Abstract
The long-term consequences of the coronavirus disease 19 (COVID-19) are likely to be frequent but results hitherto are inconclusive. Therefore, we aimed to define the incidence of long-term COVID signs and symptoms as defined by the World Health Organization, using a systematic review and meta-analysis of observational studies. A systematic search in several databases was carried out up to 12 January 2022 for observational studies reporting the cumulative incidence of long COVID signs and symptoms divided according to body systems affected. Data are reported as incidence and 95% confidence intervals (CIs). Several sensitivity and meta-regression analyses were performed. Among 11,162 papers initially screened, 196 were included, consisting of 120,970 participants (mean age: 52.3 years; 48.8% females) who were followed-up for a median of six months. The incidence of any long COVID symptomatology was 56.9% (95% CI 52.2–61.6). General long COVID signs and symptoms were the most frequent (incidence of 31%) and digestive issues the least frequent (7.7%). The presence of any neurological, general and cardiovascular long COVID symptomatology was most frequent in females. Higher mean age was associated with higher incidence of psychiatric, respiratory, general, digestive and skin conditions. The incidence of long COVID symptomatology was different according to continent and follow-up length. Long COVID is a common condition in patients who have been infected with SARS-CoV-2, regardless of the severity of the acute illness, indicating the need for more cohort studies on this topic.
Similar content being viewed by others
Introduction
Since the beginning of the COVID-19 pandemic on 8 March 2020, more than 500 million cases of SARS-CoV-2 infection have been reported worldwide with a daily global increase of approximately 500,000 cases per day [1]. While global health strategies, vaccines, antivirals and new monoclonal antibodies have significantly reduced COVID-19 mortality and severe illness, long consequences after the acute phase of the disease remain an unresolved issue.
During the first pandemic wave, several articles highlighted the possible medium-to long-term devasting consequences of SARS-CoV2 infection, for patients and healthcare systems [2, 3]. Article conclusions were based on follow-up studies of people who had coronavirus infections including SARS-CoV-1 in 2003 and MERS-CoV in 2012 [4, 5] and who, after months and years of follow-up, still had symptoms and signs linked to previous infection. There is an increasing body of global literature reporting the long-term sequelae of patients with previous SARS-CoV-2 infection [2, 3]. Reported symptoms vary and include, for example, dyspnea, hair loss, anxiety, depression, asthenia, fatigue and loss of appetite [2, 3].
Furthermore, the terminology relating to long COVID in the literature is not standardized. Researchers have used different terms to describe the prolonged symptoms following COVID-19 disease, for example: Long COVID, Long-haulers, Post-acute COVID-19 syndrome, and Chronic COVID-19. Moreover, different time cut-offs have been used (from 2–3 weeks to months after COVID-19) [4]. In October 2021, the World Health Organization (WHO) defined the long COVID as “a condition that occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19 with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis” [5]. The real number of people living with long COVID is unknown, as well as the real incidence and which organs or systems are most frequently involved. Knowing the real incidence of long COVID is critical for addressing the problem and examining possible therapeutic approaches, preventative efforts, and global health policy. The definition and inclusion criteria of previous studies on long/post COVID conditions may have masked the true burden. However, to explain the real incidence without the confounding influence of different follow-up lengths, we used the WHO definition. Importantly, our study is the first to include exclusively papers using the WHO proposed period to define long COVID [6,7,8,9].
Given this background, we carried out a systematic review and meta-analysis regarding the incidence of signs and symptoms typical of long COVID, with a minimum follow-up time longer than at least 3 months and according to the WHO definition.
Materials and methods
Protocol registration
This study was conducted following the recommendations in the Cochrane handbook for systematic literature reviews to conduct the screening and selection of studies [10]. The original protocol was registered in https://osf.io/5b2tv.
This systematic review and meta-analysis was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, updated version to 2021 [11].
Research question
The research question for this systematic review is as follows: “What is the incidence of long COVID signs and symptoms?” To guide the identification of adequate keywords to build search strategies to search bibliographic databases, the research question was framed into the PICO(S) (Participants, Intervention, Comparison, Outcome, Study design) format: (P) laboratory confirmed and/or clinically diagnosed COVID-19: long COVID was defined as the presence of signs and/or symptoms after three months and lasting at least two months and that cannot be explained by other medical conditions, in agreement with the indications of the WHO [5]; (I): none; (C) none; (O) incidence of signs and symptoms of long COVID; (S) observational studies.
Information sources and search strategies
We searched Medline (via Ovid) and Web of Science from database inception to 12 January 2022, through OVID. The search for individual studies in these bibliographic databases was supplemented by a manual search of references included in relevant systematic reviews already published regarding this topic.
Considering the main PICOS elements, we built the following search strategy for Medline: “(“COVID-19” OR “Novel Coronavirus–Infected Pneumonia” OR “2019 novel coronavirus” OR “2019-nCoV” OR “SARS-CoV-2”) AND (“lingering symptoms” OR “persistent symptoms” OR “long-term symptoms” OR "long-term Covid" OR “long-term” OR “long term” OR “long”)”. Then we adapted the search strategy for Web of Science.
The management of potentially eligible references was carried out using the Rayyan website (https://www.rayyan.ai/).
Eligibility criteria
Inclusion criteria comprised the following: (1) observational studies (case–control, cohort, longitudinal studies); (2) studies that investigated the diagnosis of long COVID according to the criteria mentioned previously; (3) presence of long COVID for at least 12 weeks [5]. Only articles written in English were included.
Studies with a follow-up shorter than 12 weeks or with an unclear follow-up, case series and case reports were excluded.
Study selection
We followed the recommendations reported in the Cochrane handbook for Systematic reviews to select studies that were finally included in this review [10]. The selection of the articles was performed independently by six authors (OT, AB, LD, DFB, RB, VG), in couples. The agreement within the couples, evaluated with the K was 0.85 in couple 1, 0.81 in couple 2 and 0.86 in couple 3. Consensus meetings were held with all reviewers to discuss the studies for which divergent selection decisions were made. Two additional senior members (NV, FDG) of the review team were involved, when necessary. The studies selection process involved, first, a selection based on title and/or abstracts, then a selection of studies retrieved from this first step based on the full-text manuscripts.
Data collection and data items
We collected the following information: data regarding the identification of the manuscript (e.g., first author name and affiliation, year of publication, journal name, title of the manuscript), data on the characteristics of the population considered (e.g., sample size, mean age, location, gender, etc.), setting (e.g., hospital, intensive care unit, etc.), method of follow-up visit, follow-up in months, type of diagnosis of COVID-19 and signs and symptoms recorded during the follow-up period. These data were collected using a standard data extraction form. The data extraction was carried out independently by the six authors, in couples, with one author for each couple extracting the data and the other checking, with the senior authors checking the quality of the data extraction.
Risk of bias evaluation
The Newcastle–Ottawa Scale (NOS) was used to assess the study quality/risk of bias [12]. The NOS assigns a maximum of 9 points based on following three quality parameters: selection, comparability, and outcome. The evaluation was made by one author and checked by another, independently. The risk of bias was then categorized as high (< 5/9 points), moderate (6–7) or low (8–9) [13]. The investigators solved any discrepancies by jointly re-assessing an article (NV, AB and FDG).
Data synthesis
Signs and symptoms were grouped into anatomical clusters, i.e., neurological, dermatological, and psychiatric conditions. The cumulative incidence of symptoms and 95% confidence intervals (CIs) were estimated using a meta-analysis, under a random-effect model [14]. Heterogeneity between estimates was assessed using the I2 statistic. In case of an I2 over 50% a series of meta-regression analyses (taking as moderators if the participants were hospitalized, the percentage of females, and the mean age of the sample size) was conducted. Several sensitivity analyses (continent, mean age, using the WHO classification in children, adults, older people [15], follow-up period, stayed in intensive care unit, hospitalized, type of follow-up, and risk of bias) were also conducted [16]. Moderators and strata were chosen based on clinical judgment. Publication bias was assessed by visually inspecting funnel plots and using Egger bias test, with a p-value < 0.05 indicative of possible publication bias [17].
All analyses were performed using “metaprop”, a command available in STATA 14.0
Results
Search results
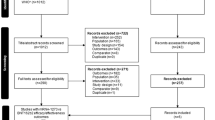
As shown in Supplementary Fig. 1, among 11,167 records initially screened, 346 full-texts were retrieved, with a final selection of 196 articles (see the list in Supplementary Table 1).
Descriptive characteristics
As shown in Supplementary Table 2, the 196 studies included 120,970 participants (median per study: 190 participants, range 17–31,013) with a mean age of 52.3 years. The participants were more frequently males (percentage of females = 48.8%) (p < 0.0001, Chi Square test). The majority of the studies took place in Europe (n = 126, 64.3%) and used the polymerase chain reaction for the identification of SARS-CoV-2 (n = 185, 94.4%). Furthermore, most studies considered only hospitalized patients (n = 128, 65.3%) including people admitted to intensive care unit (n = 101, 51.5%). Follow-up with a median of six months (range 3–12 months) was predominantly conducted via outpatients’ visits (n = 86, 43.9%). Among the 196 articles included, only two reported data on the vaccination status against SARS-CoV-2.
Risk of bias
As reported in Supplementary Table 2, the risk of bias, evaluated with the NOS, was overall low in 129 (65.8) studies and moderate for the other works included. No study was at high risk of bias evaluated as a NOS score less than 5.
Incidence of long COVID signs and symptoms
Figure 1 and Table 1 show the incidence of long COVID signs and symptoms. In the 196 studies included, comprising 120,970 people, the cumulative incidence of any long COVID symptomatology was 56.9% (95% CI 52.2–61.6).
By grouping into anatomical clusters, we observed that in 156 cohorts (106,284 participants), the overall incidence of neurological signs/symptoms was 19.7% (95% CI 17.4–22.1). In this cluster the most frequent sign/symptom was difficulty in concentrating (14.6%), and the least frequent was seizures (0.6%). The incidence of headache, taste and smell disorders, cognitive impairment, memory deficits, dizziness, and cramps were over 10%. Psychiatric conditions affected 20.3% of the participants (95% CI 17.4–23.3), in 117 cohorts and for a total of 65,156 people. All the four signs and symptoms considered in this cluster (post-traumatic stress disorder [PTSD], depression, sleep disorder, anxiety) had an incidence over 10%.
Respiratory conditions affected approximately one quarter of the participants with long COVID (154 cohorts, 101,849 participants, 24.5%; 95% CI 21.3–27.9). Among the respiratory signs or symptoms, the most frequent was dyspnea (142 cohorts, 97,065 participants, incidence of 24.1%). Mobility impairment disorders affected 13.7% (10.6–17.2) of the 19,747 participants included in 34 different cohorts, with a decreased exercise tolerance (incidence of 16.6%), being the most frequent. Heart conditions were also particularly frequent, affecting 11.0% of the participants. Palpitations were identified in 11.2% of the 32,784 participants considered. Among the clusters considered, digestive (incidence: 7.7%; 95% CI 6.4–9.1) and skin disorders (incidence: 8.5%, 95% CI 6.8–10.3) were the least represented.
Finally, general signs and symptoms, i.e., not includible in any of the clusters cited before, affected approximately one-third of the 113,802 people included in 166 cohorts. Of particular interest, fatigue affected 31.4% (95% CI 27.1–35.8) of the people included, being the most common symptom in the general cluster.
Meta-regression analyses
Considering the incidence of signs and symptoms clusters, all were affected by a high heterogeneity (I2 = 99%). Therefore, we tried to explain the heterogeneity observed using a series of meta-regression and sensitivity analyses.
Supplementary Table 3 shows the meta-regression analyses. Higher percentage of females moderated the onset of any, neurological, general, and cardiovascular long COVID symptomatology. Each increase in one percent of females in the sample size was associated with a small increase in any long COVID symptomatology (beta = 0.02 ± 0.01; p = 0.047), neurological (beta = 0.003 ± 0.0009; p = 0.001), general (beta = 0.02 ± 0.01; p = 0.05), and cardiovascular (beta = 0.003 ± 0.0009; p = 0.001) signs and symptoms. However, this moderator explained only a small proportion of the heterogeneity of the various outcomes (less than 10%, except for cardiovascular outcomes) (Supplementary Table 3).
Finally, higher mean age of the cohorts included was associated with higher incidence of psychiatric (beta = 0.003 ± 0.001; p = 0.007), respiratory (beta = 0.004 ± 0.001; p = 0.009), general (beta = 0.004 ± 0.002; p = 0.03), digestive (beta = 0.002 ± 0.0009; p = 0.04) and skin conditions (beta = 0.002 ± 0.0009; p = 0.02) (Supplementary Table 3). Again, except for the last outcome, higher mean age explained only a small proportion of the heterogeneity found in the various outcomes.
Sensitivity analyses
Supplementary Table 4 shows the cumulative incidence stratified by some potential factors, i.e., continent, mean age and follow-up. Overall, the incidence of any long COVID was significantly higher in studies carried out in Oceania (63.4%) vs. Europe (48.5%) (p for the interaction < 0.0001), whilst no significant differences were observed by mean age or by follow-up. When considering neurological conditions, the incidence was, again, significantly higher in Oceania and in Europe compared to North America (with an incidence almost doubled). Moreover, the incidence of neurological conditions was significantly higher in adults than in children (p for interaction = 0.03) and in studies having a follow-up of 3 months compared to those with a longer follow-up (Supplementary Table 4). Similarly, psychiatric conditions affected more frequently African participants than Asians (p for the interaction < 0.0001) and participants older than 60 years, with an incidence approximately four times higher than children/youth. Similarly, respiratory conditions were more frequent in Europe than in the other continents and in the studies with a follow-up of 3 months. Another point of importance is that the incidence of mobility issues was significantly higher in adults than the other ages considered and in studies having a follow-up over six months. Finally, general and cardiovascular symptomatology was higher in studies carried out in Africa than in other continents and in adults (Supplementary Table 4).
Finally, Supplementary Table 5 reports the data stratified by ICU admission, hospitalization status, type of follow-up and presence of risk of bias. Overall, patients previously admitted in ICU reported a significantly lower incidence of neurological conditions and mobility issues than their counterparts. Similarly, patients not hospitalized reported a significantly higher presence of neurological and psychiatric conditions. When considering the type of follow-up method used for evaluating long COVID symptomatology patients interviewed in person usually reported lower incidence of several long COVID signs and symptoms. Finally, considering the presence of risk of bias, we observed a significantly higher incidence of neurological, psychiatric, respiratory, cardiovascular, digestive, skin conditions and mobility issues in studies having a moderate risk of bias compared to low.
Discussion
According to the WHO definition for long COVID, we carried out a systematic review of all the studies reporting data on long COVID symptomatology including 196 studies for a total of 120,970 patients with a previous SARS-CoV-2 infection. A key finding of this study was that more than half of the patients previously having COVID-19 had some form of long COVID symptomology, further strengthening the importance of this emergent condition.
Comparing our results with those reported in three previously published systematic reviews with meta-analyses [3, 18, 19], we observed that the incidence of any sign or symptom of long COVID remained high when only including studies having a follow-up of at least 3 months according to the new WHO definition [5]. Respiratory symptomatology, such as dyspnea, and general signs and symptoms, such as fatigue, may affect between one quarter and one-third of all long CVOID patients. Moreover, different inclusion/exclusion criteria indicated that long COVID is a long-term condition that will likely be experienced over coming years and with current limited therapeutical options [20].
These findings support the idea that COVID-19 could lead to persistence of symptoms even after the end of acute infection, as has already been demonstrated for SARS-CoV-1 and MERS-CoV. In 2003, after the end of the outbreak of SARS-CoV-1, Herridge et al. evaluated the respiratory function of 109 survivors at 3, 6 and 12 months after discharge, reporting a relevant reduction in respiratory function and quality of life [21]. Most patients had also extrapulmonary conditions, with muscle wasting and fatigue being the most frequent, similar to long COVID [21]. In addition, Ahmed et al. conducted a systematic review and meta-analysis investigating persistent symptoms of both SARS-CoV-1 and MERS-COV, demonstrating that up to 6 months after discharge impaired respiratory function was present in 27% of patients, PTSD in 39%, depression in 33%, and anxiety in 30%. Moreover, a reduction in exercise capacity was noted with a mean 6-min walking distance of 461 m in the cohort of patients analysed [22]. It is important to remark that some studies demonstrated the persistence of symptoms for several years from SARS-CoV-1 infection. In particular, Ngai et al. performed a respiratory function-test 2 years after discharge on 55 SARS-CoV-1 infected patients, showing a significant impairment of diffusing capacity of the lungs for carbon monoxide (DLCO), exercise capacity and health status, with a more marked adverse impact among health care workers [23]. Moldofsky et al., evaluated the neuropsychiatric disorders that occurred in SARS-CoV-1-infected patients, demonstrating that chronic fatigue, pain, weakness, depression, and sleep disturbance, were still present over a 20-month follow-up [24]. This evidence suggests that for COVID-19 we should expect similar long-term consequences.
Another result of importance of our systematic review and meta-analysis was that long COVID signs and symptoms, and particularly general, neurological and cardiovascular symptoms, were more frequent in females than in males supporting other literature which found that females appear to be at higher risk of long COVID than males, even though females are less represented in the present systematic review [25]. Moreover, higher mean age also represents an important risk factor to develop long COVID symptoms, particularly general, psychiatric, respiratory, digestive and skin issues indicating that long COVID could be of epidemiological importance in older people. Sudre et al. in a cohort of 558 patients described a greater risk for people aged over 70 years of developing ongoing symptoms. Indeed, 22% of people aged over 70 reported symptoms lasting 4 weeks or more, compared to 10% of patients aged 18 to 49 years [26]. Notably, in our systematic review, there was not an increased risk of long COVID for patients who had been hospitalized or had stayed in intensive care units, contrary to what is reported by Jovanoski et al., who described an increased risk of respiratory, cardiovascular, and mental health outcomes up to 6 months after discharge in patients hospitalized with severe/critical COVID-19 [27]. Overall, these findings indicate that people living in the community and not hospitalized can have a similar incidence of long COVID symptomatology, demonstrating the importance of follow-up among these patients.
Furthermore, the incidence of any and general signs and symptoms was significantly higher in Oceania, whilst respiratory symptoms were more commonly reported in Europe and Africa. North America reported the lowest incidence among all categories of symptoms. Even if a definitive conclusion cannot be drawn, we can hypothesize that genetic and environmental factors can justify these different incidences. We can also report that this difference is partially ascribable to the process of symptoms’ definition and perception, and data collection across countries that could greatly vary. However, future studies are needed to better understand these significant differences. Among all the results reported in the sensitivity analyses, we would like to underline the importance of mobility issues that were more frequent in adults than in the other ages. Since mobility issues are often a precursor to disability, our meta-analysis further indicates the need to approach long COVID with non-pharmacological approaches, such as promoting physical activity [28]. When stratifying patients for mean age, it is interesting that children and adolescents presented long COVID symptoms, particularly respiratory and general symptoms: taken together, these significant findings encourage follow-up of children previously affected by COVID-19 for better understanding of the long-term sequalae of this condition.
In the opinion of the present authors, long COVID represents a major public-health problem, both because of its incidence in patients with SARS-CoV-2 infection and because of the lack of effective therapeutic strategies to date [20]. Published literature regarding the possible treatment is still limited, and studies published until now were limited by lack of homogeneity owing to varying study designs, settings, populations, follow-up period and symptoms description. Potentially, mass vaccination and the use of new therapies aimed at rapidly reducing viral load and limiting disease progression could play a crucial role in preventing long COVID and long-term symptoms persistence, but future studies are urgently needed. In addition to characteristics of patients, the SARS-CoV-2 variant of concern involved in acute infection is often missing, but it may also play an important role in the type of symptoms that occur in long COVID.
The results of our systematic review with meta-analysis must be interpreted within its limitations. First, some long COVID symptoms may be missing because they were not identified and not investigated in patient questionnaires. This limitation determines the need to standardize questionnaires and to better define some symptoms as follows: for example, the symptom “fatigue” may be exaggerated by some patients or underestimated by others. The use of objective and precise scales, such as the Visual Analogue Scale for pain or the Fatigue Assessment Scale for fatigue would facilitate harmonization of symptom descriptions. The studies included in this meta-analysis often used only self-reported information or physical examination. Second, all the outcomes were characterized by a high heterogeneity, only partly explained by our meta-regression or sensitivity analyses. These findings suggest that other factors are probably important in determining a higher or lower incidence of long COVID. Unfortunately, we were not able to explore the role of vaccinations on the incidence of long COVID: further studies are urgently needed in this sense. Another important problem is the presence of publication bias in our findings, likely owing to the choice to screen papers written only in English and the fact that only two databases were screened [29]. Finally, the maximum follow-up reported by the studies included in our systematic review was only one year. Future studies are needed regarding long-term consequences of COVID-19.
In conclusion, our systematic review and meta-analysis indicates that long COVID is a common condition in patients who have been infected with SARS-CoV-2 and often regardless of the severity of the acute illness. Therefore, more long-term studies are needed to understand the real long-term impact on quality of life, but also to develop optimal therapeutic and long COVID prevention strategies.
Data availability
The database is available upon reasonable request to the corresponding author.
References
World Health Organization (2022) Weekly operational update on COVID-19; Issue No. 97. https://www.who.int/publications/m/item/weekly-operational-update-on-covid-19---30-march-2022#.YkwrvaHCh8g.link. Accessed 03/30/2022
Sanchez-Ramirez DC, Normand K, Zhaoyun Y, Torres-Castro R (2021) Long-term impact of COVID-19: a systematic review of the literature and meta-analysis. Biomedicines 9:900
Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, Villapol S (2021) More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep 11:1–12
Baig AM (2020) Chronic COVID syndrome: need for an appropriate medical terminology for long-COVID and COVID long-haulers. J Med Virol. https://doi.org/10.1002/jmv.26624
World Health Organization (2021) A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021. World Health Organization
Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B (2022) Global prevalence of post COVID-19 condition or long COVID: a meta-analysis and systematic review. J Infect Dis. https://doi.org/10.1093/infdis/jiac136
Stavem K, Ghanima W, Olsen MK, Gilboe HM, Einvik G (2021) Prevalence and determinants of fatigue after COVID-19 in non-hospitalized subjects: a population-based study. Int J Environ Res Public Health 18:2030
Natarajan A, Shetty A, Delanerolle G, Zeng Y, Zhang Y, Raymont V, Rathod S, Halabi S, Elliot K, Phiri P (2022) A systematic review and meta-analysis of long COVID symptoms. medRxiv. https://doi.org/10.1101/2022.03.08.22272091
Han Q, Zheng B, Daines L, Sheikh A (2022) Long-term sequelae of COVID-19: a systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens 11:269
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (2019) Cochrane handbook for systematic reviews of interventions. John Wiley & Sons
Sarkis-Onofre R, Catalá-López F, Aromataris E, Lockwood C (2021) How to properly use the PRISMA statement. Syst Rev 10:1–3
Luchini C, Stubbs B, Solmi M, Veronese N (2017) Assessing the quality of studies in meta-analyses: advantages and limitations of the Newcastle Ottawa scale. World J Meta-Anal 5:80–84
Luchini C, Veronese N, Nottegar A, Shin JI, Gentile G, Granziol U, Soysal P, Alexinschi O, Smith L (2021) Assessing the quality of studies in meta-research: review/guidelines on the most important quality assessment tools. Pharm Stat 20:185–195
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Dyussenbayev A (2017) Age periods of human life. Adv Soc Sci Res J. https://doi.org/10.14738/assrj.46.2924
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Michelen M, Manoharan L, Elkheir N, Cheng V, Dagens A, Hastie C, O’Hara M, Suett J, Dahmash D, Bugaeva P (2021) Characterising long COVID: a living systematic review. BMJ Glob Health 6:e005427
Groff D, Sun A, Ssentongo AE, Ba DM, Parsons N, Poudel GR, Lekoubou A, Oh JS, Ericson JE, Ssentongo P (2021) Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open 4:e2128568–e2128568
Veronese N, Bonica R, Cotugno S, Tulone O, Camporeale M, Smith L, Trott M, Bruyere O, Mirarchi L, Rizzo G (2022) Interventions for improving long COVID-19 symptomatology: a systematic review. Viruses 14:1863
Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S (2003) One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 348:683–693
Ahmed H, Patel K, Greenwood DC, Halpin S, Lewthwaite P, Salawu A, Eyre L, Breen A, O’Connor R, Jones A (2020) Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med. https://doi.org/10.2340/16501977-2694
Ngai JC, Ko FW, Ng SS, To KW, Tong M, Hui DS (2010) The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology 15:543–550
Moldofsky H, Patcai J (2011) Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol 11:1–7
Stewart S, Newson L, Briggs TA, Grammatopoulos D, Young L, Gill P (2021) Long COVID risk-a signal to address sex hormones and women’s health. Lancet Regional Health-Eur 11:100242
Sudre C, Murray B, Varsavsky T, Graham M, Penfold R, Bowyer R, Pujol JC, Klaser K, Antonelli M, Canas L (2020) Attributes and predictors of long-COVID: analysis of COVID cases and their symptoms collected by the COVID symptoms study app. Nat Med 27(4):626–631
Jovanoski N, Chen X, Becker U, Zalocusky K, Chawla D, Tsai L, Borm M, Neighbors M, Yau V (2021) Severity of COVID-19 and adverse long-term outcomes: a retrospective cohort study based on a US electronic health record database. BMJ Open 11:e056284
Fernández-Lázaro D, González-Bernal JJ, Sánchez-Serrano N, Navascués LJ, Ascaso-del-Río A, Mielgo-Ayuso J (2020) Physical exercise as a multimodal tool for COVID-19: could it be used as a preventive strategy? Int J Environ Res Public Health 17:8496
Gilbody SM, Song F, Eastwood AJ, Sutton A (2000) The causes, consequences and detection of publication bias in psychiatry. Acta Psychiatr Scand 102:241–249
Acknowledgements
We acknowledge the illustrators Marco Rossetti and Giuseppina Maria Cozzolino for the drawing provided to us.
Author information
Authors and Affiliations
Contributions
FDG and NV conceived the study topic and design. AB, LD, DFB, FDG, OT, VG and RB carried out the study selection and data extraction. The data were analysed by NV and the manuscript drafted by FDG, AB and NV. LS, MT, OB, LM, CC, MB, LJD, AS contributed significantly to the revision of the manuscript. All authors approved the final version of the text. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Statements
Not needed.
Human and animal rights
Not needed since this study did not involve any human or animal.
Informed consent
Not needed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary Material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Di Gennaro, F., Belati, A., Tulone, O. et al. Incidence of long COVID-19 in people with previous SARS-Cov2 infection: a systematic review and meta-analysis of 120,970 patients. Intern Emerg Med 18, 1573–1581 (2023). https://doi.org/10.1007/s11739-022-03164-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-022-03164-w