Abstract
The present study evaluated an integrated biomonitoring approach based on three different bioindicators: tree rings, lichens, and beetles in a complex environment (urban-industrial-forest). In Central Italy, four sampling sites were selected to assess the anthropogenic impact of cement plants taking into account (1) long-term exposure (1988–2020) through the analysis of trace elements in tree rings of Quercus pubescens; (2) medium-term exposure (2020–2021) through the analysis of trace elements in thalli (outermost portions) of the lichen Xanthoria parietina; (3) short-term exposure in spring 2021 through the bioaccumulation and evaluation of sample vitality in transplants of the lichen Evernia prunastri and a periodic survey of entomological biodiversity carried out during spring and summer 2021. Trace elements of industrial origin were found in tree rings, with different levels of accumulation between 1988 and 2020 and a maximum in 2012. Native thalli of the lichen X. parietina showed an overall low bioaccumulation of trace elements except for Cr, probably reflecting the influence of national lockdown measures. The transplants of E. prunastri showed a weak stress response in the industrial and urban sites, but not in the forest, and identified Tl and V as the main elements contributing to atmospheric contamination, with peaks at the industrial sites. Concerning the beetles, a significantly lower number of species was found at the Semonte industrial site.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental pollution is a global issue, where anthropogenic impact is the main cause of pollutant availability in the environment (Briffa et al. 2020). Environmental pollution, determined by the accumulation of gases (e.g., NOx, SO2, VOC, and NH3) and/or particulate matter (PM), defines significant negative impacts and risks for human and ecosystem health (EEA 2018). Therefore, the monitoring of environmental quality in urban areas is a sensible topic that generates significant interest. Environmental safety is determined by World directives, such as the World Health Organization’s (WHO) guidelines, and by National Emission Ceilings Directive that regulate environmental quality monitoring through regional environmental agencies (WHO 2017; De Marco et al. 2019). Traditional air quality monitoring is widely available with each country housing environmental agencies tasked with this responsibility (https://aqicn.org/sources/). Nevertheless, several factors prevent the complete data collection necessary for the establishment of suitable policies. In some cases, monitoring stations are set upped to only measure specific pollutants and/or in defined periods not continuous. Moreover, the resource-intensive maintenance of instruments requires significant financial investment that may generate the reduction of the quality of data acquisition.
Biomonitoring relies on the sensitivity of biological organisms (bioindicators) that interact with the environment where they live to gather valuable information (Costa and Teixeira 2014). Plants and animals are continuously exposed to environmental conditions that regulate their growth, physiology, productivity, and distribution (Malmstrom 2010). Although data interpretation in biomonitoring can be a complex undertaking, biomonitoring offers numerous advantages as a valuable tool for ecological and human health monitoring, primarily due to the widespread availability of bioindicators.
Several organisms are currently used as bioindicators, among which were tree rings (Ballikaya et al. 2022), lichens (Abas 2021), and beetles (Parisi et al. 2018). According to current dendrochemistry applications, trees serve as effective monitor of pollutants, offering data for past decades (Binda et al. 2021; Ballikaya et al. 2022). Trees are subjected to continuous exposure to trace elements, which can be taken up through their roots, leaves, and bark. These elements are subsequently deposited in tree rings, allowing for the decoding of environmental signals corresponding to each year of wood formation through the dendrochemistry approach (Perone et al. 2018; Cocozza et al. 2021). Lichens, given their close dependence on the atmosphere for water supply and mineral nutrients, are sensitive to the presence of substances that alter the normal atmospheric composition. Trace elements can be absorbed directly through the surface of thalli and accumulated (e.g., Vannini et al. 2019; Anderson et al. 2022) thus indicating differences in the elements’ availability during their exposure to the environment. Insects, abundant and widely distributed in all habitats, can act as bioaccumulators and indicators of air pollution (Gutiérrez et al. 2020). Their response can be observed through alterations in life cycle duration, mortality rate, and overall abundance. Trees, lichens, and insects that grow in urban areas, namely in parks and gardens, ensure an effective distribution in the landscape, which can compensate for the lack of both historical pollution time series and artificial air monitoring networks (Baroni et al. 2023).
While biomonitoring studies usually focus on one specific taxon, a combined approach proved to be effective in forest (Burrascano et al. 2021, and reference therein) and urban environment (Pinho et al. 2016). The integrated response of several biological groups can reveal temporal and spatial patterns of environmental variables since different ecological indicators, with varied characteristics, respond differently to human disturbance and can be present in the study sites in different times.
The study was aimed to detect the trace elements in a complex urban environment by using three different bioindicators. The sampling of different biomonitors was designed to consider different durations of exposure in the environment, namely, long-term (ca. 30 years) exposure in wood (trees cores), medium-term (ca. one year) exposure in native foliose lichens, and short-term exposure in insects (beetles sampled from June to October 2021) and lichen transplants (exposed from April to July 2021). Lichens and tree cores are used as bioaccumulators, where bioaccumulation refers to the process whereby a substance present in the environment accumulates at the surface of an organism and/or penetrates it (ISPRA Guidelines, Giordani et al. 2020).
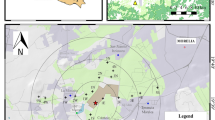
Four different sites were selected by identifying as potential sources of environmental pollution two cement plants, listed by EEA (Holland et al. 2011) among the industries with the greatest environmental and health impact in Europe. Thus, an urban, a forest, and two different industrial sites were considered.
Materials and methods
Study area
The study area is in the Gubbio Plain, located in Central Italy (43°21′ N, 12°34′ E, 495 m a.s.l.). This extensive intermontane basin includes a valley region and the foothills of the Monti di Gubbio, situated to the north of the town. The primary sources of air pollutants (i.e., NOx, SOx, As, Cd, Cr, Pb, and Ni) are two operational cement plants. Other relevant sources of air pollutants (CO, VOCs, PM2.5, and PM10) are heating systems, followed by vehicular traffic (mostly CO and NOx) and agriculture (the principal source of NH3).
The first cement plant, the Ghigiano site, is a manufacturing facility established in 1966, located 8 km away from the urban center of Gubbio. The amount of cement produced in the plant in the Ghigiano site is around 4 million tons per year (https://www.colacem.com/media/userfiles/files/Colacem_RS_2021(2).pdf). The second cement plant, the Semonte site, houses a cement factory built in 1926 and is located less than 2 km from downtown Gubbio, along a high-traffic regional road, with a cement production of 1.3 million tons per year. Besides those two sites, the study area includes an urban site, Parco Ranghiasci, a park located downtown within a limited traffic zone, which was chosen as the reference for the urban environment. Finally, a forest site, namely, a control site, known as S. Bartolomeo, was selected in a rural environment situated 7 km away from the town center and Semonte site and 15 km away from the Ghigiano site. The S. Bartolomeo Forest site was specifically chosen to characterize environmental conditions that are less influenced by urban disturbances (Fig. 1).
Data on the deposition of some airborne pollutants in the study area are available at https://apps.arpa.umbria.it/webgis/emissioni/index.asp.
Sampling
Tree cores, lichens, and insects were sampled at the four sampling sites: Ghigiano and Semonte (industrial), Parco Ranghiasci (urban), and S. Bartolomeo (forest).
Three trees of Quercus pubescens Willd. characterized by a mean diameter at breast height (DBH) of 28 ± 3 cm and average age of 30 ± 2 years were selected in four sampling sites. Two cores per tree were collected at DBH by using an incremental borer (Haglof Company Group, Sweden) in April 2021 and then cut using a microtome (Gärtner and Nievergelt 2010). The tree ring width was measured using the LINTAB instrument (Rinntech, Heidelberg, Germany) and a Leica MS5 stereoscope (Leica Microsystems, Germany). The raw tree-ring width chronologies were obtained by the software TSAP Win (Rinn 1996) and then statistically cross-dated to identify the year of tree-ring formation (Speer 2012). The Gleichläufigkeit statistical index and the relative significance value were calculated (Schweingruber 1988; Speer 2012). The signature of the temporal evolution of environmental conditions was obtained over a long period through the trace element measurements in tree rings from 1988 to 2020 (the common period of tree ring chronologies within sampling sites).
To consider medium- and short-term exposure, the lichen species were chosen among those most widely used in biomonitoring studies (e.g., Cocozza et al. 2016; Vannini et al. 2017; Contardo et al. 2021). Regarding medium-term exposure, fragments of the foliose lichen Xanthoria parietina (L.) Th. Fr., were collected in April 2021 from the tree trunks used for dendrochemical analysis, following ISPRA Guidelines (Giordani et al. 2020). Specifically, the outermost portions (3 mm), corresponding to about 1 year of metabolic activity (Tretiach and Carpanelli 1992), were selected to obtain three analytical samples for each site.
To consider a short-term exposure, thalli of the fruticose lichen Evernia prunastri (L.) Ach. were collected from deciduous oak trees in a forested area of the Umbria Region (Stroncone), far from any local pollution sources. Prior to transplantation, extraneous residues such as bark and insects were removed from the thalli. Sample vitality was then randomly checked by analyzing the photosynthetic efficiency. Lichen thalli were transplanted at the four sampling sites (three samples per plot), suspended to the branches of the trees used for the dendrochemical analysis, following ISPRA Guidelines (Giordani et al. 2020). An exposure of 12 weeks is regarded as optimal for E. prunastri (Loppi et al. 2019).
In the four sampling sites, the capture of saproxylic and non-saproxylic adult beetles was performed. Forest beetle community was considered because saproxylic species are closely related to tree wood (Speight 1989) and include threatened taxa (see, Carpaneto et al. 2015), whereas non-saproxylic beetles refer to all remaining species occupying other ecological niches. Many of the non-saproxylic species occupy unknown trophic niches (Audisio et al. 2015). The beetle sampling was carried out using four window flight traps (WFTs) per site for flying beetles suspended to the branches of the trees used for the dendrochemical analysis (in total 16 traps). At each site, the WFTs were located approximately 10 m from each other. Traps were checked four times, approximately every 30 days, from June to October 2021 and then removed. Systematics and nomenclature followed Bouchard et al. (2011).
Trace element analysis
The tree core samples were measured with particle-induced X-ray emission (PIXE) technique at the INFN LABEC ion beam laboratory in Florence (Chiari et al. 2021), which is located a 3 MV Tandetron accelerator. A 3.00 MeV proton beam was used, extracted into atmosphere through a 200-nm-thick Si3N4 membrane. Beam size was defined by a 1.0-mm-diameter collimator, placed in vacuum before the extraction window. The tree core samples were positioned roughly at 1 cm from the beam exit window (the proton beam energy on the sample surface was 2.95 MeV considering the energy loss in the Si3N4 window and the path in external atmosphere) and moved by micrometric stepper motors to allow aiming at a specific tree ring. Beam current intensity, indirectly measured by means of a rotating chopper (Chiari et al. 2002), varied between 3 and 4 nA (to keep dead time at few percent at most) and the measurements lasted 300 s each. Two X-ray detectors were used for PIXE measurements, a 10 mm2 (collimated to 3.5 mm2) Silicon Drift Detector (SDD), 4.5 cm from sample with He flow in front in order to reduce the absorption of lower energy X-rays in air, for light elements (Na–Ca) analysis, and a 150 mm2 SDD, 2 cm from sample, with 450 μm Mylar absorber, for heavy (> Ca) and trace elements. The collected PIXE spectra were then analyzed with the GUPIXWin software package (Campbell et al. 2010) using the trace element solution option, applying an instrumental parameter obtained by the analysis of X-ray spectra of external reference standards (NIST 1412 and NIST 610). The analysis of tree rings was performed in latewood, more uniform than earlywood in oak (Perone et al. 2018), and in one ring every four to obtain a consistent signal and check it within the intra-ring variability (Ballikaya et al. 2023) by collecting data from 1988 to 2020. The analysis defined trace element concentrations in earlywood of tree rings formed in 1988, 1992, 1996, 2000, 2004, 2008, 2012, 2016, and 2020.
The trace element concentrations to be compared within sampling sites were normalized, using the following equation:
where valuex refers to the level of a specific year, and valuelowest and valuehighest refer to the lowest and the highest element concentrations, respectively, measured in tree rings of each site.
To determine trace elements in lichens, samples of X. parietina and E. prunastri were frozen, powdered and homogenized by grinding in a mill with Teflon balls. Concerning E. prunastri, transplants (three samples, each composed by 300 mg of powdered lichen material, for the control and each site) were analyzed in the University of Siena. Samples were mineralized with a mixture of 3 mL of 70% HNO3, 0.2 mL of 60% HF, and 0.5 mL of 30% H2O2. Digestion of samples was carried out in a microwave digestion system (Milestone Ethos 900) for a total time of 30 min. Concentrations of trace elements (Ba, Ce, Co, Cr, Cu, Dy, Fe, Ga, I, Mn, Nb, Ni, Pb, Pr, Rb, Sr, Tb, Tl, U, V, Zn, Zr), expressed on a dry weight basis, were determined by inductively coupled plasma mass spectrometry (ICP-MS, Perkin-Elmer Sciex Elan 6100) using the “Total Quant” method with both standard (STD) and KED (kinetic energy dispersion) operating modes. This method was chosen in order to have the widest possible overview of the trace elements present in the study area at the time of the research. One procedural blank and one sample of the certified material GBW-07604 “Poplar leaves” were also analyzed.
The values of bioaccumulation in lichens, namely, the ratio between species-specific element concentration values in (i) native samples of X. parietina and the corresponding background element concentration values (B-Ratio) and (ii) exposed E. prunastri samples and the corresponding element concentration values measured in unexposed samples (EU-Ratio) was calculated.
The interpretation of the ratios followed ISPRA Guidelines (Giordani et al. 2020). The attribution of a sampling site i to a class of the bioaccumulation scale (i.e., absence of bioaccumulation, low bioaccumulation, moderate bioaccumulation, high bioaccumulation, severe bioaccumulation) has to be performed on the basis of the mean value of the B ratio or EU ratio diminished by its uncertainty (e.g., for transplants: EU(i) − Δ(EU(i)).
Chlorophyll a fluorescence measurements
Lichen vitality was assessed in thalli of the lichen E. prunastri before and after a 12-week exposure in the study area by measuring the chlorophyll a fluorescence emission with a plant efficiency analyzer fluorimeter (Hand PEA, Hansatech, Norfolk, UK). The maximum quantum yield of primary photochemistry calculated as Fv/Fm = (Fm – F0)/Fm, where F0 and Fm are minimum and maximum chlorophyll a fluorescence and Fv = (Fm − F0) is the variable fluorescence, and the photosynthetic performance PIABS, a global indicator of the photosynthetic performance, were measured (Strasser et al. 2004). The fluorescence parameters were determined in hydrated thalli (five measurements for each of three samples for a total of 15 measurements for the control and each site), after 10 min of dark adaptation, applying a saturating flash of light of 2400 µmol s−1 m−2 for 1 s.
Statistical analysis
Descriptive statistics (means, standard errors) were calculated for all the measured trace elements in tree rings and lichens. One-way ANOVA with post hoc Tukey HSD test was applied to test the effect of site in environmental signal. Time series of the trace elements were analyzed through the Kruskal–Wallis test to test significant differences between the index levels of elements over time in relation to concentration in years. To assess the relationships among trends in different elements and investigate spatial pollution patterns, principal component analyses (PCA) were applied to trace elements. PCA is an ordination technique to project onto several dimensions, generally two, defined by the axes of maximal variance (Hammer and Harper 2006). The principal components with eigenvalues greater than 1.0 were retained. Statistical analyses were performed using OriginPro 8 program (OriginLab Corporation, Northampton, UK).
Results
Tree rings
Mean tree-ring chronologies ranged from 1988, 1986, 1978, and 1988 to 2020 in Ghigiano, Semonte, forest, and urban sites, respectively. The mean tree-ring width was 3.42 mm (± 0.15 mm) (Table 1). Cross-dating between site-mean chronologies showed a Gleichläufigkeit value of 67 (p < 0.05) (Table 1).
Trace elements detected in the tree rings by PIXE analysis were Al, Br, Ca, Cl, Co, Cr, Cu, Fe, K, Mg, Mn, Na, Ni, P, Pb, Rb, S, Si, Sr, Ti, V, Zn, and Zr (Table 2). Concentrations of Al, Mg, Mn, and P resulted to be differently distributed within measured tree rings and sampling sites (Table 2). The ordination diagram (I and II components), resulting from PCA applied to biomonitors (wood), was grouped in four sampling sites (Ghigiano, Semonte, urban, and forest sites) for the measured tree rings (Fig. 2). The PCA defined an element ordination in tree rings of Q. pubescens in Ghigiano, Semonte, and urban sites in 2012 that was not observed in the forest site (Fig. 2). The ordination PCA defined 23 PCs: the first component accounted for over 31% and the second for over 16%; two grouping of elements were obtained: the first group of elements was constituted by Al, Ca, Cu, Mg, Na, P, S, Si, Sr, and Zn and the second group by Br, Cl, Cr, Fe, K, Mn, Ni, Pb, Rb, Ti, V, and Zr (Figure S1). Moreover, the ordination PCA allowed to define representative elements in tree rings of Q. pubescens for each sampling site: Ghigiano site (Br, Ca, Cl, Cr, Cu, Mg, Ni, P, Pb, Rb, V, Zn and Zr), Semonte site (Al, Br, Cl, Cu, Fe, K, Mg, Mn, Na, P, Si, Ti and Zn), urban site (Al, Ca, Cr, Cu, Fe, Mg, Mn, Ni, P, S, Sr, Ti, and Zn), and forest site (Br, Cl, Cu, Fe, K, Mg, Mn, Na, P, Pb, Rb, Ti, V, Zn, and Zr) (Fig. S1). Therefore, elements were identified as differently distributed between sampling sites, except for Cu, Mg, P, and Zn which were found in all sites.
Biplot diagrams from the principal component analysis of elements detected in tree rings of Q. pubescens on spatial (Ghigiano, Semonte, forest, and urban sites) and temporal scale (1988, 1992, 1996, 2000, 2004, 2008, 2012, 2016, 2020). The biplot in the first and second component planes is shown with the elements (dots) and parameters (vectors) measured. Each dot indicates the average for the three samples
By observing the common elements (Cu, Mg, P, Zn) found in four sites, the highest normalized concentrations were found in 2012 in Ghigiano, Semonte, and urban sites (Fig. 3). A common high levels of elements were in 2020 in four sites, while Cu and Zn resulted higher in 1992 and 1996, respectively, in forest sites than others (Fig. 3).
Heat map resuming the representative elements (Cu, Mg, P, Zn, the common elements detected in four sites) in tree rings of Q. pubescens for each sampling site, Ghigiano, Semonte, urban, and forest sites, obtained by PCA elements grouping (Fig. S1). Values are mean normalized data of element concentrations in tree-rings (standard error is < 0.1). The highest values are represented by dark red, whereas the lowest values are represented by dark blue
Native lichens
The analysis of X. parietina samples revealed the presence of Cr, Cu, Fe, Mn, Ni, and Zn in the lichen thalli, with a low level of bioaccumulation of Cr in the industrial sites and of Zn in the urban site (Table 3). The Ghigiano site exhibited the highest concentration of Cr, which was 1.4 times higher than the concentration observed at the forest site, along with elevated levels of Fe. At the Semonte site, the highest concentrations of Mn and Ni were recorded, while the forest site showed the highest levels of Cu and Zn.
Lichens transplants
After 12 weeks of exposure to the environmental conditions of the sampling sites, all elements presented a certain degree of bioaccumulation at least in one sampling site, except for Dy and Rb (Table 4). Mostly, the elements showed a low level of bioaccumulation while a moderate bioaccumulation was found for Ce and Fe in Ghigiano, Zr in the forest site, Pr in Ghigiano and the urban sites, and U and V in Semonte and the urban site (Table 4). High bioaccumulation of V was observed only at the Ghigiano site, while severe bioaccumulation of Tl, the main air pollutant in the area among the considered elements, was found in both industrial sampling sites of Ghigiano and Semonte (Table 4).
Photosynthetic efficiency of lichen transplants
After 12 weeks of exposure to the environmental conditions of the sampling sites, the physiological parameter of E. prunastri were lower than the control in all sites. However, Fv/Fm showed a statistically significant decrease with respect to the control value only in the urban site (− 17%, p level = 0.012) while the PIABS was significantly lower in the industrial and urban sites (up to − 29% with respect to the control) but not in the forest site (Fig. 4).
Physiological parameters of E. prunastri, Fv/FM, and PI (N = 15; mean ± standard deviation), in control samples and sampling sites after 12 weeks of exposure: Ghigiano, Semonte, urban, and forest sites. One-way ANOVA defines the significance of the effects of the site. Different letters indicate statistical differences between sites at P < 0.05
Insects
The abundance of specimens, species, and threatened categories for each site was identified (Table 2). All the taxa collected during the field activities are alphabetically listed in Table 5 together with the proportion of the collected species, grouped according to the prevalent trophic categories. We collected 478 specimens belonging to 57 species referring to 23 families of Coleoptera (Table 5). Species strictly considered as saproxylic (sensu Carpaneto et al. 2015) are also reported in Table 5, together with their risk category at the Italian level (see Carpaneto et al. 2015). The beetle assemblages were grouped according to the prevalent trophic categories, defined by Carpaneto et al. (2015). The most abundant species were Cetonia aurata and Protaetia morio (Cetoniidae), respectively, with 94 and 80 specimens. Regarding the IUCN risk categories, the sampled saproxylic beetles were classified as follows: near threatened (NT; 3 species) and least concern (LC; 18 species). Xylophagous and saproxylophagous accounted for 5.7% of the total sampled species, followed by Sap-feeder (4%), predators (2.28%), and only two specimens of mycophagous were collected.
Discussion
Environmental signal by trees
The study revealed distinct patterns of trace element accumulation in tree rings across time and space. The detection of trace elements in tree rings provided insight into the availability of several elements (Al, Br, Ca, Cl, Co, Cr, Cu, Fe, K, Mg, Mn, Na, Ni, P, Pb, Rb, S, Si, Sr, Ti, V, Zn, and Zr) in the environment, information that was not previously available. The variability in tree ring composition over time allowed the assessment of temporal patterns of pollutant accumulation in different sampling sites. The bioaccumulation of elements in tree rings presents some challenges in pinpointing the exact source, as the production of certain pollutants cannot be attributed exclusively to specific anthropogenic activities. However, compelling evidence of trace elements in tree rings was found in relation to the industrial activity of the study area. Chlorine and Br were detected in both the Ghigiano and Semonte sites. Chlorine is known as a potentially harmful element typically derived by the combustion of waste-derived fuel (e.g., Gerassimidou et al. 2021), while Br is used as halogen additives to reduce mercury emissions (UNEP 2011). In the Ghigiano site, relevant concentrations of Cr, Cu, Pb, Rb, and V were found, indicating the area as a potential source of heavy metals and metalloids, a major threat to environmental and human health (Fan et al. 2021). In the Semonte site, the presence of Cu, Fe, Mg, Mn, Si, Tl, and Zn confirmed the existence of a contamination chain like heavy metals, metalloids, and “”non-metals, usually released from industries into the atmosphere, soil, water, and contaminating food (Pourret et al. 2021). Signals were detected in tree rings in 2012 in industrial and urban sites by highlighting the pollution signals characteristic of antropizated ecosystems, deriving from anthropogenic contamination and traffic emission (Bibi et al. 2023). On the other hand, the forest site showed elements characteristics with different time frame. The common element accumulation in tree rings of 2020 on four sites might be attributed to various production processes, including combustion in manufacturing industries, energy and transformation industries, and residential combustion (Capon and de Saulles 2023), suggesting a process of elements’ diffusion. However, it is worth noting that extremely toxic and hazardous metals like As and Hg (Index 2018) were not found in the tree rings in any of the sampling sites in Gubbio.
Environmental signal by lichens
The dual approach used for lichens provided information pertaining to two distinct time spans: 1 year, approximately corresponding to 2020—first months of 2021, in the case of native samples collected in situ and a shorter subsequent period (spring 2021) in the case of the transplants.
The analysis of thalli of X. parietina revealed enrichment of Cr in both industrial sites, which has been associated with the impact of cement production in previous studies (Paoli et al. 2017). An enrichment in Zn was found in the urban and forest sites and the latter also showed the highest concentration of Cu. The occurrence of these elements can be attributed to agricultural practices, primarily linked to the olive groves surrounding the forest site. Indeed, insecticides and fungicides often contain Cu and Zn, like the Bordeaux mixture (CuSO4) and the Mancozeb® (Zn) (National Center for Biotechnology Information 2017).
When examining the medium-term contamination of the study area, it is important to consider the significant reduction in air pollution that occurred in 2020 resulting of the widespread lockdown measures implemented to curb the spread of the coronavirus infection (Donzelli et al. 2020; Ravina et al. 2021). This reduction in air pollution could be a contributing factor to the relatively low levels of trace element bioaccumulation (e.g., Tl which is instead highly accumulated in lichen transplants) observed in the lichen material corresponding to this period. However, it was not observed in tree rings perhaps due to the selectivity of element absorption regulated by root, for example.
The analysis of the transplants identified Tl among the measured elements, as the primary contributing factors to atmospheric contamination in the study area. These findings agree with similar studies carried out around cement factories (Demiray et al. 2012; Gallo et al. 2014; Paoli et al. 2014, 2017). In fact, Tl can be present as an impurity in raw materials, and its compounds are volatile at high temperatures (ATSDR 1992) making cement factories significant anthropogenic sources of this element (IPCS 1996). Vanadium appears to be predominantly associated with combustion activities that involve heavy petroleum products, e.g., petcoke used as main fuel by the two cement factories (as reported in their 2021 Environmental Performance Indicators). Although it is possible that residential combustion contributed to some extent to atmospheric contamination in the urban site, previous studies have shown that high concentrations of V are primarily linked to the use of petcoke as fuel in plant factories (e.g., Gallo et al. 2014), and V and Tl have been identified as reliable markers of cement factory activity. In addition, among the trace elements bioaccumulated by transplants, a few lanthanoids (Ce, Pr, Tb) have been also detected. The accumulation of lanthanoids in mosses and lichens is commonly attributed to the deposition of soil dust (Wu et al. 2020), along with Fe. The presence of Zr, a traffic-related element (Lyubomirova et al. 2011), and U in the study area is likely attributed to the same origin, namely, the resuspension of soil particles from the many dirty roads in the surroundings. The presence of U is explained by the natural radioactivity of the study area (data from Regione Umbria, available at: https://www.regione.umbria.it/documents/18/20164312/radioattivita+naturale/086b8f8b-82ec-476a-8894-66eb5f013d87?t=1586461453107). The effect of a high-traffic road near the industrial site of Semonte is suggested by the higher concentrations of Mn and Zn, in agreement with similar studies carried out in urban areas (Paoli et al. 2013; Wu et al. 2020).
Transplanted lichen thalli were still alive at the end of the exposure period, confirming the ongoing functionality of the active component within the bioaccumulation process.
A decrease in fluorescence parameters is a non-specific physiological response to stress factors. In our study, this alteration can be likely attributed to the increased availability of pollutants since the samples transplanted to the forest site did not show significant difference from the control samples. A comparable reduction of the chlorophyll a fluorescence emission in transplants of E. prunastri has been recorded in the center of Milan (Contardo et al. 2021).
Environmental signal by beetles
The beetle community was not characterized to demonstrate environmental pollution. Beetles were characterized by wood-related populations and the presence of threatened species in urban environments (e.g., Forister et al. 2019; Kotze et al. 2022; Kitahara and Fujii 1997). Usually, these species are very sensitive to disturbances and can be valid indicators of environmental health. The beetle community sampled at the four sites showed differences both in terms of specimens and species collected. In particular, the Semonte and Ghigiano sites included most of the specimens collected. The Semonte site was integrated into an agroecosystem with oak trees arranged along a road and agricultural fields. The abundance of specimens attributable to a few species is typical of over-anthropized ecosystems where human activities have an impact on biodiversity levels (Chowdhury et al. 2023). As it is known in the scientific literature, natural environments that are little subject to human activities present constant levels of biodiversity. In fact, in the forest site, the relationship between the number of beetle species and trophic categories (i.e., predators and prey) seems to be in balance (see Table 5). A similar relationship, between predators and prey, was observed in the Ghigiano site (abandoned oak forest) and in the urban site. In the latter, the trees in the site (mainly holm oaks) probably favored a good presence of species despite the urban park management activities (Sabatelli et al. 2023
Conclusions
Combined biomonitoring generally might offer several advantages in environmental assessment and monitoring compared to single-taxon biomonitoring. Different species have varying sensitivities and ecological roles, making them indicators of diverse aspects of environmental quality. Furthermore, the use of different organisms helps overcome the limitations of relying solely on a single group, which might not be present in the entire study area. This work highlights how combining the response of taxonomic groups with different life cycles can provide information about environmental conditions across various time spans or at specific moments. In a complex environment like the study area, including several ecosystems and pollution sources, this approach provides a more comprehensive and nuanced understanding of environmental conditions and changes. The sampling design highlighted the different effects of anthropized ecosystems to element diffusion in the environment. In detail, the sampling in forest sites resulted necessary to isolate signal of industrial and urban activities. Integrating data from various biomonitoring approaches, despite discrepancies, offers a holistic understanding of environmental changes, identifies pollution sources, and informs conservation and management efforts. Additionally, such studies should focus on providing data for mapping and visualizing biomonitoring, aiding in raising awareness, guiding land management decisions, and supporting conservation strategies. However, to be effective, this combined approach requires support from multiple case studies, and consistent and numerous sampling sites are essential.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abas A (2021) A systematic review on biomonitoring using lichen as the biological indicator: a decade of practices, progress and challenges. Ecol Indic 121:107197
Anderson J, Lévesque N, Caron F, Beckett P, Spiers GA (2022) A review on the use of lichens as a biomonitoring tool for environmental radioactivity. J Environ Radioact 243:106797. https://doi.org/10.1016/j.jenvrad.2021.106797
ATSDR (1992) Toxicological profile for thallium. Agency for Toxic Substances and Disease Registry, Atlanta, Georgia, pp 1–90. (ATSDR/TP-91/26). https://www.inchem.org/documents/ehc/ehc/ehc182.htm
Audisio P, Alonso Zarazaga M, Slipinski A, Nilsson A, Jelínek J, Taglianti A, Turco F, Otero C, Canepari C, Kral D, Liberti G, Sama G, Nardi G, Löbl I, Horak J, Kolibac J, Háva J, Sapiejewski M, Jäch M, Bologna M, Biondi M, Nikitsky N, Mazzoldi P, Zahradnik P, Wegrzynowicz P, Constantin R, Gerstmeier R, Zhantiev R, Fattorini S, Tomaszewska W, Rücker W, Vazquez-Albalate X, Cassola F, Angelini F, Johnson C, Schawaller W, Regalin R, Baviera C, Rocchi S, Cianferoni F, Beenen R, Schmitt M, Sassi D, Kippenberg H, Zampetti M, Trizzino M, Chiari S, Carpaneto G, Sabatelli S, de Jong Y (2015) Fauna Europaea: Coleoptera 2 (excl. series Elateriformia, Scarabaeiformia, Staphyliniformia and superfamily Curculionoidea). Biodivers Data J 3:e4750
Ballikaya P, Marshall J, Cherubini P (2022) Can tree-ring chemistry be used to monitor atmospheric nanoparticle contamination over time? Atmos Environ 268:118781
Ballikaya P, Song W, Bachmann O, Guillong M, Wang X, Cherubini P (2023) Chemical elements recorded by Quercus mongolica Fisch ex Ledeb. tree rings reveal trends of pollution history in Harbin, China. Forests 14:187
Baroni D, Ancora S, Franzaring J, Loppi S, Monaci F (2023) Tree-rings analysis to reconstruct atmospheric mercury contamination at a historical mining site. Front Plant Sci 14:1260431
Bibi D, Tőzsér D, Sipos B, Tóthmérész B, Simon E (2023) Heavy metal pollution of soil in Vienna, Austria. Water Air Soil Pollut 234:232
Binda G, Di Iorio A, Monticelli D (2021) The what, how, why, and when of dendrochemistry:(paleo) environmental information from the chemical analysis of tree rings. Sci Total Environ 758:143672. https://doi.org/10.1016/j.scitotenv.2020.143672
Bouchard P, Bousquet Y, Davies AE, Alonso-Zarazaga MA, Lawrence JF, Lyal CHC, Newton AF, Reid CAM, Schmitt M, Slipinski SA, Smith ABT (2011) Family-group names in Coleoptera (Insecta). ZooKeys 88:1–972
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6:e04691
Burrascano S, Trentanovi G, Paillet Y, Heilmann-Clausen J, Giordani P, Bagella S, Bravo-Oviedo A, Campagnaro T, Campanaro A, Chianucci F, De Smedt P, Garcia-Mijangos I, Matošević D, Sitzia T, Aszalós R, Brazaitis G, Cutini A, D’Andrea E, Doerfler I, Hofmeister J, Hošek J, Janssen P, Rojas SK, Korboulewsky N, Kozák D, Lachat T, Lõhmus A, Lopez R, Mårell A, Matula R, Mikoláš M, Munzi S, Nordén B, Pärtel M, Penner J, Runnel K, Schall P, Svoboda M, Tinya F, Ujházyová M, Vandekerkhove K, Verheyen K, Xystrakis F, Ódor P (2021) Handbook of field sampling for multi-taxon biodiversity studies in European forests. Ecol Indic 132:108266
Campbell JL, Boyd NI, Grassi N, Bonnick P, Maxwell JA (2010) The Guelph PIXE software package IV. Nucl Instrum Methods Phys Res, Sect B 268:3356–3363
Capon R, de Saulles T (2023) K GHG inventory improvement: carbonation of concrete emissions sink modelling. Uk Air, Department for Environment Food & Rural Affairs. https://uk-air.defra.gov.uk/library/reports?report_id=1114
Carpaneto GM, Baviera C, Biscaccianti AB, Brandmayr P, Mazzei A, Mason F, Battistoni A, Teofili C, Rondinini C, Fattorini S, Audisio P (2015) A red list of Italian saproxylic beetles: taxonomic overview, ecological features and conservation issues (Coleoptera). Fragem Entomol 47:53–126
Chiari M, Migliori A, Mandò PA (2002) Measurement of low currents in an external beam set-up. Nucl Instrum Methods Phys Res, Sect B 188:162–165
Chiari M, Barone S, Bombini A, Calzolai G, Carraresi L, Castelli L, Czelusniak C, Fedi ME, Gelli N, Giambi F, Giardi F, Giuntini L, Lagomarsino S, Liccioli L, Lucarelli F, Manetti M, Massi M, Mazzinghi A, Nava S, Ottanelli P, Sciortino S, Ruberto C, Sodi L, Taccetti F, Mandò PA (2021) LABEC, the INFN ion beam laboratory of nuclear techniques for environment and cultural heritage. Eur Phys J plus 136:472
Chowdhury S, Ahmed S, Alam S, Callaghan CT, Das P, Di Marco M, Di Minin E, Jarić I, Labi MM, Rokonuzzaman M, Roll U, Sbragaglia V, Siddika A, Bonn A (2023) A standard protocol for harvesting biodiversity data from Facebook. EcoEvoRxiv. https://doi.org/10.32942/X2XS4F
Cocozza C, Ravera S, Cherubini P, Lombardi F, Marchetti M, Tognetti R (2016) Integrated biomonitoring of airborne pollutants over space and time using tree rings, bark, leaves and epiphytic lichens. Urban for Urban Green 17:177–191
Cocozza C, Alterio E, Bachmann O, Guillong M, Sitzia T, Cherubini P (2021) Monitoring air pollution close to a cement plant and in a multi-source industrial area through tree-ring analysis. Environ Sci Pollut Res 28:54030–54040
Contardo T, Gazzotti S, Ortenzi MA, Vannini A, Loppi S (2021) Biological effects of air pollution on sensitive bioindicators: a case study from Milan. Italy Urban Sci 5:64
Costa C, Teixeira JP (2014) Biomonitoring. Encyclopedia of toxicology, 3rd edn. pp 483–484. https://repositorio.insa.pt/handle/10400.18/2761
De Marco A, Proietti C, Anav A, Ciancarella L, D’Elia I, Fares S, Fornasier MF, Fusaro L, Gualtieri M, Manes F, Marchetto A, Mircea M, Paoletti E, Piersanti A, Rogora M, Salvati L, Salvatori E, Screpanti A, Vialetto G, Vitale M, Leonardi C (2019) Impacts of air pollution on human and ecosystem health, and implications for the National Emission Ceilings Directive: Insights from Italy. Environ Int 125:320–333
Demiray AD, Yolcubal I, Akyol NK, Çobanoğlu G (2012) Biomonitoring of airborne metals using the Lichen Xanthoria parietina in Kocaeli Province, Turkey. Ecol Ind 18:632–643
Donzelli G, Cioni L, Cancellieri M, Llopis Morales A, Morales Suárez-Varela MM (2020) The effect of the Covid-19 lockdown on air quality in three Italian medium-sized cities. Atmosphere 11(10):1118
EEA (European Environment Agency) (2018) Air quality in Europe. Report N° 12:83. https://doi.org/10.2800/777411
Fan X, Lu X, Yu B, Zuo L, Fan P, Yang Y, Zhuang S, Liu H, Qin Q (2021) Risk and sources of heavy metals and metalloids in dust from university campuses: a case study of Xi’an. China Environ Res 202:111703
Forister ML, Pelton EM, Black SH (2019) Declines in insect abundance and diversity: we know enough to act now. Conserv Sci Pract 1(8):e80
Gallo L, Corapi A, Loppi S, Lucadamo L (2014) Element concentrations in the lichen Pseudevernia furfuracea (L.) Zopf transplanted around a cement factory (S Italy). Ecol Ind 46:566–574
Gärtner H, Nievergelt D (2010) The core-microtome: a new tool for surface preparation on cores and time series analysis of varying cell parameters. Dendrochronologia 28:85–92
Gerassimidou S, Velis CA, Williams PT, Castaldi MJ, Black L, Komilis D (2021) Chlorine in waste-derived solid recovered fuel (SRF), co-combusted in cement kilns: a systematic review of sources, reactions, fate and implications. Crit Rev Environ Sci Technol 51(2):140–186
Giordani P et al (2020) Guidelines for the use of lichens as bioaccumulators. ISPRA, Manuali e linee guida 189/2019. ISBN: 978–88–448–0966–9. https://www.isprambiente.gov.it/en/publications/handbooks-and-guidelines/guidelines-for-use-of-lichens-as-bioindicators-1. Accessed 25 June 2024
Gutiérrez M, Molero R, Gaju M, van der Steen J, Porrini C, Ruiz JA (2020) Assessing heavy metal pollution by biomonitoring honeybee nectar in Córdoba (Spain). Environ Sci Poll Res 27(10):10436–10448
Hammer Ø, Harper DAT (2006) Palaeontological data analysis. Blackwell, Cambridge, p p351
Holland M, Wagner A, Davies T, Spadaro J, Adams M (2011) Revealing the costs of air pollution from industrial facilities in Europe. EEA Technical report No 15/2011. https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.eea.europa.eu/publications/cost-of-air-pollution/revealing-the-costs-of-air&ved=2ahUKEwjHgve9xPaGAxUsGBAIHVW5C04QFnoECBMQAQ&usg=AOvVaw1wVGcBnDwAzN64lfXeJEl6
Index EP (2018) EPI report. Yale University. https://epi.envirocenter.yale.edu/2018-epi-report/ introduction. Accessed 17 Oct 2018.
IPCS (1996) Thallium International Program on Chemical Safety, Environmental Health Criteria: 182, WHO, Geneva. https://inchem.org/documents/ehc/ehc/ehc182.htm. Accessed 25 June 2024
Kitahara M, Fujii K (1997) An island biogeographical approach to the analysis of butterfly community patterns in newly designed parks. Res Popul Ecol 39:23–35
Kotze DJ, Lowe EC, MacIvor JS, Ossola A, Norton BA, Hochuli DF, Mata L, Moretti M, Gagné SA, Handa IT, Jones TM, Threlfall CG, Hahs AK (2022) Urban forest invertebrates: how they shape and respond to the urban environment. Urban Ecosystems 25(6):1589–1609
Loppi S, Ravera S, Paoli L (2019) Coping with uncertainty in the assessment of atmospheric pollution with lichen transplants. Environ Forensics 20(3):228–233
Lyubomirova V, Djingova R, van Elteren JT (2011) Fractionation of traffic-emitted Ce, La and Zr in road dusts. J Environ Monit 13(6):1823–1830
Malmstrom C (2010) Ecologists study the interactions of organisms and their environment. Nat Educ Knowl 3(10):88
National Center for Biotechnology Information (2017) PubChem Compound database; CID=3034368 [WWW Document]. URL https://pubchem.ncbi.nlm.nih.gov/compound/3034368. Accessed 25 June 2024
Paoli L, Munzi S, Fiorini E, Gaggi C, Loppi S (2013) Influence of angular exposure and proximity to vehicular traffic on the diversity of epiphytic lichens and the bioaccumulation of traffic-related elements. Environ Sci Pollut Res 20(1):250–259
Paoli L, Guttová L, Grassi A, Lackovičová A, Senko D, Loppi S (2014) Biological effects of airborne pollutants released during cement production assessed with lichens (SW Slovakia). Ecol Ind 40:127–135
Paoli L, Winkler A, Guttová A, Sagnotti L, Grassi A, Lackovičová A, Senko D, Loppi S (2017) Magnetic properties and element concentrations in lichens exposed to airborne pollutants released during cement production. Environ Sci Pollut Res 24:12063–12080
Parisi F, Pioli S, Lombardi F, Fravolini G, Marchetti M, Tognetti R (2018) Linking deadwood traits with saproxylic invertebrates and fungi in European forests – a review. iForest 11:423–436
Perone A, Cocozza C, Cherubini P, Bachmann O, Guillong M, Lasserre B, Marchetti M, Tognetti R (2018) Oak tree-rings record spatial-temporal pollution trends from different sources in Terni (Central Italy). Environ Poll 233:278–289
Pinho P, Correia O, Lecoq M, Munzi S, Vasconcelos S, Gonçalves P, Rebelo R, Antunes C, Silva P, Freitas C, Lopes N, Santos-Reis M, Branquinho C (2016) Evaluating green infrastructure in urban environments using a multi-taxa and functional diversity approach. Environ Res 147:601–610
Pourret O, Bollinger JC, Hursthouse A (2021) Heavy metal: a misused term? Acta Geochim 40(3):466–471
Ravina M, Esfandabadi ZS, Panepinto D, Zanetti M (2021) Traffic-induced atmospheric pollution during the COVID-19 lockdown: Dispersion modeling based on traffic flow monitoring in Turin, Italy. J Clean Prod 317:128425. https://doi.org/10.1016/j.jclepro.2021.128425. (ISSN 0959-6526)
Rinn F (1996) TSAP-Win. Time series analysis and presentation for dendrochronology and 409 related applications
Sabatelli S, Parisi F, Vangi E, Francini S, D’Amico G, Chirici G, Travaglini D, Rignanese D, Muambalo K, Audisio P (2023) Relationship between tree-related microhabitats and the captures obtained using different trap methods for large threatened saproxylic beetles. REDIA 106:45–56
Schweingruber FH (1988) Tree rings: basics and applications of dendrochronology. Springer, Netherlands
Speer JH (2012) Fundamentals of tree-ring research. University of Arizona Press
Speight MCD (1989) Saproxylic invertebrates and their conservation. Nature and environment. Council of Europe, Strasbourg
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence; Springer: Berlin/Heidelberg, Germany, pp. 321–362.
Tretiach M, Carpanelli A (1992) Morphology and chlorophyll content as factors influencing the photosynthetic rates of Parmelia caperata (L.) Ach. Lichenologist 24:81–90
UNEP (2011) Process optimization guidance for reducing mercury emissions from coal combustion in power plants. Division of Technology, Industry and Economics (DTIE) Chemicals Branch, Geneva, Switzerland.https://wedocs.unep.org/bitstream/handle/20.500.11822/11418/POG_FINAL_2011_edited_07_Jan_2011.pdf?isAllowed=y&sequence=1. Accessed 25 June 2024
Vannini A, Paoli L, Nicolardi V, Di Lella LA, Loppi S (2017) Seasonal variations in intracellular trace element content and physiological parameters in the lichen Evernia prunastri transplanted to an urban environment. Acta Bot Croat 76(2):171e176
Vannini A, Paoli L, Russo A, Loppi S (2019) Contribution of submicronic (PM1) and coarse (PM> 1) particulate matter deposition to the heavy metal load of lichens transplanted along a busy road. Chemosphere 231:121–125
World Health Organization (2017) Economic cost of the health impact of air pollution in Europe: clean air, health and wealth. World Health Organization, Copenhagen
Yy Wu, Gao J, Zhang GZ, Zhao RK, Liu AQ, Sun LW, Li X, Tang HL, Zhao LC, Guo XP, Liu HJ (2020) Two lichens differing in element concentrations have similar spatial patterns of element concentrations responding to road traffic and soil input. Sci Rep 10:19001
Acknowledgements
Thanks are extended to Susanna Pucci for laboratory analysis.
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement. This work has been funded by a private Committee “Comitato NO CSS nelle cementerie di Gubbio.” SM received the grant UIBD/00329/2020 for the Centre for Ecology, Evolution and Environmental Changes (cE3c) from FCT (Foundation for Science and Technology).
Author information
Authors and Affiliations
Contributions
Claudia Cocozza, Francesco Parisi, and Sonia Ravera contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by all authors. The first draft of the manuscript was written by Claudia Cocozza, Francesco Parisi, and Sonia Ravera, and all authors commented on previous versions of the manuscript. Claudia Cocozza, Francesco Parisi, Sonia Ravera, Massimo Chiari, Stefano Loppi, and Silvana Munzi have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The researchers sought informed consent of all participants prior to recruitment prior to recruitment for data collection.
Consent for publication
The explicit consent for publication was also obtained from participants.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cocozza, C., Parisi, F., Chiari, M. et al. Integration of ecological indicators to assess a multitemporal impact of cement industries. Environ Sci Pollut Res 31, 48233–48249 (2024). https://doi.org/10.1007/s11356-024-34079-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-34079-y