-020-00180-6.
Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, Hodges EL, Ungvari Z, Csiszar A, Chen S, et al. IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience. 2017;39:129–45. https://doi.org/10.1007/s11357-017-9971-0.
Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:313–29. https://doi.org/10.1093/gerona/glr164.
Fulop GA, Ramirez-Perez FI, Kiss T, Tarantini S, Valcarcel Ares MN, Toth P, Yabluchanskiy A, Conley SM, Ballabh P, Martinez-Lemus LA, et al. IGF-1 deficiency promotes pathological remodeling of cerebral arteries: a potential mechanism contributing to the pathogenesis of intracerebral hemorrhages in aging. J Gerontol A Biol Sci Med Sci. 2018. https://doi.org/10.1093/gerona/gly144.
Gulej R, Csik B, Faakye J, Tarantini S, Shanmugarama S, Chandragiri SS, Mukli P, Conley S, Csiszar A, Ungvari Z, et al. Endothelial deficiency of insulin-like growth factor-1 receptor leads to blood-brain barrier disruption and accelerated endothelial senescence in mice, mimicking aspects of the brain aging phenotype. Microcirculation. 2023;e12840. https://doi.org/10.1111/micc.12840
Tarantini S, Balasubramanian P, Yabluchanskiy A, Ashpole NM, Logan S, Kiss T, Ungvari A, Nyul-Toth A, Schwartzman ML, Benyo Z, et al. IGF1R signaling regulates astrocyte-mediated neurovascular coupling in mice: implications for brain aging. Geroscience. 2021;43:901–11. https://doi.org/10.1007/s11357-021-00350-0.
Tarantini S, Nyul-Toth A, Yabluchanskiy A, Csipo T, Mukli P, Balasubramanian P, Ungvari A, Toth P, Benyo Z, Sonntag WE, et al. Endothelial deficiency of insulin-like growth factor-1 receptor (IGF1R) impairs neurovascular coupling responses in mice, mimicking aspects of the brain aging phenotype. Geroscience. 2021;43:2387–94. https://doi.org/10.1007/s11357-021-00405-2.
Tarantini S, Tucsek Z, Valcarcel-Ares MN, Toth P, Gautam T, Giles CB, Ballabh P, Wei JY, Wren JD, Ashpole NM, et al. Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging. Age (Dordr). 2016;38:273–89. https://doi.org/10.1007/s11357-016-9931-0.
Toth L, Czigler A, Hegedus E, Komaromy H, Amrein K, Czeiter E, Yabluchanskiy A, Koller A, Orsi G, Perlaki G, et al. Age-related decline in circulating IGF-1 associates with impaired neurovascular coupling responses in older adults. Geroscience. 2022;44:2771–83. https://doi.org/10.1007/s11357-022-00623-2.
Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015;14:1034–44. https://doi.org/10.1111/acel.12372.
Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab. 2014;34:1887–97. https://doi.org/10.1038/jcbfm.2014.156.
Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-TNFalfa treatment in aging. Am J Pathol. 2007;170:388–698.
Ludwig FC, Elashoff RM. Mortality in syngeneic rat parabionts of different chronological age. Trans N Y Acad Sci. 1972;34:582–7. https://doi.org/10.1111/j.2164-0947.1972.tb02712.x.
Horrington EM, Pope F, Lunsford W, Mc CC. Age changes in the bones, blood pressure, and diseases of rats in parabiosis. Gerontologia. 1960;4:21–31. https://doi.org/10.1159/000210970.
Lunsford WR, Mc CC, Lupien PJ, Pope FE, Sperling G. Parabiosis as a method for studying factors which affect aging in rats. Gerontologia. 1963;7:1–8. https://doi.org/10.1159/000211170.
McCay CM, Pope F, Lunsford W, Sperling G, Sambhavaphol P. Parabiosis between old and young rats. Gerontologia. 1957;1:7–17. https://doi.org/10.1159/000210677.
Gulej R, Nyul-Toth A, Csik B, Petersen B, Faakye J, Negri S, Chandragiri SS, Mukli P, Yabluchanskiy A, Conley S, et al. Rejuvenation of cerebromicrovascular function in aged mice through heterochronic parabiosis: insights into neurovascular coupling and the impact of young blood factors. Geroscience. 2023. https://doi.org/10.1007/s11357-023-01039-2
Ximerakis M, Holton KM, Giadone RM, Ozek C, Saxena M, Santiago S, Adiconis X, Dionne D, Nguyen L, Shah KM, et al. Heterochronic parabiosis reprograms the mouse brain transcriptome by shifting aging signatures in multiple cell types. Nat Aging. 2023;3:327–45. https://doi.org/10.1038/s43587-023-00373-6.
Chen MB, Yang AC, Yousef H, Lee D, Chen W, Schaum N, Lehallier B, Quake SR, Wyss-Coray T. Brain endothelial cells are exquisite sensors of age-related circulatory cues. Cell Rep. 2020;30:4418-4432.e4414. https://doi.org/10.1016/j.celrep.2020.03.012.
Harris RB. Loss of body fat in lean parabiotic partners of ob/ob mice. Am J Physiol. 1997;272:R1809-1815. https://doi.org/10.1152/ajpregu.1997.272.6.R1809.
Harris RB. Parabiosis between db/db and ob/ob or db/+ mice. Endocrinology. 1999;140:138–45. https://doi.org/10.1210/endo.140.1.6449.
Coleman DL. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia. 1973;9:294–8. https://doi.org/10.1007/bf01221857.
Coleman DL, Hummel KP. Effects of parabiosis of normal with genetically diabetic mice. Am J Physiol. 1969;217:1298–304. https://doi.org/10.1152/ajplegacy.1969.217.5.1298.
Bitto A, Kaeberlein M. Rejuvenation: it’s in our blood. Cell Metab. 2014;20:2–4. https://doi.org/10.1016/j.cmet.2014.06.007.
Cannata A, Marcon G, Cimmino G, Camparini L, Ciucci G, Sinagra G, Loffredo FS. Role of circulating factors in cardiac aging. J Thorac Dis. 2017;9:S17–29. https://doi.org/10.21037/jtd.2017.03.95.
Conboy MJ, Conboy IM, Rando TA. Heterochronic parabiosis: historical perspective and methodological considerations for studies of aging and longevity. Aging Cell. 2013;12:525–30. https://doi.org/10.1111/acel.12065.
Fan X, Wheatley EG, Villeda SA. Mechanisms of hippocampal aging and the potential for rejuvenation. Annu Rev Neurosci. 2017;40:251–72. https://doi.org/10.1146/annurev-neuro-072116-031357.
Flemming A. Cardiovascular disease: rejuvenating the ageing heart. Nat Rev Drug Discov. 2013;12:503. https://doi.org/10.1038/nrd4064.
Ghosh AK, O’Brien M, Mau T, Qi N, Yung R. Adipose tissue senescence and inflammation in aging is reversed by the young milieu. J Gerontol A Biol Sci Med Sci. 2019;74:1709–15. https://doi.org/10.1093/gerona/gly290.
Gontier G, Iyer M, Shea JM, Bieri G, Wheatley EG, Ramalho-Santos M, Villeda SA. Tet2 rescues age-related regenerative decline and enhances cognitive function in the adult mouse brain. Cell Rep. 2018;22:1974–81. https://doi.org/10.1016/j.celrep.2018.02.001.
Harrison DE, Astle CM. Loss of stem cell repopulating ability upon transplantation Effects of donor age, cell number, and transplantation procedure. J Exp Med. 1982;156:1767–79. https://doi.org/10.1084/jem.156.6.1767.
Hirayama R, Takemura K, Nihei Z, Ichikawa W, Takagi Y, Mishima Y, Utsuyama M, Hirokawa K. Differential effect of host microenvironment and systemic humoral factors on the implantation and the growth rate of metastatic tumor in parabiotic mice constructed between young and old mice. Mech Ageing Dev. 1993;71:213–21. https://doi.org/10.1016/0047-6374(93)90085-6.
Katsimpardi L, Kuperwasser N, Camus C, Moigneu C, Chiche A, Tolle V, Li H, Kokovay E, Lledo PM. Systemic GDF11 stimulates the secretion of adiponectin and induces a calorie restriction-like phenotype in aged mice. Aging Cell. 2019:e13038. https://doi.org/10.1111/acel.13038
Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–4. https://doi.org/10.1126/science.1251141.
Rebo J, Mehdipour M, Gathwala R, Causey K, Liu Y, Conboy MJ, Conboy IM. A single heterochronic blood exchange reveals rapid inhibition of multiple tissues by old blood. Nat Commun. 2016;7:13363. https://doi.org/10.1038/ncomms13363.
Sousa-Victor P, Neves J, Cedron-Craft W, Ventura PB, Liao CY, Riley RR, Soifer I, van Bruggen N, Kolumam GA, Villeda SA, et al. MANF regulates metabolic and immune homeostasis in ageing and protects against liver damage. Nat Metab. 2019;1:276–90. https://doi.org/10.1038/s42255-018-0023-6.
Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–4. https://doi.org/10.1038/nature10357.
Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik D, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20:659–63. https://doi.org/10.1038/nm.3569.
Zhang H, Cherian R, Jin K. Systemic milieu and age-related deterioration. Geroscience. 2019;41:275–84. https://doi.org/10.1007/s11357-019-00075-1.
DeCarolis NA, Kirby ED, Wyss-Coray T, Palmer TD. The role of the microenvironmental niche in declining stem-cell functions associated with biological aging. Cold Spring Harb Perspect Med. 2015;5. https://doi.org/10.1101/cshperspect.a025874
Middeldorp J, Lehallier B, Villeda SA, Miedema SS, Evans E, Czirr E, Zhang H, Luo J, Stan T, Mosher KI, et al. Preclinical assessment of young blood plasma for Alzheimer disease. JAMA Neurol. 2016;73:1325–33. https://doi.org/10.1001/jamaneurol.2016.3185.
Smith LK, He Y, Park JS, Bieri G, Snethlage CE, Lin K, Gontier G, Wabl R, Plambeck KE, Udeochu J, et al. beta2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat Med. 2015;21:932–7. https://doi.org/10.1038/nm.3898.
Rodriguez SL, Carver CM, Dosch AJ, Huffman DM, Duke Boynton FD, Ayasoufi K, Schafer MJ. An optimized mouse parabiosis protocol for investigation of aging and rejuvenative mechanisms. Front Aging. 2022;3:993658. https://doi.org/10.3389/fragi.2022.993658.
Yousefzadeh MJ, Robbins PD, Huffman DM. Heterochronic parabiosis: a valuable tool to investigate cellular senescence and other hallmarks of aging. Aging (Albany NY). 2022;14:3325–8. https://doi.org/10.18632/aging.204015.
Morrison EJ, Champagne DP, Dzieciatkowska M, Nemkov T, Zimring JC, Hansen KC, Guan F, Huffman DM, Santambrogio L, D’Alessandro A. Parabiosis incompletely reverses aging-induced metabolic changes and oxidant stress in mouse red blood cells. Nutrients. 2019;11. https://doi.org/10.3390/nu11061337
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: an open-source platform for biological-image analysis. Nature Methods. 2012;9:676–82. https://doi.org/10.1038/nmeth.2019.
Arganda-Carreras I, Kaynig V, Rueden C, Eliceiri KW, Schindelin J, Cardona A, Sebastian Seung H. Trainable Weka Segmentation: a machine learning tool for microscopy pixel classification. Bioinformatics. 2017;33:2424–6. https://doi.org/10.1093/bioinformatics/btx180.
Hussain B, Fang C, Chang J. Blood–brain barrier breakdown: an emerging biomarker of cognitive impairment in normal aging and dementia. Front Neurosci. 2021;15. https://doi.org/10.3389/fnins.2021.688090
Erickson MA, Banks WA. Transcellular routes of blood-brain barrier disruption. Exp Biol Med (Maywood). 2022;247:788–96. https://doi.org/10.1177/15353702221080745.
Schroer AB, Ventura PB, Sucharov J, Misra R, Chui MKK, Bieri G, Horowitz AM, Smith LK, Encabo K, Tenggara I, et al. Platelet factors attenuate inflammation and rescue cognition in ageing. Nature. 2023;620:1071–9. https://doi.org/10.1038/s41586-023-06436-3.
Kumar A. Editorial: neuroinflammation and cognition. Front Aging Neurosci. 2018;10:413. https://doi.org/10.3389/fnagi.2018.00413.
Wang Q, Chen G, Schindler SE, Christensen J, McKay NS, Liu J, Wang S, Sun Z, Hassenstab J, Su Y, et al. Baseline microglial activation correlates with brain amyloidosis and longitudinal cognitive decline in Alzheimer disease. Neurol Neuroimmunol Neuroinflamm. 2022;9:e1152. https://doi.org/10.1212/NXI.0000000000001152.
Reeson P, Choi K, Brown CE. VEGF signaling regulates the fate of obstructed capillaries in mouse cortex. Elife. 2018;7. https://doi.org/10.7554/eLife.33670
Banki E, Sosnowska D, Tucsek Z, Gautam T, Toth P, Tarantini S, Tamas A, Helyes Z, Reglodi D, Sonntag WE, et al. Age-related decline of autocrine pituitary adenylate cyclase-activating polypeptide impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci. 2015;70:665–74. https://doi.org/10.1093/gerona/glu116.
Csiszar A, Sosnowska D, Tucsek Z, Gautam T, Toth P, Losonczy G, Colman RJ, Weindruch R, Anderson RM, Sonntag WE, Ungvari Z. Circulating factors induced by caloric restriction in the nonhuman primate Macaca mulatta activate angiogenic processes in endothelial cells. J Gerontol A Biol Sci Med Sci. 2013;68:235–49. https://doi.org/10.1093/gerona/gls158.
Liu F, Austin TR, Schrack JA, Chen J, Walston J, Mathias RA, Grams M, Odden MC, Newman A, Psaty BM, et al. Late-life plasma proteins associated with prevalent and incident frailty: a proteomic analysis. Aging Cell. 2023;22:e13975. https://doi.org/10.1111/acel.13975.
Benndorf RA, Schwedhelm E, Gnann A, Taheri R, Kom G, Didie M, Steenpass A, Ergun S, Boger RH. Isoprostanes inhibit vascular endothelial growth factor-induced endothelial cell migration, tube formation, and cardiac vessel sprouting in vitro, as well as angiogenesis in vivo via activation of the thromboxane A(2) receptor: a potential link between oxidative stress and impaired angiogenesis. Circ Res. 2008;103:1037–46. https://doi.org/10.1161/CIRCRESAHA.108.184036.
Kiss T, Balasubramanian P, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Csipo T, Lipecz A, Reglodi D, Zhang XA, Bari F, et al. Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for the prevention of vascular cognitive impairment. Geroscience. 2019;41:619–30. https://doi.org/10.1007/s11357-019-00074-2.
Ungvari Z, Tucsek Z, Sosnowska D, Toth P, Gautam T, Podlutsky A, Csiszar A, Losonczy G, Valcarcel-Ares MN, Sonntag WE. Aging-induced dysregulation of Dicer1-dependent microRNA expression impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci. 2013;68:877–91. https://doi.org/10.1093/gerona/gls242.
Valcarcel-Ares MN, Gautam T, Warrington JP, Bailey-Downs L, Sosnowska D, de Cabo R, Losonczy G, Sonntag WE, Ungvari Z, Csiszar A. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:821–9. https://doi.org/10.1093/gerona/glr229.
Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab. 2007;27:1908–18. https://doi.org/10.1038/sj.jcbfm.9600491.
Csiszar A, Tarantini S, Yabluchanskiy A, Balasubramanian P, Kiss T, Farkas E, Baur JA, Ungvari ZI. Role of endothelial NAD+ deficiency in age-related vascular dysfunction. Am J Physiol Heart Circ Physiol. 2019. https://doi.org/10.1152/ajpheart.00039.2019.
Kiss T, Nyul-Toth A, Balasubramanian P, Tarantini S, Ahire C, Yabluchanskiy A, Csipo T, Farkas E, Wren JD, Garman L, et al. Nicotinamide mononucleotide (NMN) supplementation promotes neurovascular rejuvenation in aged mice: transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects. Geroscience. 2020. https://doi.org/10.1007/s11357-020-00165-5
Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009. https://doi.org/10.1016/j.mad.2009.06.004.
Tarantini S, Nyul-Toth A, Yabluchanskiy A, Csipo T, Mukli P, Balasubramanian P, Ungvari A, Toth P, Benyo Z, Sonntag WE, et al. Endothelial deficiency of insulin-like growth factor-1 receptor (IGF1R) impairs neurovascular coupling responses in mice, mimicking aspects of the brain aging phenotype. Geroscience. 2021https://doi.org/10.1007/s11357-021-00405-2
Farias Quipildor GE, Mao K, Hu Z, Novaj A, Cui MH, Gulinello M, Branch CA, Gubbi S, Patel K, Moellering DR, et al. Central IGF-1 protects against features of cognitive and sensorimotor decline with aging in male mice. Geroscience. 2019;41:185–208. https://doi.org/10.1007/s11357-019-00065-3.
Sonntag WE, Deak F, Ashpole N, Toth P, Csiszar A, Freeman W, Ungvari Z. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front Aging Neurosci. 2013;5:27. https://doi.org/10.3389/fnagi.2013.00027.
Gocmez SS, Yazir Y, Gacar G, Demirtas Sahin T, Arkan S, Karson A, Utkan T. Etanercept improves aging-induced cognitive deficits by reducing inflammation and vascular dysfunction in rats. Physiol Behav. 2020;224:113019. https://doi.org/10.1016/j.physbeh.2020.113019.
Heringa SM, van den Berg E, Reijmer YD, Nijpels G, Stehouwer CD, Schalkwijk CG, Teerlink T, Scheffer PG, van den Hurk K, Kappelle LJ, et al. Markers of low-grade inflammation and endothelial dysfunction are related to reduced information processing speed and executive functioning in an older population - the Hoorn Study. Psychoneuroendocrinology. 2014;40:108–18. https://doi.org/10.1016/j.psyneuen.2013.11.011.
Li Y, Kracun D, Dustin CM, El Massry M, Yuan S, Goossen CJ, DeVallance ER, Sahoo S, St Hilaire C, Gurkar AU, et al. Forestalling age-impaired angiogenesis and blood flow by targeting NOX: Interplay of NOX1, IL-6, and SASP in propagating cell senescence. Proc Natl Acad Sci USA. 2021;118. https://doi.org/10.1073/pnas.2015666118
Franco CA, Mericskay M, Parlakian A, Gary-Bobo G, Gao-Li J, Paulin D, Gustafsson E, Li Z. Serum response factor is required for sprouting angiogenesis and vascular integrity. Dev Cell. 2008;15:448–61. https://doi.org/10.1016/j.devcel.2008.07.019.
Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009;118:103–13. https://doi.org/10.1007/s00401-009-0522-3.
Bolte C, Ren X, Tomley T, Ustiyan V, Pradhan A, Hoggatt A, Kalin TV, Herring BP, Kalinichenko VV. Forkhead box F2 regulation of platelet-derived growth factor and myocardin/serum response factor signaling is essential for intestinal development. J Biol Chem. 2015;290:7563–75. https://doi.org/10.1074/jbc.M114.609487.
Chai J, Jones MK, Tarnawski AS. Serum response factor is a critical requirement for VEGF signaling in endothelial cells and VEGF-induced angiogenesis. FASEB J. 2004;18:1264–6. https://doi.org/10.1096/fj.03-1232fje.
Chai J, Tarnawski AS. Serum response factor: discovery, biochemistry, biological roles and implications for tissue injury healing. J Physiol Pharmacol. 2002;53:147–57.
Chen J, Yuan K, Mao X, Miano JM, Wu H, Chen Y. Serum response factor regulates bone formation via IGF-1 and Runx2 signals. J Bone Miner Res. 2012;27:1659–68. https://doi.org/10.1002/jbmr.1607.
Chow N, Bell RD, Deane R, Streb JW, Chen J, Brooks A, Van Nostrand W, Miano JM, Zlokovic BV. Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer’s phenotype. Proc Natl Acad Sci USA. 2007;104:823–8. https://doi.org/10.1073/pnas.0608251104.
Galmiche G, Labat C, Mericskay M, Aissa KA, Blanc J, Retailleau K, Bourhim M, Coletti D, Loufrani L, Gao-Li J, et al. Inactivation of serum response factor contributes to decrease vascular muscular tone and arterial stiffness in mice. Circ Res. 2013;112:1035–45. https://doi.org/10.1161/CIRCRESAHA.113.301076.
Lahoute C, Sotiropoulos A, Favier M, Guillet-Deniau I, Charvet C, Ferry A, Butler-Browne G, Metzger D, Tuil D, Daegelen D. Premature aging in skeletal muscle lacking serum response factor. PLoS One. 2008;3:e3910. https://doi.org/10.1371/journal.pone.0003910.
Werth D, Grassi G, Konjer N, Dapas B, Farra R, Giansante C, Kandolf R, Guarnieri G, Nordheim A, Heidenreich O. Proliferation of human primary vascular smooth muscle cells depends on serum response factor. Eur J Cell Biol. 2010;89:216–24. https://doi.org/10.1016/j.ejcb.2009.12.002.
Han X, Aenlle KK, Bean LA, Rani A, Semple-Rowland SL, Kumar A, Foster TC. Role of estrogen receptor alpha and beta in preserving hippocampal function during aging. J Neurosci. 2013;33:2671–83. https://doi.org/10.1523/JNEUROSCI.4937-12.2013.
Sandoval KE, Witt KA. Age and 17beta-estradiol effects on blood-brain barrier tight junction and estrogen receptor proteins in ovariectomized rats. Microvasc Res. 2011;81:198–205. https://doi.org/10.1016/j.mvr.2010.12.007.
Hillmer L, Erhardt EB, Caprihan A, Adair JC, Knoefel JE, Prestopnik J, Thompson J, Hobson S, Rosenberg GA. Blood-brain barrier disruption measured by albumin index correlates with inflammatory fluid biomarkers. J Cereb Blood Flow Metab. 2023;43:712–21. https://doi.org/10.1177/0271678X221146127.
Liberale L, Bonetti NR, Puspitasari YM, Vukolic A, Akhmedov A, Diaz-Canestro C, Keller S, Montecucco F, Merlini M, Semerano A, et al. TNF-alpha antagonism rescues the effect of ageing on stroke: perspectives for targeting inflamm-ageing. Eur J Clin Invest. 2021;51:e13600. https://doi.org/10.1111/eci.13600.
Rather HA, Almousa S, Craft S, Deep G. Therapeutic efficacy and promise of stem cell-derived extracellular vesicles in Alzheimer’s disease and other aging-related disorders. Ageing Res Rev. 2023;92:102088. https://doi.org/10.1016/j.arr.2023.102088.
Ozansoy M, Mikati H, Velioglu HA, Yulug B. Exosomes: a missing link between chronic systemic inflammation and Alzheimer’s disease? Biomed Pharmacother. 2023;159:114161. https://doi.org/10.1016/j.biopha.2022.114161.
Ozek C, Krolewski RC, Buchanan SM, Rubin LL. Growth differentiation factor 11 treatment leads to neuronal and vascular improvements in the hippocampus of aged mice. Sci Rep. 2018;8. https://doi.org/10.1038/s41598-018-35716-6
Kim T-W, Park S-S, Park J-Y, Park H-S. Infusion of plasma from exercised mice ameliorates cognitive dysfunction by increasing hippocampal neuroplasticity and mitochondrial functions in 3xTg-AD mice. Int J Mol Sci. 2020;21:3291. https://doi.org/10.3390/ijms21093291.
Horowitz AM, Fan X, Bieri G, Smith LK, Sanchez-Diaz CI, Schroer AB, Gontier G, Casaletto KB, Kramer JH, Williams KE, Villeda SA. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science. 2020;369:167–73. https://doi.org/10.1126/science.aaw2622.
Yousef H, Conboy MJ, Morgenthaler A, Schlesinger C, Bugaj L, Paliwal P, Greer C, Conboy IM, Schaffer D. Systemic attenuation of the TGF-beta pathway by a single drug simultaneously rejuvenates hippocampal neurogenesis and myogenesis in the same old mammal. Oncotarget. 2015; 6:11959-11978 https://doi.org/10.18632/oncotarget.3851
Heine VM, Maslam S, Joëls M, Lucassen PJ. Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus–pituitary–adrenal axis activation. Neurobiol Aging. 2004;25:361–75. https://doi.org/10.1016/S0197-4580(03)00090-3.
Mehdipour M, Skinner C, Wong N, Lieb M, Liu C, Etienne J, Kato C, Kiprov D, Conboy MJ, Conboy IM. Rejuvenation of three germ layers tissues by exchanging old blood plasma with saline-albumin. Aging. 2020;12:8790–819. https://doi.org/10.18632/aging.103418.
Mehdipour M, Mehdipour T, Skinner CM, Wong N, Liu C, Chen C-C, Jeon OH, Zuo Y, Conboy MJ, Conboy IM. Plasma dilution improves cognition and attenuates neuroinflammation in old mice. GeroScience. 2021;43:1–18. https://doi.org/10.1007/s11357-020-00297-8.
Acknowledgements
We sincerely thank the Division of Comparative Medicine team at the University of Oklahoma Health Sciences Center for their invaluable support in supervising animal care and sharing their extensive expertise. Special recognition is extended to Dr. Shawn Lane, DVM, for his invaluable guidance and expertise in surgical and postsurgical care. We are grateful to Dr. Wendy Williams, DVM, for her instrumental role in designing appropriate pre- and postsurgical treatments. We also acknowledge Ms. Carlye Yancey, BS, for her exceptional animal husbandry knowledge and contributions to parabiosis housing. Furthermore, we wish to express our gratitude to Mr. Chad Cunningham, Electronic & Instrument Shop Supervisor Building Manager of the University of Oklahoma’s Department of Physics and Engineering, for his essential assistance in fabricating the parabiont-adjusted components of the stereotactic frame, which was instrumental in facilitating simultaneous measurements of neurovascular coupling in parabionts. The 4.0 version of ChatGPT, developed by OpenAI, was used as a language tool to refine our writing, enhancing the clarity of our work.
Funding
This work was supported by grants from the American Heart Association (RG: 916225, ANT: AHA834339, and ST: AHA CDA941290), the Oklahoma Center for the Advancement of Science and Technology, the National Institute on Aging (RF1AG072295, R01AG055395, R01AG068295; R01AG070915, K01AG073614, K01AG073613, R03AG070479), the National Institute of Neurological Disorders and Stroke (R01NS100782), the National Cancer Institute (R01CA255840), the Oklahoma Shared Clinical and Translational Resources (U54GM104938) with an Institutional Development Award (IDeA) from NIGMS, the Presbyterian Health Foundation, the Reynolds Foundation, the Oklahoma Nathan Shock Center (P30AG050911), the Cellular and Molecular GeroScience CoBRE (P20GM125528), the NCI Cancer Center Support Grant (P30 CA225520), and the Oklahoma Tobacco Settlement Endowment Trust. DMH is supported by P30AG038072. ANT was supported by TKP2021-NKTA-47, implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-NKTA funding scheme, and by funding through the National Cardiovascular Laboratory Program (RRF-2.3.1-21-2022-00003) provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund; Project no. 135784 implemented with the support provided from the National Research, Development and Innovation Fund of Hungary, financed under the K20 funding scheme and the European University for Well-Being (EUniWell) program (grant agreement number: 101004093/ EUniWell/EAC-A02-2019 / EAC-A02-2019-1). The funding sources had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
The study’s conception, design, and data interpretation involved contributions from all authors. Parabiosis surgeries were executed by RG and BC, while the post-surgical monitoring of animals was carried out by RG, BC, BP, JF, and SS. The assessment of blood-brain barrier permeability and microvascular density and subsequent data analysis were undertaken by RG, ANT, SN, and RP The initial draft of the manuscript was jointly composed by RG, ANT, ST, and ZU. Subsequent revisions to the manuscript were conducted by all authors, who also collectively reviewed and provided their approval for the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Dr. Anna Csiszar serves as Associate Editor for The Journal of Gerontology, Series A: Biological Sciences and Medical Sciences and GeroScience. Dr. Zoltan Ungvari serves as Editor-in-Chief for GeroScience. Dr. Stefano Tarantini, Dr. Adam Nyul-Toth, Dr. Shannon Conley, Dr. Peter Mukli, Dr. Derek M. Huffman, and Dr. Andriy Yabluchanskiy serve as Associate Editors for GeroScience.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the American Heart Association, or the Presbyterian Health Foundation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Gulej, R., Nyúl-Tóth, Á., Csik, B. et al. Young blood-mediated cerebromicrovascular rejuvenation through heterochronic parabiosis: enhancing blood-brain barrier integrity and capillarization in the aged mouse brain. GeroScience (2024). https://doi.org/10.1007/s11357-024-01154-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11357-024-01154-8