Abstract
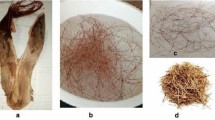
This research was conducted to evaluate the methylene blue dye adsorption by piaçava fibers. The effects of adsorbent amount, pH, kinetics, equilibrium, and thermodynamics were analyzed, as well as the adsorbent performance in the treatment of synthetic textile effluents. The adsorbent characterization was also performed. Experimental kinetic data were fitted with pseudo-first-order, pseudo-second-order, and Elovich models. The equilibrium tests were done at 298, 308, and 318 K, and the models of Freundlich, Langmuir, Redlich–Peterson, and Sips were used. The adsorption was favored using 0.025 g of adsorbent, pH 10, and 318 K. The Elovich model provided better fit to kinetic data. The equilibrium experimental points were well represented by the Sips model. The maximum experimental adsorption capacity of methylene blue dye was 427.3 mg g−1. It was verified a spontaneous, favorable, and endothermic adsorption. Piaçava fiber was a promising low-cost material to be used for color removal in effluents containing methylene blue.









Similar content being viewed by others
References
Alves CCO (2012) Removal of aromatic Amino Acids from aqueous solutions by adsorbent prepared from agricultural residue. Thesis, Federal University of Minas Gerais
Avelar FF (2008) Use of piaçava fiber in activated charcoal preparation. Dissertation, Federal University of Lavras
Brazilian Association of Textile and Clothing Industry (2015) Sector Profile. Communication Department. http://www.abit.org.br/cont/perfil–do–setor. Accessed 23 Feb 2018
Crini G, Badot PM (2008) Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: a review of recent literature. Prog Polym Sci 33:399–447
De Rossi A, Rigon MR, Zaparoli M, Braido RD, Colla LM, Dotto GL, Piccin JS (2018) Chromium (VI) biosorption by Saccharomyces cerevisiae subjected to chemical and thermal treatments. Environ Sci Pollut Res 25:19179–19186
Dotto GL, Costa JAV, Pinto LAA (2013) Kinetic studies on the biosorption of phenol by nanoparticles from Spirulina sp. LEB 18. J Environ Chem Eng 1:1137–1143
Dotto GL, Santos JMN, Rodrigues IL, Rosa R, Pavan FA, Lima EC (2015) Adsorption of methylene blue by ultrasonic surface modified chitin. J Colloid Interface Sci 446:133–115
Falamarzpour P, Behzad T, Zamani A (2017) Preparation of nanocellulose reinforced chitosan films, cross–linked by adipic acid. Int J Mol Sci 18:1–12
Foust SA, Wenzel LA, Clump CW, Maus L, Andersen LB (1982) Unitary operation principles. LTC, Rio de Janeiro
Freundlich H (1906) Over the adsorption in solution. Z Phys Chemie 7:358–471
Goldstein JI, Newbury DE, Echil P, Joy DC, Romig AD Jr, Lyman CE, Fiori C, Lifshin E (1992) Scanning electron microscopy and X–ray microanalysis. Plenum Press, New York
Gurung M, Adhikari BB, Kawakita H, Ohto K, Inoue K, Alam S (2012) Selective recovery of precious metals from acidic leach liquor of circuit boards of spent mobile phones using chemically modified persimmon tannin gel. Ind Eng Chem Res 51:11901–11913
Han X, Wang W, Ma X (2011) Adsorption characteristics of methylene blue onto agricultural wastes lotus leaf in bath and column modes. Chem Eng J 171:1–8
Ho YS, Mckay G (1998) Kinetic models for the sorption of dye from aqueous solution by wood. Proc Saf Environ Protect 76:183–119
Kumar KV, Porkodi K, Rocha F (2008) Isotherms and thermodynamics by linear and non–linear regression analysis for the sorption of methylene blue onto activated carbon: comparison of various error functions. J Hazard Mater 151:794–804
Lagergren S (1898) About the theory of so–called adsorption of soluble substances. Kung Svenska Vetenskap 24:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Lima DR, Klein L, Dotto GL (2017) Application of ultrasound modified corn straw as adsorbent for malachite green removal from synthetic and real effluents. Environ Sci Pollut Res 24:21484–21495
Magdy YH, Altaher H (2018) Kinetic analysis of the adsorption of dyes from high strength wastewater on cement kiln dust. J Environ Chem Eng 6:834–841
Milonjic SK (2007) A consideration of the correct calculation of thermodynamic parameters of adsorption. J Serb Chem Soc 72:1363–1367
Miranda CS, Fiuza RP, Carvalho RF, José NM (2015) Effect of surface treatment on properties of bagasse piassava fiber Attalea funifera martius. Quim Nova 38:161–165
Mohamed NA, El-Ghany NAA (2017) Pyromellitimide benzoyl thioureacross linkedcarboxymethyl chitosan hydrogels as antimicrobial agents. Int J Polym Mater Polym Biomater 66:861–870
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low–cost adsorbents: a review. J Hazard Mater 177:70–80
Redlich O, Peterson DL (1959) A useful adsorption isotherm. J Chem Phys 63:1024–1027
Ruthven DM (1984) Principles of adsorption and adsorption processes. Wiley, New York
Sellaoui L, Dotto GL, Peres EC, Benguerba Y, Lima EC, Ben Lamine A, Erto A (2017) New insights into the adsorption of crystal violet dye on functionalized multi–walled carbon nanotubes: experiments, statistical physics and COSMO–RS models application. J Mol Liq 248:890–897
Silva JM, Farias BS, Gründmann DDR, Cadaval TRS Jr, Moura JM, Dotto GL, Pinto LAA (2017) Development of chitosan/Spirulinabio–blends and its application as dye biosorbents potential for dyes. J Appl Polym Sci 134:1–8
Silverstein RM, Webster FX, Kiemle DJ (2007) Spectrometric identification of organic compounds. Wiley, New York
Sips RJ (1948) On the structure of a catalyst surface. J Chem Phys 16:490–495
Wu FC, Tseng RL, Juang RS (2009) Characteristics of Elovich equation used for the analysis of adsorption kinetics in dye–chitosan systems. Chem Eng J 150:366–373
Acknowledgements
The authors would like to thank CAPES (Brazilian Agency for Improvement of Graduate Personnel) and CNPq (National Council of Science and Technological Development) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Marques, B.S., Frantz, T.S., Sant’Anna Cadaval Junior, T.R. et al. Adsorption of a textile dye onto piaçava fibers: kinetic, equilibrium, thermodynamics, and application in simulated effluents. Environ Sci Pollut Res 26, 28584–28592 (2019). https://doi.org/10.1007/s11356-018-3587-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3587-5