Abstract
Chronic COVID syndrome is characterized by chronic fatigue, myalgia, depression and sleep disturbances, similar to chronic fatigue syndrome (CFS) and fibromyalgia syndrome. Implementations of mitochondrial nutrients (MNs) with diet are important for the clinical effects antioxidant. We examined if use of an association of coenzyme Q10 and alpha lipoic acid (Requpero®) could reduce chronic covid symptoms. The Requpero study is a prospective observational study in which 174 patients, who had developed chronic-covid syndrome, were divided in two groups: The first one (116 patients) received coenzyme Q10 + alpha lipoic acid, and the second one (58 patients) did not receive any treatment. Primary outcome was reduction in Fatigue Severity Scale (FSS) in treatment group compared with control group. complete FSS response was reached most frequently in treatment group than in control group. A FSS complete response was reached in 62 (53.5%) patients in treatment group and in two (3.5%) patients in control group. A reduction in FSS core < 20% from baseline at T1 (non-response) was observed in 11 patients in the treatment group (9.5%) and in 15 patients in the control group (25.9%) (p < 0.0001). To date, this is the first study that tests the efficacy of coenzyme Q10 and alpha lipoic acid in chronic Covid syndrome. Primary and secondary outcomes were met. These results have to be confirmed through a double blind placebo controlled trial of longer duration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was isolated for the first time in China in December 2019. Since then, more than 175 million people worldwide have been infected and over 3.8 million people died for severe ARDS due to interstitial bilateral pneumonia linked to the SARS-CoV-2 disease (COVID-19) [1]
The most common symptoms of patients with COVID-19 are fever, cough, shortness of breath, and myalgia/fatigue [2]. Anosmia and dysgeusia have been reported in 33–80% of patients with COVID-19 [3].
Clinical manifestations of COVID-19 disease range from asymptomatic infection to fatal ARDS syndrome [4, 5].
After more than two years, to date, numerous studies have shown that some patients who recovered from COVID-19 develop a chronic post-viral syndrome, characterized by chronic fatigue, variable nonspecific myalgia, depression and sleep disturbances. Other persistent symptoms may include cognitive and mental disturbances, chest and joint pain, palpitations, dysfunction of smell and taste, cough, headache, and gastrointestinal dysfunction. [6]. All laboratory examinations are normal, inflammation markers included.
This new entity has been called “Long Covid-19” (other name has been used for this entity, such as “Long COVID-19,” “post-acute COVID-19,” “persistent COVID-19 symptoms,” “chronic COVID-19,” “post-COVID-19 manifestations,” “long-term COVID-19 effects,” “post COVID-19 syndrome,” “ongoing COVID-19,” “long-term sequelae,” or “long-haulers” as synonyms [7,8,9]).
Recently, The National Institute for Health and Care Excellence (NICE) defined the stages of SARS-CoV-2 disease in relation to the time of onset of symptoms [10, 11] and proposed the following definition.
-
1.
Acute COVID-19 infection: Signs and symptoms of COVID-19 for up to 4 weeks. [11]
-
2.
Symptomatic COVID-19: Signs and symptoms of COVID-19 from 4 to 12 weeks not explained by an alternative diagnosis [11]
-
3.
Post-COVID-19 syndrome: Signs and symptoms that develop during or following an infection consistent with COVID-19, continue for > 12 weeks and are not explained by an alternative diagnosis [11]
Long-term symptoms following acute COVID-19 have been observed across the whole spectrum of acute disease severity.
These long-term adverse effects of COVID-19 are very similar to those experienced by patients with chronic fatigue syndrome (CFS) and fibromyalgia syndrome [12, 13].
Several studies were conducted in these years to clarify the pathogenesis of SARS-CoV-2 infection and mechanisms of cellular damage, both for acute illness and for chronic covid syndrome.
SARS-CoV-2 enters cells via the angiotensin-converting enzyme 2 (ACE2) receptor [14].
The presence of the ACE-2 receptor in numerous organs and tissues such as the oral and nasal mucosa, lungs, heart, gastrointestinal tract, liver, kidneys, spleen, brain, and arterial and venous endothelial cells is linked to the cellular damage of the COVID-19 virus to different organs and tissues [15, 16]
Once inside the cells, SARS-CoV-2 replicates and matures. Activation of immune response results in an inflammatory response that ends in the recruitment of inflammatory cells and the release of cytokines [17].
The COVID-19 cytokine storm (overproduction of > 150 inflammatory cytokines and chemical mediators released by immune or nonimmune cells) [18] determines rapid proliferation and hyperactivation of T cells, macrophages, and natural killer cells.
Oxidative stress is also recognized as a major pathogenetic factor in several viral infections.
The main role of mitochondria is to supply cells with energy. The main role of mitochondria is to supply cells with energy, Inflammation related to COVID-19 could lead to a decrease in the synthesis of ATP.
Inefficient of ATP synthesis as well as dysregulation in fatty acid and amino acid metabolism have been suggested to be also implicated in CFS pathogenesis [19]
The anti-inflammatory properties of mitochondrial nutrients (MNs) are well documented in the literature [20, 21]. The dietary implementation of MNs seems to prevent the uncontrolled production of mitochondrial reactive oxygen species (mtROS), responsible of mitochondrial damage and mitochondrial dysfunction.
Recent reviews have been focused on the potential clinical effects of dietary implementations with coenzyme Q10 (CoQ10) [22, 23] and α-lipoic acid (ALA) [24, 25]. They have different and complementary roles in mitochondrial function, associated with a strong antioxidant actions.
Coenzyme Q10 (CoQ10- ubiquinone), characterized by a side chain consisting of ten isoprenoid units, is an integral component of the mitochondrial respiratory chain [26] and a gene expression modulator [27]. CoQ10 is also introduced through the diet. These properties inspired its use in clinical practice as food supplement. Levels of CoQ10 can decrease both in acute and chronic illness leading to a decreased cellular energy production and to free radical overproduction. As dietary supplement, CoQ10 has low toxicity and does not induce serious adverse effects in humans [28, 29]. As documented by Cordero and colleagues, the inflammasome complex activation and release of proinflammatory cytokines are implicated in the pathophysiology of fibromyalgia. This activation is mediated by CoQ10 deficiency and oral CoQ10 treatment reduces inflammasome activity. [30]
α-lipoic acid (ALA) is a powerful endogenous and exogenous antioxidant. The active metabolite is represented by its reduced form (dihydrolipoic acid). ALA is part of Krebs cycle, as a co-factor of mitochondrial enzymes [31, 32]. Its dietary supplementation is safety [33]. Its antioxidant and immunomodulatory activity has been well studied.
ALA regulates several processes, such as nucleic acid synthesis and ATP production, via the citric acid cycle [34].
As recently summarized [35], ALA has been tested in several chronic disease correlated to immunoinflammatory conditions like metabolic syndrome and diabetic neuropathy. Additionally, an antiviral effect has been postulated, suggesting its clinical use for co-treatment of several viral infections.
ALA acting as a free radical scavenger and co-factor of ATP production, so that could modulate the course of an infection.
Moreover, a recent study by Sadeghiyan Galeshkalami et al. [34] reported the benefits of ALA and coQ10 combination on experimental diabetic neuropathy by modulating oxidative stress and apoptosis.
These pharmacological properties suggested us to use ALA via dietary implementation in patients with, acting as booster of CoQ10.
The combined use of these two therapeutic agents could provide a synergic effect in treatment of chronic covid syndrome, increasing energy production and reducing cellular oxidative stress.
Given these assumptions, we decided to investigate whether the use of coenzyme Q10 and alpha lipoic acid could reduce chronic covid symptoms.
Methods
Starting from these clinical and pathogenetic considerations and evaluating the multidisciplinary nature of chronic COVID-19 disease, in March 2021, a post-covid syndrome clinic was founded at our internal medicine department.
The mission of our clinic was to identify patients who had developed post-covid syndrome and give them a pharmacologic and non-pharmacologic support to alleviate symptoms, mostly fatigue.
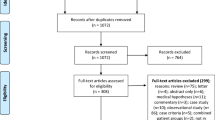
The Requpero study, approved by the Ethics committee of “ASL Brindisi,” is a prospective observational study in which, of 200 consecutive patients, evaluated from June 2021 to October 2021, we enrolled 174 ones who met inclusion criteria (Table 1).
Inclusion criteria were patients aged 18–81 who contracted COVID-19 and who met the 2015 National Academy of Medicine diagnostic criteria for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) (Fig. 1).
Patients with fatigue secondary to pathological clinical conditions justifying this symptomatology and/or patients with a previous diagnosis of chronic fatigue syndrome or fibromyalgia were excluded (Table 1). Other clinical conditions were excluded by an anamnestic questionnaire, complete physical examination, vital parameters at rest (blood pressure, O2 saturation, heart rate, body temperature), blood tests, first level cancer screening.
Study population was divided in two groups: the first one (116 patients) received coenzyme Q10 + alpha lipoic acid—given every day for two months at a dose of 100 mg of coenzyme Q10 + 100 mg of lipoic acid bid—and the second one (58 patients) not received this treatment.
Patients in both groups received multimodal therapy, based on analgesic drugs (mostly paracetamol ore codeine), COXIB/NSAIDs, antidepressant drugs (mostly duloxetine, for its pain modulation effect), anticonvulsant with analgesic effect (pregabalin and gabapentin) psychological/psychiatric counselling, physio-kinesiotherapy, physical reconditioning, yoga/pilates.
At baseline (T0) and after 60 days (T1), patients were administered the following questionnaires.
-
1.
Fatigue severity scale (FSS) (Fig. 2)
-
2.
WPI (Fig. 3)
-
3.
SSS (Fig. 4)
-
4.
Visual Analogue Scale (VAS) pain (Fig. 5)
-
5.
VAS fatigue (Fig. 6)
-
6.
VAS sleep (Fig. 7)
The primary end-point was to evaluate the efficacy of the association of coenzyme Q10 and alpha lipoic acid in reducing fatigue, expressed as a reduction of at least 50% (total response) from the baseline or at least 20% (partial response) in Fatigue Severity Scale (FSS) (Fig. 2) from T0 to T1. Patients were defined “non-responders” if FSS score improvement at T1 was < 20%.
Secondary end-points were to evaluate the modification in VAS fatigue, VAS pain, VAS sleep, Widespread Pain Index (WPI), Symptoms Severity Scale (SSS) and modified Fibromyalgia Assessment Status (FAS) from baseline at the second month. Response was assessed for this scores at the same way of FSS response (responders, partial responders, non-responders).
All patients signed a consent form before study.
Statistical analysis
Data were analyzed with “Stata MP17” software. Continuous variables between groups were evaluated with the Student’s t test. Continuous variables between T0, T1 and groups were evaluated with repeated measures ANOVA test. Chi-square test was used for categorical variables.
The outcome differences between treatment and control group were evaluated with multivariate linear regression, adjusted for age, sex, BMI, hospitalization, time passed from SARS-CoV-2 molecular test positivity. Correlation coefficients were calculated, with a confidence interval of 95% (95% CI). For all test, a significant p value was reported as < 0.05.
Results
From June to October 2021, we evaluated 174 patients with previous SARS-CoV-2 infection with persistence of systemic symptoms, such as fatigue, mental confusion, sleep disturbances, arthromyalgia, dyspnea, Headache.
The mean age of the participants was 51 years (interquartile range (IQR) 18–81 years). The sample was composed of 51% (89/174) of male patients and 49% (85/180) female patients.
52% had comorbidities. The most frequent ones were chronic lung disease (16%, 28/174), diabetes mellitus (13%, 23/174), psychiatric diseases (7.5%, 13/174), rheumatic diseases (9.8%, 17/174).
Only 17.8% (31) of patients had been previously hospitalized for severe respiratory SARS-CoV-2 pneumonia.
The remaining 82.2% had mild/moderate symptoms during the acute phase.
The mean duration of chronic covid symptoms was 5.9 months (IQR 1–19 months).
The most common symptoms were fatigue (80%), impaired concentration (68%), sleep disorders (85%) disturbed smell and/or taste (60%), memory loss (45%), dyspnea (21%) and arthromyalgias (64%).
Of 174 patients recruited, 116 (66.7%) were assigned to treatment group and 58 were assigned to control group. The characteristics of the patients in the two groups were similar at baseline (Table 2).
The primary end-point was to evaluate the effectiveness of the association of coenzyme Q10 and alpha lipoic acid in reducing fatigue, expressed as a reduction in Fatigue Severity Scale (FSS), at the second month (T1), of at least 50% (complete response) from the baseline (T0) or at least 20% (partial response) from the baseline (T0). A reduction in FSS < 20% from baseline at T1 was considered as a non-response.
There were no differences between control group and treatment group at baseline in mean FSS, VAS fatigue, VAS pain, VAS sleep, WPI, FAS, SSS (Table 3).
Overall, mean FSS at T1 was lower in treatment group (p < 0.0001). (Table 3).
The differences in reduction in clinimetric indices between groups were confirmed by ANOVA test (Table 4).
A complete FSS response was reached most frequently in treatment group than in control group. A FSS complete response was reached in 62 (53.5%) patients in treatment group and in two (3.5%) patients in control group. A reduction in FSS score < 20% from baseline at T1 (non-response) was observed in 11 patients in the treatment group (9.5%) and in 15 patients in the control group (25.9%) (p < 0.0001).
Similar results have been obtained by analyzing all the other scores, with the biggest differences observed between groups for VAS pain, WPI, FAS. (Table 5).
Multivariate linear regression detected significant differences between groups in:
-
− FSS (coef. = -9.8; 95%CI = -12.7–69; p < 0.0001)
-
− SSS (coef. = -2.2; 95%CI = -2.7–16; p < 0.0001)
-
− WPI (coef. = -2.0; 95%CI = -2.5–14; p < 0.0001)
-
− FAS (coef. = -6.6; 95%CI = -7.7–5.4; p < 0.0001)
-
− VAS pain coef. = -2.0; 95%CI = -2.4–1.5; p < 0.0001)
-
− VAS sleep (coef. = -2.3; 95%CI = -2.8–1.8; p < 0.0001)
-
− VAS fatigue (coef. = -1.9; 95%CI = -2.5–1.4; p < 0.0001)
Discussion
SARS-CoV-2 is a new viral entity; the clinical presentation is variable [3,4,5].
A subset of patients with acute SARS-CoV-2 infection develop some persistent symptoms lasting many months (6), the so-called chronic covid syndrome [36,37,38,39,40].
A recent study [41] published in the Lancet by Bin Cao shows how the medium- and long-term effects of COVID-19 disease are very disabling and not always diagnosed. The study was conducted on over 1,700 patients discharged between January and May from Wuhan, the Chinese city that hosted the first outbreak of the pandemic.
After 6 months from the acute phase of the infection, a proportion of patients, ranging from 60 to 80% [9,10,11], continued to live with at least one symptom related to the disease, mostly fatigue, muscle weakness, but also depression, anxiety and insomnia and other constitutional symptoms.
Some patients developed visible pulmonary damage or, for example, renal dysfunctions, but the most frequent symptom was the so-called fatigue, conditioning severe limitations in activities of daily living. After acute phase, 63% of patients suffered from it. 26% developed sleep disturbances and 23% experience anxiety or depression.
These symptoms are described also in chronic fatigue syndrome (also called myalgic encephalomyelitis) [13]. This syndrome is also described among the possible sequelae of other infectious diseases, such as SARS coronavirus [42], Epstein-Barr virus [43,44,45], enteroviruses [46], human herpesvirus-6 [47], Ebola virus [46], West Nile virus [48], Dengue virus [49], parvovirus [50]; Borrelia burgdorferi [51], Coxiella burnetii [52], Mycoplasma pneumoniae [53], Giardia lamblia [54].
Many study groups compared long covid syndrome with chronic fatigue syndrome. More patients hospitalized with SARS-CoV-2 disease may develop a “post-viral syndrome which is strikingly similar to myalgic encephalomyelitis/chronic fatigue syndrome [46]”.
The lack of a known etiology or pathophysiology, the variability of symptoms, the lack of clear physical signs, the normality of laboratory tests, the association with anxiety and depression, have all contributed to the social stigma of ME/CFS. [46]. It has often been called an “invisible disease”, as patients may appear healthy, when, in reality, they are seriously ill.
In the comparison between long Covid syndrome and ME/CFS, the only difference is that chronic fatigue syndrome, to be diagnosed, requires the persistence of the symptoms described above for at least 6 months, while the duration of symptoms in the long covid syndrome is not yet well known.
Immune and metabolic dysregulation may be a driver of chronic inflammation in recovered COVID-19 patients [55], that might be responsible to mitochondrial dysfunction [56, 57] and oxidative stress.
Many studies have shown that mitochondrial dysfunction is involved in persistence and severity of symptoms associated with SARS-CoV-2 infection [56,57,58,59]. Their dysfunction and aging contribute to the production of oxygen radicals (mtROS) which increase cell oxidative damage [60,61,62], inflammasome hyperactivation and apoptosis [63,64,65]. For these reasons, mitochondria are now considered central hubs in regulating innate immunity and inflammatory responses [66, 67]
Many studies seem to show that the improvement of mitochondrial turnover contributes to the decrease in inflammation and the restoration of the immune system activity [68,69,70,71].
In SARS-CoV-2 infection, mitochondrial dysfunction could be one of the most important element that determines not only the severity of the clinical manifestations but also the chronicization of the disease [72,73,74].
In line with these data and assuming that the pathogenesis of chronic covid syndrome, at least partially, is linked with a mitochondrial dysfunction and cellular oxidative stress, we tested in a group of patients the oral administration of 100 mg bid of coenzyme Q10 and 100 mg bid of alpha lipoic acid.
Despite the short follow-up period, we demonstrated a clinical benefit, suggesting the rapid effect of this therapy. On the other hand, because of the short follow-up duration, we do not know if this clinical benefit persists over time.
Our results, all based on subjective indices, were definitely in favor of the treatment group.
Considering clinical response extent is to be remarked that the majority of patients in the control group (41 patients, 70.6%) still had a partial response (− 20/50% of FSS at T1). Considering that non-response, partial response and complete response ranges have been established arbitrarily, effective response differences between Group are mitigated. The rate of response reported also in control group is probably due to multimodal therapy used in both groups, based, as mentioned, on analgesic drugs (mostly paracetamol ore codeine), COXIB/NSAIDs, intrarticular/intrabursal/peritendinous steroid/hyaluronic acid injections, antidepressant drugs (mostly duloxetine, for its pain modulation effect), anticonvulsant with analgesic effect (pregabalin and gabapentin) psychological/psychiatric counselling, physio-kinesiotherapy, physical reconditioning, yoga/pilates.
The most important difference is in the percentage of total responders (reduction in FSS at T1 > 50%) between treatment and control group (62% vs 2%). Notably, response differences were also confirmed in all composite scores, in VAS pain and in VAS fatigue.
This study has several limitations.
For what concerns scientific rationale, a mitochondrial dysfunction in chronic Covid syndrome has not been demonstrated yet. As mentioned above, patients’ clinical characteristics and symptoms closely resemble those of ME/CFS and fibromyalgia. Pathogenesis of these syndromes, however, has also not yet been clarified.
Considering the study design, clinimetric indices used in our study were borrowed from ME/CSF and fibromyalgia and, they are not validated in chronic Covid syndrome. Remarkably, they are very appropriate to monitor the large majority of chronic Covid syndrome clinical aspects (fatigue, arthromyalgias, headache, mood disturbance, sleep disturbances, mental fog).
Another limit of our study is that it is not placebo controlled.
To date, this is the first study that tests the efficacy of coenzyme Q10 and alpha lipoic acid in chronic Covid syndrome. Primary and secondary outcomes were met. These results have to be confirmed through a double blind placebo controlled trial of longer duration.
Abbreviations
- ALA:
-
α-Lipoic acid
- CFS:
-
Chronic fatigue syndrome
- CoQ10:
-
Coenzyme Q10
- FAS:
-
Modified fibromyalgia assessment status
- FSS:
-
Fatigue severity scale
- ME/CFS:
-
Myalgic encephalomyelitis/chronic fatigue syndrome
- SARS-CoV-2:
-
Severe acute respiratory syndrome
- SSS:
-
Symptoms severity scale
- VAS:
-
Visual analog scale
- WPI:
-
Widespread pain index
References
Ritchie, H, Ortiz-Ospina, E, Beltekian, D, Mathieu, E, Hasell, J, Macdonald B, Giattino C, Appel C, Rodés-Guirao, L, & Roser, M. Coronavirus Pandemic (COVID-19). 2021
Baj J, Karakuła-Juchnowicz H, Teresiński G, et al. COVID-19: specific and non-specific clinical manifestations and symptoms: the current state of knowledge. J Clin Med. 2020;9(6):1753.
Yang W, Cao Q, Qin L, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): A multi-center study in Wenzhou city, Zhejiang. China J Infect. 2020;80(4):388–93. https://doi.org/10.1016/j.jinf.2020.02.016.
Kim GU, Kim MJ, Ra SH, et al. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin Microbiol Infect. 2020;26(7):948e1–3.
Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;26(368): m1091. https://doi.org/10.1136/bmj.m1091.
Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus a first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–7. https://doi.org/10.1002/path.1570.
Li W, Moore M, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–4.
Hofmann H, Pöhlmann S. Cellular entry of the SARS coronavirus. Trends Microbiol. 2004;12(10):466–72.
Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J Infect. 2020;80(6):607–13. https://doi.org/10.1016/j.jinf.2020.03.037.
Fara A, Mitrev Z, Rosalia RA, Assas BM. Cytokine storm and COVID-19: a chronicle of pro-inflammatory cytokines. Open Biol. 2020;10(9): 200160. https://doi.org/10.1098/rsob.200160.
Carfì A, Bernabei R, Landi F. for the gemelli against COVID-19 post-acute care study group persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–5. https://doi.org/10.1001/jama.2020.12603.
Rubin R. As Their numbers grow, COVID-19 “long haulers” stump Experts. JAMA. 2020;324(14):1381–3. https://doi.org/10.1001/jama.2020.17709.
Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626–31. https://doi.org/10.1038/s41591-021-01292-y.
Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al More than 50 Long-term effects of COVID-19: a systematic review and meta-analysis. medRxiv. 2021: 32: 1613–1617. doi https://doi.org/10.1101/2021.01.27.21250617
Daugherty SE, Guo Y, Heath K, Dasmariñas MC, Jubilo KG, Samranvedhya J, et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ. 2021. https://doi.org/10.1136/bmj.n1098.
Venkatesan P. NICE guideline on long COVID. Lancet Respir Med. 2021;9(2):129. https://doi.org/10.1016/S2213-2600(21)00031-X.
National Institute for Health and Care Excellence. COVID-19 rapid guideline: managing the long-term effects of COVID-19 NICE guideline; c20
Wong TL, Weitzer DJ. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)-a systemic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas). 2021;57(5):418. https://doi.org/10.3390/medicina57050418.
Komaroff AL, Bateman L. Will COVID-19 Lead to Myalgic Encephalomyelitis/Chronic Fatigue Syndrome? Front Med. 2021;7:606824. https://doi.org/10.3389/fmed.2020.606824.
Missailidis D, Annesley SJ, Fisher PR. Pathological mechanisms underlying myalgic encephalomyelitis/chronic fatigue syndrome. Diagnostics (Basel). 2019;9(3):80. https://doi.org/10.3390/diagnostics9030080.
Bested AC, Marshall LM. Review of myalgic encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223–49. https://doi.org/10.1515/reveh-2015-0026.
Pagano G, Manfredi C, Pallardó FV, et al. Potential roles of mitochondrial cofactors in the adjuvant mitigation of proinflammatory acute infections, as in the case of sepsis and COVID-19 pneumonia. Inflamm Res. 2021;70:159–70. https://doi.org/10.1007/s00011-020-01423-0.
Haas RH. Mitochondrial dysfunction in aging and diseases of aging. Biology (Basel). 2019;8(2):48. https://doi.org/10.3390/biology8020048.
Fan L, Feng Y, Chen GC, Qin LQ, Fu CL, Chen LH. Effects of coenzyme Q10 supplementation on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2017;119:128–36. https://doi.org/10.1016/j.phrs.2017.01.032.
Zhai J, Bo Y, Lu Y, Liu C, Zhang L. Effects of coenzyme Q10 on markers of inflammation: a systematic review and meta-analysis. PLoS ONE. 2017. https://doi.org/10.1371/journal.pone.0170172.
Tibullo D, Li Volti G, Giallongo C, et al. Biochemical and clinical relevance of alpha lipoic acid: antioxidant and anti-inflammatory activity, molecular pathways and therapeutic potential. Inflamm Res. 2017;66(11):947–59. https://doi.org/10.1007/s00011-017-1079-6.
Rahimlou M, Asadi M, Banaei Jahromi N, Mansoori A. Alpha-lipoic acid (ALA) supplementation effect on glycemic and inflammatory biomarkers: a Systematic Review and meta- analysis. Clin Nutr ESPEN. 2019;32:16–28. https://doi.org/10.1016/j.clnesp.2019.03.015.
Crane FL. Biochemical functions of coenzyme Q10. J Am Coll Nutr. 2001;20(6):591–8. https://doi.org/10.1080/07315724.2001.10719063.
Groneberg DA, Kindermann B, Althammer M, et al. Coenzyme Q10 affects expression of genes involved in cell signalling, metabolism and transport in human CaCo-2 cells. Int J Biochem Cell Biol. 2005;37(6):1208–18. https://doi.org/10.1016/j.biocel.2004.11.017.
Hidaka T, Fujii K, Funahashi I, Fukutomi N, Hosoe K. Safety assessment of coenzyme Q10 (CoQ10). BioFactors. 2008;32(1–4):199–208. https://doi.org/10.1002/biof.5520320124.
Ikematsu H, Nakamura K, Harashima S, Fujii K, Fukutomi N. Safety assessment of coenzyme Q10 (Kaneka Q10) in healthy subjects: a double-blind, randomized, placebo-controlled trial. Regul Toxicol Pharmacol. 2006;44(3):212–8. https://doi.org/10.1016/j.yrtph.2005.12.002.
Yao JK, Keshavan MS. Antioxidants, redox signaling, and pathophysiology in schizophrenia: an integrative view. Antioxid Redox Signal. 2011;15(7):2011–35. https://doi.org/10.1089/ars.2010.360.
Packer L, Cadenas E. Lipoic acid: energy metabolism and redox regulation of transcription and cell signaling. J Clin Biochem Nutr. 2011;48(1):26–32. https://doi.org/10.3164/jcbn.11-005FR.
Bustamante J, Lodge JK, Marcocci L, Tritschler HJ, Packer L, Rihn BH. Alpha-lipoic acid in liver metabolism and disease. Free Radic Biol Med. 1998;24(6):1023–39. https://doi.org/10.1016/s0891-5849(97)00371-7.
Cremer DR, Rabeler R, Roberts A, Lynch B. Safety evaluation of alpha-lipoic acid (ALA). Regul Toxicol Pharmacol. 2006;46(1):29–41. https://doi.org/10.1016/j.yrtph.2006.06.004.
Dragomanova S, Miteva S, Nicoletti F, et al. Therapeutic potential of alpha-lipoic acid in viral infections, including COVID-19. Antioxid (Basel). 2021;10(8):1294. https://doi.org/10.3390/antiox10081294.
Sadeghiyan Galeshkalami N, Abdollahi M, Najafi R, et al. Alpha-lipoic acid and coenzyme Q10 combination ameliorates experimental diabetic neuropathy by modulating oxidative stress and apoptosis. Life Sci. 2019;1(216):101–10. https://doi.org/10.1016/j.lfs.2018.10.055.
Sweetman E, Kleffmann T, Edgar C, de Lange M, Vallings R, Tate W. A SWATH-MS analysis of myalgic encephalomyelitis/chronic fatigue syndrome peripheral blood mononuclear cell proteomes reveals mitochondrial dysfunction. J Transl Med. 2020;18(1):365. https://doi.org/10.1186/s12967-020-02533-3.
Wood E, Hall KH, Tate W. Role of mitochondria, oxidative stress and the response to antioxidants in myalgic encephalomyelitis/chronic fatigue syndrome: A possible approach to SARS-CoV-2 “long-haulers”? Chronic Dis Transl Med. 2021;7(1):14–26. https://doi.org/10.1016/j.cdtm.2020.11.002.
Booth NE, Myhill S, McLaren-Howard J. Mitochondrial dysfunction and the pathophysiology of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Int J Clin Exp Med. 2012;5(3):208–20.
Holden S, Maksoud R, Eaton-Fitch N, et al. A systematic review of mitochondrial abnormalities in myalgic encephalomyelitis/chronic fatigue syndrome/systemic exertion intolerance disease. J Transl Med. 2020;18:290. https://doi.org/10.1186/s12967-020-02452-3.
Filler K, Lyon D, Bennett J, et al. Association of mitochondrial dysfunction and fatigue: a review of the literature. BBA Clin. 2014;1:12–23. https://doi.org/10.1016/j.bbacli.2014.04.001.
Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–32. https://doi.org/10.1016/S0140-6736(20)32656-8.
Tizenberg BN, Brenner LA, Lowry CA, et al. Biological and psychological factors determining neuropsychiatric outcomes in COVID-19. Curr Psychiatry Rep. 2021;23(10):68. https://doi.org/10.1007/s11920-021-01275-3.
Jones JF, Ray CG, Minnich LL, Hicks MJ, Kibler R, Lucas DO. Evidence for active Epstein-barr virus infection in patients with persistent, unexplained illnesses: elevated anti-early antigen antibodies. Ann Intern Med. 1985;102(1):1–7. https://doi.org/10.7326/0003-4819-102-1.
Hickie I, Davenport T, Wakefield D, Vollmer-Conna U, Cameron B, Vernon SD, Reeves WC, Lloyd A. Dubbo Infection Outcomes Study Group Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006;333(7568):575. https://doi.org/10.1136/bmj.38933.585764.AE.
Gold D, Bowden R, Sixbey J, Riggs R, Katon WJ, Ashley R, Obrigewitch RM, Corey L. Chronic fatigue a prospective clinical and virologic study. JAMA. 1990;264(1):48–53. https://doi.org/10.1001/jama.264.1.48.
Komaroff AL, Bateman L. Will COVID-19 Lead to Myalgic Encephalomyelitis/Chronic Fatigue Syndrome? Front Med (Lausanne). 2021;7:606824. https://doi.org/10.3389/fmed.2020.606824.
Komaroff AL. Is human herpesvirus-6 a trigger for chronic fatigue syndrome? J Clin Virol. 2006;37(Suppl 1):S39-46. https://doi.org/10.1016/S1386-6532(06)70010-5.
Sejvar JJ, et al. Neurocognitive and functional outcomes in persons recovering from West Nile virus illness. J Neuropsychol. 2008;22:477–99.
Seet RCS, Quek AML, Lim ECH. Post-infectious fatigue syndrome in dengue infection. J Clini Virol. 2007;38(1):1–6.
Kerr JR, Gough J, Richards SC, Main J, Enlander D, McCreary M, Komaroff AL, Chia JK. Antibody to parvovirus B19 nonstructural protein is associated with chronic arthralgia in patients with chronic fatigue syndrome/myalgic encephalomyelitis. J Gen Virol. 2010;91(Pt 4):893–7. https://doi.org/10.1099/vir.0.017590-0.
Sigal LH. Summary of the first 100 patients seen at a Lyme disease referral center. Am J Med. 1990;88(6):577–81.
Morroy G, et al. Fatigue following acute Q-fever: a systematic literature review. PLoS ONE. 2016;11(5):e0155884. https://doi.org/10.1371/journal.pone.0155884.
Nicolson GL, Gan R, Haier J. Multiple co-infections (Mycoplasma, Chlamydia, human herpes virus-6) in blood of chronic fatigue syndrome patients: association with signs and symptoms. APMIS. 2003;111(5):557–66. https://doi.org/10.1034/j.1600-0463.2003.1110504.x.
Litleskare S, Rortveit G, Eide GE, Hanevik K, Langeland N, Wensaas KA. Prevalence of Irritable Bowel Syndrome and Chronic Fatigue 10 Years After Giardia Infection. Clin Gastroenterol Hepatol. 2018;16(7):1064-1072.e4. https://doi.org/10.1016/j.cgh.2018.01.022.
Ong SWX, Fong S-W, Young BE, et al. Persistent symptoms and association with inflammatory cytokine signatures in recovered coronavirus disease 2019 patients. Open Forum Infect Dis. 2021. https://doi.org/10.1093/ofid/ofab156.
Moreno Fernández-Ayala DJ, Navas P, López-Lluch G. Age-related mitochondrial dysfunction as a key factor in COVID-19 disease. Exp Gerontol. 2020;142: 111147. https://doi.org/10.1016/j.exger.2020.111147.
Babbar M, Basu S, Yang B, Croteau DL, Bohr VA. Mitophagy and DNA damage signaling in human aging. Mech Ageing Dev. 2020;186: 111207. https://doi.org/10.1016/j.mad.2020.111207.
Desdín-Micó G, Soto-Heredero G, Aranda JF, et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science. 2020;368(6497):1371–6. https://doi.org/10.1126/science.aax0860.
Bordallo B, Bellas M, Cortez AF, Vieira M, Pinheiro M. Severe COVID-19: what have we learned with the immunopathogenesis? Adv Rheumatol. 2020;60(1):50. https://doi.org/10.1186/s42358-020-00151-7.
Brenner D, Mak TW. Mitochondrial cell death effectors. Curr Opin Cell Biol. 2009;21(6):871–7. https://doi.org/10.1016/j.ceb.2009.09.004.
McGuire PJ. Mitochondrial dysfunction and the aging immune system. Biology. 2019;8(2):26.
Spierings D, McStay G, Saleh M, Bender C, Chipuk J, Maurer U, Green DR. Connected to death: the (unexpurgated) mitochondrial pathway of apoptosis. Science. 2005;310(5745):66–7. https://doi.org/10.1126/science.1117105.
Moro L. Mitochondrial dysfunction in aging and cancer. J Clin Med. 2019;8(11):1983. https://doi.org/10.3390/jcm8111983.
Natarajan V, Chawla R, Mah T, Vivekanandan R, Tan SY, Sato PY, Mallilankaraman K. Mitochondrial dysfunction in age-related metabolic disorders. Proteomics. 2020;20(5–6):e1800404. https://doi.org/10.1002/pmic.201800404.
Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305(5684):626–9. https://doi.org/10.1126/science.1099320.
Missiroli S, Genovese I, Perrone M, Vezzani B, Vitto VAM, Giorgi C. The role of mitochondria in inflammation: from cancer to neurodegenerative disorders. J Clini Med. 2020;9(3):740. https://doi.org/10.3390/jcm9030740.
Abhishek M, et al. Mitochondria: the indispensable players in innate immunity and guardians of the inflammatory response. J Cell Comm Signal. 2019;6:1–16.
Sandhir R, Halder A, Sunkaria A. Mitochondria as a centrally positioned hub in the innate immune response. Biochim Biophys Acta Mol Basis Dis. 2017;1863(5):1090–7. https://doi.org/10.1016/j.bbadis.2016.10.020.
Weinberg SE, Sena LA, Chandel NS. Mitochondria in the regulation of innate and adaptive immunity. Immunity. 2015;42(3):406–17. https://doi.org/10.1016/j.immuni.2015.02.002.
Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–35. https://doi.org/10.1038/nature07201.
Ganji R, Reddy PH. Impact of COVID-19 on mitochondrial-based immunity in aging and age-related diseases. Front Aging Neurosci. 2021;12(12): 614650. https://doi.org/10.3389/fnagi.2020.614650.
Holder K, Reddy PH. The COVID-19 effect on the immune system and mitochondrial dynamics in diabetes, obesity, and dementia. Neuroscientist. 2021;27(4):331–9. https://doi.org/10.1177/1073858420960443.
Acknowledgements
We are grateful to Luca Spagnolo & Barbara Spagnolo, chief of “Officina Speziale” of Taranto, for production of Requpero®.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare that are relevant to the content of this article.
Consent to participate
Informed consent was obtained from all patients included in the study.
Consent for publication
Not applicable.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics Committee of the ASL Brindisi.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barletta, M.A., Marino, G., Spagnolo, B. et al. Coenzyme Q10 + alpha lipoic acid for chronic COVID syndrome. Clin Exp Med 23, 667–678 (2023). https://doi.org/10.1007/s10238-022-00871-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-022-00871-8