Abstract
2,4-Dichlorophenoxyacetic acid (2,4-D) is an herbicide and is among the most widely distributed pollutant in the environment and wastewater. Herein is presented a complete comparison of adsorption performance between two different magnetic carbon nanomaterials: graphene oxide (GO) and its reduced form (rGO). Magnetic functionalization was performed employing a coprecipitation method, using only one source of Fe2+, requiring low energy, and potentially allowing the control of the amount of incorporated magnetite. For the first time in literature, a green reduction approach for GO with and without Fe3O4, maintaining the magnetic behavior after the reaction, and an adsorption performance comparison between both carbon nanomaterials are demonstrated. The nanoadsorbents were characterized by FTIR, XRD, Raman, VSM, XPS, and SEM analyses, which demonstrates the successful synthesis of graphene derivate, with different amounts of incorporate magnetite, resulting in distinct magnetization values. The reduction was confirmed by XPS and FTIR techniques. The type of adsorbent reveals that the amount of magnetite on nanomaterial surfaces has significant influence on adsorption capacity and removal efficiency. The procedure demonstrated that the best performance, for magnetic nanocomposites, was obtained by GO∙Fe3O4 1:1 and rGO∙Fe3O4 1:1, presenting values of removal percentage of 70.49 and 91.19%, respectively. The highest adsorption capacity was reached at pH 2.0 for GO∙Fe3O4 1:1 (69.98 mg g−1) and rGO∙Fe3O4 1:1 (89.27 mg g−1), through different interactions: π-π, cation-π, and hydrogen bonds. The adsorption phenomenon exhibited a high dependence on pH, initial concentration of adsorbate, and coexisting ions. Sips and PSO models demonstrate the best adjustment for experimental data, suggesting a heterogeneous surface and different energy sites, respectively. The thermodynamic parameters showed that the process was spontaneous and exothermic. Finally, the nanoadsorbents demonstrated a high efficiency in 2,4-D adsorption even after five adsorption/desorption cycles.
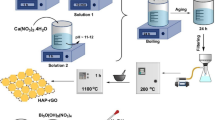
Graphical Abstract















Similar content being viewed by others
Data availability
Data will be made available on request.
References
Alayli A, Nadaroglu H, Turgut E (2021) Nanobiocatalyst beds with Fenton process for removal of methylene blue. Appl Water Sci 11. https://doi.org/10.1007/s13201-021-01367-8
Al-Gaashani R, Najjar A, Zakaria Y et al (2019) XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram Int 45:14439–14448. https://doi.org/10.1016/j.ceramint.2019.04.165
Al-Ghouti MA, Da’ana DA (2020) Guidelines for the use and interpretation of adsorption isotherm models: a review. J Hazard Mater 393:122383. https://doi.org/10.1016/j.jhazmat.2020.122383
Alshorifi FT, Alswat AA, Salama RS (2022) Gold-selenide quantum dots supported onto cesium ferrite nanocomposites for the efficient degradation of rhodamine B. Heliyon 8. https://doi.org/10.1016/j.heliyon.2022.e09652
Aparecida Matias C, Vilela PB, Becegato VA, Paulino AT (2019) Adsorption kinetic, isotherm and thermodynamic of 2,4-dichlorophenoxyacetic acid herbicide in novel alternative natural adsorbents. Water Air Soil Pollut 230:276. https://doi.org/10.1007/s11270-019-4324-5
Aunkor MTH, Mahbubul IM, Saidur R, Metselaar HSC (2016) The green reduction of graphene oxide. RSC Adv 6:27807–27828. https://doi.org/10.1039/C6RA03189G
Binh QA, Nguyen HH (2020) Investigation the isotherm and kinetics of adsorption mechanism of herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) on corn cob biochar. Bioresour Technol Rep 11. https://doi.org/10.1016/j.biteb.2020.100520
Björk J, Hanke F, Palma C-A et al (2010) Adsorption of aromatic and anti-aromatic systems on graphene through π−π stacking. J Phys Chem Lett 1:3407–3412. https://doi.org/10.1021/jz101360k
Bortolozzo LS, Côa F, Khan LU et al (2021) Mitigation of graphene oxide toxicity in C. elegans after chemical degradation with sodium hypochlorite. Chemosphere 278. https://doi.org/10.1016/j.chemosphere.2021.130421
Bruckmann FS, Rhoden CRB (2024) Applications of magnetic graphene oxide in water decontamination. Compr Anal Chem 104:1–17. https://doi.org/10.1016/bs.coac.2023.10.002
Chen H, Du W, Liu J et al (2019) Efficient room-temperature production of high-quality graphene by introducing removable oxygen functional groups to the precursor. Chem Sci 10:1244–1253. https://doi.org/10.1039/c8sc03695k
Cheng Y, Yang S, Tao E (2021) Magnetic graphene oxide prepared via ammonia coprecipitation method: the effects of preserved functional groups on adsorption property. Inorg Chem Commun 128. https://doi.org/10.1016/j.inoche.2021.108603
Cui S, Wang X, Zhang X et al (2018) Preparation of magnetic MnFe2O4-cellulose aerogel composite and its kinetics and thermodynamics of Cu(II) adsorption. Cellulose 25:735–751. https://doi.org/10.1007/s10570-017-1598-x
da Salles RT, de Rodrigues BH, da Bruckmann SF et al (2020) Graphene oxide optimization synthesis for application on laboratory of Universidade Franciscana. Disciplinarum Scientia - Ciências Naturais e Tecnológicas 21:15–26. https://doi.org/10.37779/nt.v21i3.3632
da Salles RT, Schnorr C, da Bruckmann SF et al (2023) Effective diuretic drug uptake employing magnetic carbon nanotubes derivatives: adsorption study and in vitro geno-cytotoxic assessment. Sep Purif Technol 315. https://doi.org/10.1016/j.seppur.2023.123713
da Silv Bruckmann BF, Viana AR, Lopes LQS et al (2022) Synthesis, characterization, and biological activity evaluation of magnetite-functionalized eugenol. J Inorg Organomet Polym Mater 32:1459–1472. https://doi.org/10.1007/s10904-021-02207-7
Davoodi S, Dahrazma B, Goudarzi N, Gorji HG (2019) Adsorptive removal of azithromycin from aqueous solutions using raw and saponin-modified nano diatomite. Water Sci Technol 80:939–949. https://doi.org/10.2166/wst.2019.337
de Castro Marcato AC, de Souza CP, Fontanetti CS (2017) Herbicide 2,4-D: a review of toxicity on non-target organisms. Water Air Soil Pollut 228. https://doi.org/10.1007/s11270-017-3301-0
de Oliveira ÉC et al (2022) In vitro and in vivo safety profile assessment of graphene oxide decorated with different concentrations of magnetite. J Nanoparticle Res 24:150. https://doi.org/10.1007/s11051-022-05529-w
De Silva KKH, Huang H-H, Joshi RK, Yoshimura M (2017) Chemical reduction of graphene oxide using green reductants. Carbon N Y 119:190–199. https://doi.org/10.1016/j.carbon.2017.04.025
Debebe Y, Alemayehu E, Worku Z et al (2023) Sorption of 2,4-dichlorophenoxyacetic acid from agricultural leachate using termite mound soil: optimization using response surface methodology. Water (Switzerland) 15. https://doi.org/10.3390/w15020327
Ebrahimian Pirbazari A, Saberikhah E, Badrouh M, Emami MS (2014) Alkali treated Foumanat tea waste as an efficient adsorbent for methylene blue adsorption from aqueous solution. Water Resour Ind 6:64–80. https://doi.org/10.1016/j.wri.2014.07.003
Esmaeili Y, Bidram E, Zarrabi A et al (2020) Graphene oxide and its derivatives as promising In-vitro bio-imaging platforms. Sci Rep 10. https://doi.org/10.1038/s41598-020-75090-w
Essandoh M, Wolgemuth D, Pittman CU et al (2017) Phenoxy herbicide removal from aqueous solutions using fast pyrolysis switchgrass biochar. Chemosphere 174:49–57. https://doi.org/10.1016/j.chemosphere.2017.01.105
Evy Alice Abigail M, Melvin Samuel S, Needhidasan S, Ramalingam C (2017) Stratagems employed for 2,4-dichlorophenoxyacetic acid removal from polluted water sources. Clean Technol Environ Policy 19:1607–1620
Fan S, Wang Y, Li Y et al (2018) Removal of tetracycline from aqueous solution by biochar derived from rice straw. Environ Sci Pollut Res 25:29529–29540. https://doi.org/10.1007/s11356-018-2976-0
Favela-Camacho SE, Samaniego-Benítez EJ, Godínez-García A et al (2019) How to decrease the agglomeration of magnetite nanoparticles and increase their stability using surface properties. Colloids Surf A Physicochem Eng Asp 574:29–35. https://doi.org/10.1016/j.colsurfa.2019.04.016
Feng J, Ye Y, Xiao M et al (2020) Synthetic routes of the reduced graphene oxide. Chem Pap 74:3767–3783. https://doi.org/10.1007/s11696-020-01196-0
Finkler M, Rodrigues GZP, Kayser JM et al (2022) Cytotoxic and genotoxic effects induced by associated commercial glyphosate and 2,4-D formulations using the Allium cepa bioassay. J Environ Sci Health B 57:133–141. https://doi.org/10.1080/03601234.2022.2034432
Franco D, Silva LFO, da Boit MK et al (2021) Transforming agricultural waste into adsorbent: application of Fagopyrum esculentum wheat husks treated with H2SO4 to adsorption of the 2,4-D herbicide. J Environ Chem Eng 9:106872. https://doi.org/10.1016/j.jece.2021.106872
Georgin J, Franco DSP et al (2023) Adsorption investigation of 2,4-D herbicide on acid-treated peanut (Arachis hypogaea) skins. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-12813-0/Published
Georgin J, Franco DSP, Schadeck Netto M et al (2021) Transforming shrub waste into a high-efficiency adsorbent: application of Physalis peruvian chalice treated with strong acid to remove the 2,4-dichlorophenoxyacetic acid herbicide. J Environ Chem Eng 9. https://doi.org/10.1016/j.jece.2020.104574
Goscianska J, Olejnik A (2019) Removal of 2,4-D herbicide from aqueous solution by aminosilane-grafted mesoporous carbons. Adsorption 25:345–355. https://doi.org/10.1007/s10450-019-00015-7
Gupta B, Kumar N, Panda K et al (2017) Role of oxygen functional groups in reduced graphene oxide for lubrication. Sci Rep 7:45030. https://doi.org/10.1038/srep45030
Harres A, Mikhov M, Skumryev V et al (2016) Criteria for saturated magnetization loop. J Magn Magn Mater 402:76–82. https://doi.org/10.1016/j.jmmm.2015.11.046
Jiang LH, Liu YG, Zeng GM et al (2016) Removal of 17β-estradiol by few-layered graphene oxide nanosheets from aqueous solutions: external influence and adsorption mechanism. Chem Eng J 284:93–102. https://doi.org/10.1016/j.cej.2015.08.139
Ju Z, Liu SS, Xu YQ, Li K (2019) Combined toxicity of 2,4-dichlorophenoxyacetic acid and its metabolites 2,4-dichlorophenol (2,4-DCP) on two nontarget organisms. ACS Omega 4:1669–1677. https://doi.org/10.1021/acsomega.8b02282
Kearns JP, Wellborn LS, Summers RS, Knappe DRU (2014) 2,4-D adsorption to biochars: effect of preparation conditions on equilibrium adsorption capacity and comparison with commercial activated carbon literature data. Water Res 62:20–28. https://doi.org/10.1016/j.watres.2014.05.023
Kellici S, Acord J, Ball J et al (2014) A single rapid route for the synthesis of reduced graphene oxide with antibacterial activities. RSC Adv 4:14858–14861. https://doi.org/10.1039/c3ra47573e
Kuila T, Bose S, Mishra AK et al (2012) Chemical functionalization of graphene and its applications. Prog Mater Sci 57:1061–1105. https://doi.org/10.1016/j.pmatsci.2012.03.002
Kumar NS, Suguna M, Subbaiah MV et al (2010) Adsorption of phenolic compounds from aqueous solutions onto chitosan-coated perlite beads as biosorbent. Ind Eng Chem Res 49:9238–9247. https://doi.org/10.1021/ie901171b
Kumar NS, Shaikh HM, Asif M, Al-Ghurabi EH (2021) Engineered biochar from wood apple shell waste for high-efficient removal of toxic phenolic compounds in wastewater. Sci Rep 11. https://doi.org/10.1038/s41598-021-82277-2
Lee JS, Cha JM, Yoon HY et al (2015) Magnetic multi-granule nanoclusters: a model system that exhibits universal size effect of magnetic coercivity. Sci Rep 5. https://doi.org/10.1038/srep12135
Li B, Jin X, Lin J, Chen Z (2018) Green reduction of graphene oxide by sugarcane bagasse extract and its application for the removal of cadmium in aqueous solution. J Clean Prod 189:128–134. https://doi.org/10.1016/j.jclepro.2018.04.018
Liu W, Yang Q, Yang Z, Wang W (2016) Adsorption of 2,4-D on magnetic graphene and mechanism study. Colloids Surf A Physicochem Eng Asp 509:367–375. https://doi.org/10.1016/j.colsurfa.2016.09.039
Liu G, Li L, Huang X et al (2018) Adsorption and removal of organophosphorus pesticides from environmental water and soil samples by using magnetic multi-walled carbon nanotubes @ organic framework ZIF-8. J Mater Sci 53:10772–10783. https://doi.org/10.1007/s10853-018-2352-y
Liu J, Xu D, Chen P et al (2019) Solvothermal synthesis of porous superparamagnetic RGO@Fe3O4 nanocomposites for microwave absorption. J Mater Sci: Mater Electron 30:17106–17118. https://doi.org/10.1007/s10854-019-02057-7
Magnoli K, Carranza CS, Aluffi ME et al (2020) Herbicides based on 2,4-D: its behavior in agricultural environments and microbial biodegradation aspects. A review. Environ Sci Pollut Res 27:38501–38512
Mahendran GB, Ramalingam SJ, Rayappan JBB et al (2020) Green preparation of reduced graphene oxide by Bougainvillea glabra flower extract and sensing application. J Mater Sci: Mater Electron 31:14345–14356. https://doi.org/10.1007/s10854-020-03994-4
Maletić SP, Beljin JM, Rončević SD et al (2019) State of the art and future challenges for polycyclic aromatic hydrocarbons is sediments: sources, fate, bioavailability and remediation techniques. J Hazard Mater 365:467–482
Marques BS, Frantz TS, Sant’Anna Cadaval Junior TR et al (2019) Adsorption of a textile dye onto piaçava fibers: kinetic, equilibrium, thermodynamics, and application in simulated effluents. Environ Sci Pollut Res 26:28584–28592. https://doi.org/10.1007/s11356-018-3587-5
Meftaul IM, Venkateswarlu K, Dharmarajan R et al (2020) Movement and fate of 2,4-D in urban soils: a potential environmental health concern. ACS Omega 5:13287–13295. https://doi.org/10.1021/acsomega.0c01330
Mesnage R, Brandsma I, Moelijker N et al (2021) Genotoxicity evaluation of 2,4-D, dicamba and glyphosate alone or in combination with cell reporter assays for DNA damage, oxidative stress and unfolded protein response. Food and Chemical Toxicology 157. https://doi.org/10.1016/j.fct.2021.112601
Nemati Sani O, Navaei Fezabady AA, Yazdani M, Taghavi M (2019) Catalytic ozonation of ciprofloxacin using γ-Al2O3 nanoparticles in synthetic and real wastewaters. J Water Process Eng 32.https://doi.org/10.1016/j.jwpe.2019.100894
Nunes FB, da Silva BF, da Rosa ST, Rhoden CRB (2023) Study of phenobarbital removal from the aqueous solutions employing magnetite-functionalized chitosan. Environ Sci Pollut Res 30:12658–12671. https://doi.org/10.1007/s11356-022-23075-9
Okoli CP, Naidoo EB, Ofomaja AE (2018) Role of synthesis process variables on magnetic functionality, thermal stability, and tetracycline adsorption by magnetic starch nanocomposite. Environ Nanotechnol Monit Manag 9:141–153. https://doi.org/10.1016/j.enmm.2018.02.001
Oliveira Vargas G, Schnorr C, Bastista Nunes F et al (2023) Highly furosemide uptake employing magnetic graphene oxide: DFT modeling combined to experimental approach. J Mol Liq 121652. https://doi.org/10.1016/j.molliq.2023.121652
Pattarith K, Areerob Y (2020) Fabrication of Ag nanoparticles adhered on RGO based on both electrodes in dye-sensitized solar cells (DSSCs). Renew Wind Water Sol 7. https://doi.org/10.1186/s40807-020-00058-3
Raghav S, Kumar D (2018) Adsorption equilibrium, kinetics, and thermodynamic studies of fluoride adsorbed by tetrametallic oxide adsorbent. J Chem Eng Data 63:1682–1697. https://doi.org/10.1021/acs.jced.8b00024
Rahman AJ, Ojha H, Pandey A et al (2022) Kinetic, isotherm and thermodynamic adsorption studies of organophosphorus compound (phosmet) on reduced graphene oxide. Diam Relat Mater 127. https://doi.org/10.1016/j.diamond.2022.109191
Ramos S, Homem V, Alves A, Santos L (2016) A review of organic UV-filters in wastewater treatment plants. Environ Int 86:24–44. https://doi.org/10.1016/j.envint.2015.10.004
Rhoden CRB, Bruckmann F da S, da Salles RT et al (2021) Study from the influence of magnetite onto removal of hydrochlorothiazide from aqueous solutions applying magnetic graphene oxide. J Water Process Eng 43. https://doi.org/10.1016/j.jwpe.2021.102262
Rincón-Silva NG, Moreno-Piraján JC, Giraldo LG (2015) Thermodynamic study of adsorption of phenol, 4-chlorophenol, and 4-nitrophenol on activated carbon obtained from eucalyptus seed. J Chem 2015. https://doi.org/10.1155/2015/569403
Saleem F, Khan A, Mubarak NM et al (2023) Magnetic nanoadsorbents’ potential route for heavy metals removal-a review. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-020-08711-6/Published
Sandoval S, Tobias G (2020) Tuning the nature of N-based groups from n-containing reduced graphene oxide: enhanced thermal stability using post-synthesis treatments. Nanomaterials 10:1–16. https://doi.org/10.3390/nano10081451
Serrano-Luján L, Víctor-Román S, Toledo C et al (2019) Environmental impact of the production of graphene oxide and reduced graphene oxide. SN Appl Sci 1. https://doi.org/10.1007/s42452-019-0193-1
Sharma K, Maiti K, Kim NH et al (2018) Green synthesis of glucose-reduced graphene oxide supported Ag-Cu2O nanocomposites for the enhanced visible-light photocatalytic activity. Compos B Eng 138:35–44. https://doi.org/10.1016/j.compositesb.2017.11.021
Singh K, Ohlan A, Pham VH et al (2013) Nanostructured graphene/Fe3O4 incorporated polyaniline as a high performance shield against electromagnetic pollution. Nanoscale 5:2411–2420. https://doi.org/10.1039/c3nr33962a
Siva Kumar N, Asif M, Poulose AM et al (2023) Preparation, characterization, and chemically modified date palm fiber waste biomass for enhanced phenol removal from an aqueous environment. Materials 16. https://doi.org/10.3390/ma16114057
Song M, Li M (2019) Adsorption and regeneration characteristics of phosphorus from sludge dewatering filtrate by magnetic anion exchange resin. Environ Sci Pollut Res 26:34233–34247. https://doi.org/10.1007/s11356-018-4049-9
Subedi N, Lähde A, Abu-Danso E et al (2019) A comparative study of magnetic chitosan (Chi@Fe3O4) and graphene oxide modified magnetic chitosan (Chi@Fe3O4GO) nanocomposites for efficient removal of Cr(VI) from water. Int J Biol Macromol 137:948–959. https://doi.org/10.1016/j.ijbiomac.2019.06.151
Sun J, Liang Q, Han Q et al (2015) One-step synthesis of magnetic graphene oxide nanocomposite and its application in magnetic solid phase extraction of heavy metal ions from biological samples. Talanta 132:557–563. https://doi.org/10.1016/j.talanta.2014.09.043
Tran HN, Lima EC, Juang R-S et al (2021) Thermodynamic parameters of liquid–phase adsorption process calculated from different equilibrium constants related to adsorption isotherms: a comparison study. J Environ Chem Eng 9:106674. https://doi.org/10.1016/j.jece.2021.106674
Tzabar N, ter Brake HJM (2016) Adsorption isotherms and Sips models of nitrogen, methane, ethane, and propane on commercial activated carbons and polyvinylidene chloride. Adsorption 22:901–914. https://doi.org/10.1007/s10450-016-9794-9
Vatandost E, Saraei AGH, Chekin F et al (2020) Antioxidant, antibacterial and anticancer performance of reduced graphene oxide prepared via green tea extract assisted biosynthesis. ChemistrySelect 5:10401–10406. https://doi.org/10.1002/slct.202001920
Vinayagam R, Ganga S, Murugesan G et al (2023) 2,4-Dichlorophenoxyacetic acid (2,4-D) adsorptive removal by algal magnetic activated carbon nanocomposite. Chemosphere 310. https://doi.org/10.1016/j.chemosphere.2022.136883
Viriato C, França FM, Santos DS et al (2021) Evaluation of the potential teratogenic and toxic effect of the herbicide 2,4-D (DMA® 806) in bullfrog embryos and tadpoles (Lithobates catesbeianus). Chemosphere 266. https://doi.org/10.1016/j.chemosphere.2020.129018
Wang J, Guo X (2020) Adsorption isotherm models: classification, physical meaning, application and solving method. Chemosphere 258:127279. https://doi.org/10.1016/j.chemosphere.2020.127279
Wanjeri VWO, Sheppard CJ, Prinsloo ARE et al (2018) Isotherm and kinetic investigations on the adsorption of organophosphorus pesticides on graphene oxide based silica coated magnetic nanoparticles functionalized with 2-phenylethylamine. J Environ Chem Eng 6:1333–1346. https://doi.org/10.1016/j.jece.2018.01.064
Wernke G, Shimabuku-Biadola QL, dos Santos TRT et al (2020) Adsorption of cephalexin in aqueous media by graphene oxide: kinetics, isotherm, and thermodynamics. Environ Sci Pollut Res 27:4725–4736. https://doi.org/10.1007/s11356-019-07146-y
Wijaya R, Andersan G, Permatasari Santoso S, Irawaty W (2020) Green reduction of graphene oxide using kaffir lime peel extract (Citrus hystrix) and its application as adsorbent for methylene blue. Sci Rep 10. https://doi.org/10.1038/s41598-020-57433-9
Young Lee A, Ae Hwangbo S, Seok Jeong M, Geol Lee T (2022) Eco-friendly sonochemical reduction of graphene oxide in water using TiO2 photocatalyst activated by sonoluminescence. Appl Surf Sci 605. https://doi.org/10.1016/j.apsusc.2022.154820
Acknowledgements
The authors would like to thank CAPES, FAPERGS, Laboratório de Materiais Magnéticos Nanoestruturados (LaMMaN), Laboratório de Magnetismo e Materiais Magnéticos–LMMM, UFSM, CNPEM, and Universidade Franciscana–UFN for the support.
Author information
Authors and Affiliations
Contributions
TRS, LV, FSB, LB: methodology, investigation. TRS, FSB, AHO, WJS, DSB: investigation, writing—original draft. EIM, DSTM, SRM: visualization, data curation. CRBR: conceptualization, writing—review and editing, supervision.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Rosa Salles, T., Zancanaro, L.V., da Silva Bruckmann, F. et al. Magnetic graphene derivates for efficient herbicide removal from aqueous solution through adsorption. Environ Sci Pollut Res 31, 25437–25453 (2024). https://doi.org/10.1007/s11356-024-32845-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32845-6