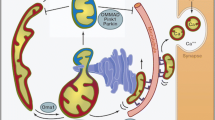
Abstract
Over the past decade, mitochondria have emerged as critical integrators of energy production, generation of reactive oxygen species (ROS), multiple cell death, and signaling pathways in the constantly beating heart. Clarification of the molecular mechanisms, underlying mitochondrial ROS generation and ROS-induced cell death pathways, associated with cardiovascular diseases, by itself remains an important aim; more recently, mitochondrial dynamics has emerged as an important active mechanism to maintain normal mitochondria number and morphology, both are necessary to preserve cardiomyocytes integrity. The two opposing processes, division (fission) and fusion, determine the cell type-specific mitochondrial morphology, the intracellular distribution and activity. The tightly controlled balance between fusion and fission is of particular importance in the high energy demanding cells, such as cardiomyocytes, skeletal muscles, and neuronal cells. A shift toward fission will lead to mitochondrial fragmentation, observed in quiescent cells, while a shift toward fusion will result in the formation of large mitochondrial networks, found in metabolically active cardiomyocytes. Defects in mitochondrial dynamics have been associated with various human disorders, including heart failure, ischemia reperfusion injury, diabetes, and aging. Despite significant progress in our understanding of the molecular mechanisms of mitochondrial function in the heart, further focused research is needed to translate this knowledge into the development of new therapies for various ailments.




Similar content being viewed by others
References
Kane LA, Youle RJ (2010) Mitochondrial fission and fusion and their roles in the heart. J Mol Med (Berl) 88:971–979
Paradies G, Petrosillo G, Pistolese M, Ruggiero FM (2002) Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene 286:135–141
Ide T, Tsutsui H, Hayashidani S et al (2001) Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res 88:529–535
Ide T, Tsutsui H, Kinugawa S et al (1999) Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res 85:357–363
Giordano FJ (2005) Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest 115:500–508
Cesselli D, Jakoniuk I, Barlucchi L et al (2001) Oxidative stress-mediated cardiac cell death is a major determinant of ventricular dysfunction and failure in dog dilated cardiomyopathy. Circ Res 89:279–286
Marin-Garcia J, Goldenthal MJ, Moe GW (2001) Abnormal cardiac and skeletal muscle mitochondrial function in pacing-induced cardiac failure. Cardiovasc Res 52:103–110
Nordberg J, Arner ES (2001) Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med 31:1287–1312
Nojiri H, Shimizu T, Funakoshi M et al (2006) Oxidative stress causes heart failure with impaired mitochondrial respiration. J Biol Chem 281:33789–33801
Huang TT, Carlson EJ, Kozy HM et al (2001) Genetic modification of prenatal lethality and dilated cardiomyopathy in Mn superoxide dismutase mutant mice. Free Radic Biol Med 31:1101–1110
Conrad M, Jakupoglu C, Moreno SG et al (2004) Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol Cell Biol 24:9414–9423
Li Y, Huang TT, Carlson EJ et al (1995) Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet 11:376–381
Shiomi T, Tsutsui H, Matsusaka H et al (2004) Overexpression of glutathione peroxidase prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation 109:544–549
Schriner SE, Linford NJ, Martin GM et al (2005) Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308:1909–1911
Mak S, Newton GE (2001) The oxidative stress hypothesis of congestive heart failure: radical thoughts. Chest 120:2035–2046
Sam F, Kerstetter DL, Pimental DR et al (2005) Increased reactive oxygen species production and functional alterations in antioxidant enzymes in human failing myocardium. J Card Fail 11:473–480
Narula J, Haider N, Virmani R et al (1996) Apoptosis in myocytes in end-stage heart failure. N Engl J Med 335:1182–1189
Olivetti G, Abbi R, Quaini F et al (1997) Apoptosis in the failing human heart. N Engl J Med 336:1131–1141
Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116:205–219
Li LY, Luo X, Wang X (2001) Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 412:95–99
Susin SA, Lorenzo HK, Zamzami N et al (1999) Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397:441–446
Liu X, Kim CN, Yang J, Jemmerson R, Wang X (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86:147–157
Kroemer G (2003) Mitochondrial control of apoptosis: an introduction. Biochem Biophys Res Commun 304:433–435
Marzo I, Brenner C, Zamzami N et al (1998) The permeability transition pore complex: a target for apoptosis regulation by caspases and bcl-2-related proteins. J Exp Med 187:1261–1271
Scorrano L, Ashiya M, Buttle K et al (2002) A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell 2:55–67
Scorrano L, Oakes SA, Opferman JT et al (2003) BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 300:135–139
Morishima N, Nakanishi K, Tsuchiya K, Shibata T, Seiwa E (2004) Translocation of Bim to the endoplasmic reticulum (ER) mediates ER stress signaling for activation of caspase-12 during ER stress-induced apoptosis. J Biol Chem 279:50375–50381
Neuss M, Monticone R, Lundberg MS, Chesley AT, Fleck E, Crow MT (2001) The apoptotic regulatory protein ARC (apoptosis repressor with caspase recruitment domain) prevents oxidant stress-mediated cell death by preserving mitochondrial function. J Biol Chem 276:33915–33922
Pi Y, Goldenthal MJ, Marin-Garcia J (2007) Mitochondrial involvement in IGF-1 induced protection of cardiomyocytes against hypoxia/reoxygenation injury. Mol Cell Biochem 301:181–189
Marín-García J, Goldenthal MJ (2008) Mitochondrial centrality in heart failure. Heart Fail Rev 13:137–150
Kirshenbaum LA, de Moissac D (1997) The bcl-2 gene product prevents programmed cell death of ventricular myocytes. Circulation 96:1580–1585
Zhu L, Yu Y, Chua BH, Ho YS, Kuo TH (2001) Regulation of sodium-calcium exchange and mitochondrial energetics by Bcl-2 in the heart of transgenic mice. J Mol Cell Cardiol 33:2135–2144
Kang PM, Haunstetter A, Aoki H, Usheva A, Izumo S (2000) Morphological and molecular characterization of adult cardiomyocyte apoptosis during hypoxia and reoxygenation. Circ Res 87:118–125
Milner DJ, Mavroidis M, Weisleder N, Capetanaki Y (2000) Desmin cytoskeleton linked to muscle mitochondrial distribution and respiratory function. J Cell Biol 150:1283–1298
Linden M, Li Z, Paulin D, Gotow T, Leterrier JF (2001) Effects of desmin gene knockout on mice heart mitochondria. J Bioenerg Biomembr 33:333–341
Weisleder N, Taffet GE, Capetanaki Y (2004) Bcl-2 overexpression corrects mitochondrial defects and ameliorates inherited desmin null cardiomyopathy. Proc Natl Acad Sci USA 101:769–774
Imahashi K, Schneider MD, Steenbergen C, Murphy E (2004) Transgenic expression of Bcl-2 modulates energy metabolism, prevents cytosolic acidification during ischemia, and reduces ischemia/reperfusion injury. Circ Res 95:734–741
Vahsen N, Cande C, Briere JJ et al (2004) AIF deficiency compromises oxidative phosphorylation. EMBO J 23:4679–4689
Joza N, Oudit GY, Brown D et al (2005) Muscle-specific loss of apoptosis-inducing factor leads to mitochondrial dysfunction, skeletal muscle atrophy, and dilated cardiomyopathy. Mol Cell Biol 25:10261–10272
Kajstura J, Cheng W, Reiss K et al (1996) Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest 74:86–107
Rayment NB, Haven AJ, Madden B et al (1999) Myocyte loss in chronic heart failure. J Pathol 188:213–219
Gill C, Mestril R, Samali A (2002) Losing heart: the role of apoptosis in heart disease—a novel therapeutic target? Faseb J 16:135–146
Honda O, Kuroda M, Joja I et al (2000) Assessment of secondary necrosis of Jurkat cells using a new microscopic system and double staining method with annexin V and propidium iodide. Int J Oncol 16:283–288
Malhi H, Gores GJ, Lemasters JJ (2006) Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology 43:S31–S44
Kim JS, He L, Lemasters JJ (2003) Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Commun 304:463–470
Lemasters JJ, Nieminen AL, Qian T et al (1998) The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta 1366:177–196
Zamzami N, Hirsch T, Dallaporta B, Petit PX, Kroemer G (1997) Mitochondrial implication in accidental and programmed cell death: apoptosis and necrosis. J Bioenerg Biomembr 29:185–193
Nakayama H, Chen X, Baines CP et al (2007) Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest 117:2431–2444
Yoshida K, Hanafusa T, Matoba R, Wakasugi C (1990) Proteolysis of myosin and troponin in human myocardium of elderly subjects. Jpn Heart J 31:683–691
Cuervo AM, Dice JF (2000) Age-related decline in chaperone-mediated autophagy. J Biol Chem 275:31505–31513
Cuervo AM (2004) Autophagy: many paths to the same end. Mol Cell Biochem 263(1–2):55–72
Levine B, Klionsky DJ (2004) Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 6:463–477
Ohsumi Y, Mizushima N (2004) Two ubiquitin-like conjugation systems essential for autophagy. Semin Cell Dev Biol 15:231–236
Kissova I, Deffieu M, Manon S, Camougrand N (2004) Uth1p is involved in the autophagic degradation of mitochondria. J Biol Chem 279:39068–39074
Lemasters JJ (2005) Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res 8:3–5
Zhu H, Tannous P, Johnstone JL et al (2007) Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest 117:1782–1793
Yan L, Vatner DE, Kim SJ et al (2005) Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci USA 102:13807–13812
Takemura G, Miyata S, Kawase Y, Okada H, Maruyama R, Fujiwara H (2006) Autophagic degeneration and death of cardiomyocytes in heart failure. Autophagy 2:212–214
Terman A, Brunk UT (1998) On the degradability and exocytosis of ceroid/lipofuscin in cultured rat cardiac myocytes. Mech Ageing Dev 100:145–156
Grune T, Merker K, Jung T, Sitte N, Davies KJ (2005) Protein oxidation and degradation during postmitotic senescence. Free Radic Biol Med 39:1208–1215
Rooyackers OE, Adey DB, Ades PA, Nair KS (1996) Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci USA 93:15364–15369
Brunk UT, Terman A (2002) The mitochondrial-lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur J Biochem 269:1996–2002
Terman A, Brunk UT (2005) Autophagy in cardiac myocyte homeostasis, aging, and pathology. Cardiovasc Res 68(3):355–365
Kurz T, Eaton JW, Brunk UT (2011) The role of lysosomes in iron metabolism and recycling. Int J Biochem Cell Biol 43:1686–1697
Brunk UT, Neuzil J, Eaton JW (2001) Lysosomal involvement in apoptosis. Redox Rep 6:91–97
Terman A, Gustafsson B, Brunk UT (2006) The lysosomal-mitochondrial axis theory of postmitotic aging and cell death. Chem Biol Interact 163:29–37
Yan L, Sadoshima J, Vatner DE, Vatner SF (2006) Autophagy: a novel protective mechanism in chronic ischemia. Cell Cycle 5:1175–1177
Kunapuli S, Rosanio S, Schwarz ER (2006) “How do cardiomyocytes die?” apoptosis and autophagic cell death in cardiac myocytes. J Card Fail 12:381–391
Iglewski M, Hill JA, Lavandero S, Rothermel BA (2010) Mitochondrial fission and autophagy in the normal and diseased heart. Curr Hypertens Rep 12:418–425
Taneike M, Yamaguchi O, Nakai A et al. (2010) Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy 6(5):600–606
Nishida K, Otsu K (2008) Cell death in heart failure. Circ J 72(Suppl A):A17–A21
Nakai A, Yamaguchi O, Takeda T et al (2007) The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 13:619–624
Chen H, Chan DC (2009) Mitochondrial dynamics–fusion, fission, movement, and mitophagy—in neurodegenerative diseases. Hum Mol Genet 18(R2):R169–R176
Liesa M, Palacin M, Zorzano A (2009) Mitochondrial dynamics in mammalian health and disease. Physiol Rev 89:799–845
Detmer SA, Chan DC (2007) Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol 8:870–879
Okamoto K, Shaw JM (2005) Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet 39:503–536
Twig G, Hyde B, Shirihai OS (2008) Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta 1777:1092–1097
Chen H, Chen DC (2010) Physiological functions of mitochondrial fusion. Ann NY Acad Sci 1201:21–25
Li J, Zhou J, Li Y, Qin D, Li P (2010) Mitochondrial fission controls DNA fragmentation by regulating endonuclease G. Free Radic Biol Med 49:622–631
Shenouda SM, Widlansky ME, Chen K et al (2011) Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 124:444–453
Meeusen S, DeVay R, Block J et al (2006) Mitochondrial inner-membrane fusion and crista maintenance requires the dynamin-related GTPase Mgm1. Cell 127:383–395
Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC (2009) Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell 20:3525–3532
Alavi MV, Bette S, Schimpf S et al (2007) A splice site mutation in the murine Opa1 gene features pathology of autosomal dominant optic atrophy. Brain 130:1029–1042
Davies VJ, Hollins AJ, Piechota MJ et al (2007) Opa1 deficiency in a mouse model of autosomal dominant optic atrophy impairs mitochondrial morphology, optic nerve structure and visual function. Hum Mol Genet 16:1307–1318
Rojo M, Legros F, Chateau D, Lombes A (2002) Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J Cell Sci 15:1663–1674
Sesaki H, Jensen RE (2001) UGO1 encodes an outer membrane protein required for mitochondrial fusion. J Cell Biol 152:1123–1134
Chen H, Chomyn A, Chan DC (2005) Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem 280:26185–26192
Amati-Bonneau P, Valentino ML, Reynier P et al (2008) OPA1 mutations induce mitochondrial DNA instability and optic atrophy ‘plus’ phenotypes. Brain 131:338–351
de Brito OM, Scorrano L (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456:605–610
Hudson G, Amati-Bonneau P, Blakely EL et al (2008) Mutation of OPA1 causes dominant optic atrophy with external ophthalmoplegia, ataxia, deafness and multiple mitochondrial DNA deletions: a novel disorder of mtDNA maintenance. Brain 131:329–337
Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ (2004) Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol 15:5001–5011
Gandre-Babbe S, van der Bliek AM (2008) The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol Biol Cell 19:2402–2412
Otera H, Wang C, Cleland MM et al (2010) Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol 191:1141–1158
Westermann B (2010) Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 11:872–884
Suen DF, Norris KL, Youle RJ (2008) Mitochondrial dynamics and apoptosis. Genes Dev 22:1577–1590
Margineantu DH, Gregory CW, Sundell L, Sherwood SW, Beechem JM, Capaldi RA (2002) Cell cycle dependent morphology changes and associated mitochondrial DNA redistribution in mitochondria of human cell lines. Mitochondrion 1:425–435
Chen H, Vermulst M, Wang YE et al (2010) Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141:280–289
Elachouri G, Vidoni S, Zanna C et al (2011) OPA1 links human mitochondrial genome maintenance to mtDNA replication and distribution. Genome Res 21:12–20
Zanna C, Ghelli A, Porcelli AM et al (2008) OPA1 mutations associated with dominant optic atrophy impair oxidative phosphorylation and mitochondrial fusion. Brain 131:352–367
Pich S, Bach D, Briones P et al (2005) The charcot-marie-tooth type 2A gene product, Mfn2, up-regulates fuel oxidation through expression of OXPHOS system. Hum Mol Genet 14:1405–1415
Bach D, Pich S, Soriano FX et al (2003) Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem 278:17190–17197
Parone PA, Da CS, Tondera D et al (2008) Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS ONE 3:e3257
Mayorov VI, Lowrey AJ, Biousse V, Newman NJ, Cline SD, Brown MD (2008) Mitochondrial oxidative phosphorylation in autosomal dominant optic atrophy. BMC Biochem 9:22
Benard G, Bellance N, James D et al (2007) Mitochondrial bioenergetics and structural network organization. J Cell Sci 120:838–848
Twig G, Elorza A, Molina AJ et al (2008) Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27:433–446
Yu T, Robotham JL, Yoon Y (2006) Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci USA 103:2653–2658
Cao H, Garcia F, McNiven MA (1998) Differential distribution of dynamin isoforms in mammalian cells. Mol Biol Cell 9:2595–2609
Ferguson SM, De Camilli P (2012) Dynamin, a membrane-remodelling GTPase. Nat Rev Mol Cell Biol 13:75–88
Nakata T, Iwamoto A, Noda Y, Takemura R, Yoshikura H, Hirokawa N (1991) Predominant and developmentally regulated expression of dynamin in neurons. Neuron 7:461–469
Ferguson SM, Raimondi A, Paradise S et al (2009) Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev Cell 17:811–822
Nakata T, Takemura R, Hirokawa N (1993) A novel member of the dynamin family of GTP-binding proteins is expressed specifically in the testis. J Cell Sci 105:1–5
Cook T, Mesa K, Urrutia R (1996) Three dynamin-encoding genes are differentially expressed in developing rat brain. J Neurochem 67:927–931
Gray NW, Fourgeaud L, Huang B (2003) Dynamin 3 is a component of the postsynapse, where it interacts with mGluR5 and Homer. Curr Biol 13:510–515
Ferguson SM, Brasnjo G, Hayashi M et al (2007) A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science 316:570–574
Ono T, Isobe K, Nakada K, Hayashi JI (2001) Human cells are protected from mitochondrial dysfunction by complementation of DNA products in fused mitochondria. Nat Genet 28:272–275
Nakada K, Inoue K, Ono T et al (2001) Inter-mitochondrial complementation: mitochondria-specific system preventing mice from expression of disease phenotypes by mutant mtDNA. Nat Med 7:934–940
Khan SM, Smigrodzki RM, Swerdlow RH (2007) Cell and animal models of mtDNA biology: progress and prospects. Am J Physiol Cell Physiol 292:C658–C669
Sharov VG, Goussev A, Lesch M, Goldstein S, Sabbah HN (1998) Abnormal mitochondrial function in myocardium of dogs with chronic heart failure. J Mol Cell Cardiol 30:1757–1762
Zak R, Rabinowitz M, Rajamanickam C, Merten S, Kwiatkowska-Patzer B (1980) Mitochondrial proliferation in cardiac hypertrophy. Basic Res Cardiol 75:171–178
Schaper J, Froede R, Hein S et al (1991) Impairment of the myocardial ultrastructure and changes of the cytoskeleton in dilated cardiomyopathy. Circulation 83:504–514
Kalra DK, Zoghbi WA (2002) Myocardial hibernation in coronary artery disease. Curr Atheroscler Rep 4:149–155
Duvezin-Caubet S, Jagasia R, Wagener J et al (2006) Proteolytic processing of OPA1 links mitochondrial dysfunction to alterations in mitochondrial morphology. J Biol Chem 281:37972–37979
Makino A, Suarez J, Gawlowski T et al (2011) Regulation of mitochondrial morphology and function by O-GlcNAcylation in neonatal cardiac myocytes. Am J Physiol Regul Integr Comp Physiol 300:R1296–R1302
Papanicolaou KN, Khairallah RJ, Ngoh GA et al (2011) Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol 31:1309–1328
Chen Y, Liu Y, Dorn GW II et al (2011) Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res 109:1327–1331
Chen H, McCaffery JM, Chan DC (2007) Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell 130:548–562
Chen H, Vermulst M, Wang YE et al (2010) Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141:280–289
Dec GW, Fuster V (1994) Idiopathic dilated cardiomyopathy. N Engl J Med 331:1564–1575
Seidman JG, Seidman C (2001) The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell 104:557–567
Karkkainen S, Peuhkurinen K (2007) Genetics of dilated cardiomyopathy. Ann Med 39:91–107
Baandrup U, Florio RA, Roters F, Olsen EG (1981) Electron microscopic investigation of endomyocardial biopsy samples in hypertrophy and cardiomyopathy. A semiquantitative study in 48 patients. Circulation 63:1289–1298
Sun CN, Dhalla NS, Olson RE (1969) Formation of gigantic mitochondria in hypoxic isolated perfused rat hearts. Experientia 25:763–764
Ashrafian H, Docherty L, Leo V et al (2010) A mutation in the mitochondrial fission gene Dnm1 l leads to cardiomyopathy. PLoS Genet 6:e1001000
Ramachandran R, Surka M, Chappie JS et al (2007) The dynamin middle domain is critical for tetramerization and higher-order self-assembly. EMBO J 26:559–566
Mears JA, Ray P, Hinshaw JE (2007) A corkscrew model for dynamin constriction. Structure 15:1190–1202
Palaniyandi SS, Qi X, Yogalingam G, Ferreira JC, Mochly-Rosen D (2010) Regulation of mitochondrial processes: a target for heart failure. Drug Discov Today Dis Mech 7:e95–e102
Chen L, Gong Q, Stice JP, Knowlton AA (2009) Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res 84:91–99
Molina AJ, Wikstrom JD, Stiles L et al (2009) Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes 58:2303–2315
Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ (2004) Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell 15:5001–5011
Arnoult D (2007) Mitochondrial fragmentation in apoptosis. Trends Cell Biol 17:6–12
Martinou JC, Youle RJ (2011) Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell 21:92–101
Parra V, Eisner V, Chiong M et al (2008) Changes in mitochondrial dynamics during ceramide-induced cardiomyocyte early apoptosis. Cardiovasc Res 77:387–397
Wasiak S, Zunino R, McBride HM (2007) Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol 177:439–450
Hoppins S, Edlich F, Cleland MM et al (2011) The soluble form of Bax regulates mitochondrial fusion via MFN2 homotypic complexes. Mol Cell 41:150–160
Guo X, Chen KH, Guo Y, Liao H, Tang J, Xiao RP (2007) Mitofusin 2 triggers vascular smooth muscle cell apoptosis via mitochondrial death pathway. Circ Res 101:1113–1122
Shen T, Zheng M, Cao C et al (2007) Mitofusin-2 is a major determinant of oxidative stress-mediated heart muscle cell apoptosis. J Biol Chem 282:23354–23361
de Brito OM, Scorrano L (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456:605–610
Neuspiel M, Zunino R, Gangaraju S, Rippstein P, McBride H (2005) Activated mitofusin 2 signals mitochondrial fusion, interferes with Bax activation, and reduces susceptibility to radical induced depolarization. J Biol Chem 280:25060–25070
Santel A, Frank S, Gaume B, Herrler M, Youle RJ, Fuller MT (2003) Mitofusin-1 protein is a generally expressed mediator of mitochondrial fusion in mammalian cells. J Cell Sci 116:2763–2774
Lukyanenko V, Chikando A, Lederer WJ (2009) Mitochondria in cardiomyocyte Ca2+ signaling. Int J Biochem Cell Biol 41:1957–1971
Papanicolaou KN, Khairallah RJ, Ngoh GA et al (2011) Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol 31:1309–1328
Tandler B, Hoppel CL (1972) Possible division of cardiac mitochondria. Anat Rec 173:309–323
Hom J, Yu T, Yoon Y, Porter G, Sheu SS (2010) Regulation of mitochondrial fission by intracellular Ca2+ in rat ventricular myocytes. Biochim Biophys Acta 1797:913–921
Hamacher-Brady A, Brady NR, Gottlieb RA, Gustafsson AB (2006) Autophagy as a protective response to Bnip3-mediated apoptotic signaling in the heart. Autophagy 2:307–309
Matsui Y, Takagi H, Qu X et al (2007) Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res 100:914–922
Gottlieb RA, Gustafsson AB (2011) Mitochondrial turnover in the heart. Biochim Biophys Acta 1813:1295–1301
Kuzmicic J, Del Campo A, Lopez-Crisosto C et al (2011) Mitochondrial dynamics: a potential new therapeutic target for heart failure. Rev Esp Cardiol 64:916–923
Ng AC (2010) Integrative systems biology and networks in autophagy. Semin Immunopathol 32:355–361
Ziviani E, Whitworth AJ (2010) How could Parkin-mediated ubiquitination of mitofusin promote mitophagy? Autophagy 6:660–662
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marín-García, J., Akhmedov, A.T. & Moe, G.W. Mitochondria in heart failure: the emerging role of mitochondrial dynamics. Heart Fail Rev 18, 439–456 (2013). https://doi.org/10.1007/s10741-012-9330-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-012-9330-2