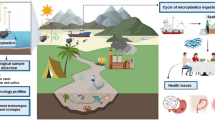
Abstract
Acrylamide (ACR) is widely applied in various industrial activities, as well as in the water purification process. Furthermore, ACR is synthesized naturally in some starchy grains exposed to high temperatures for an extended time during the cooking process. Because of its widespread industrial usage, ACR might be released into water stream sources. Also, ACR poses a high risk of contaminated surface and ground-water resources due to its high solubility and mobility in water. Furthermore, animal studies have indicated that ACR exposure may cause cancer (in many organs such as lung, prostate, uterus, and pancreas), genetic damage (in both somatic and germ cells), and severe effects on reproduction and development. Recently, numerous studies have shown that ACR has a mild acute cytotoxic impact on aquatic species, particularly during early life stages. Besides, wide-spectrum usage of ACR in many industrial activities presented higher environmental risks as well as major hazards to consumer health. This literature was designed to include all potential and accessible reports on ACR toxicity related with aquatic species. The Preferred Reporting Items for Systematic Reviews were applied to evaluate the risk effects of ACR on aquatic organisms, the ACR sub-lethal concentration in the ecosystem, and the possible protective benefits of various feed additives against ACR toxicity in fish. The major findings are summarized in Tables 2 and 3. The primary aim of this literature was to specify the hazards of ACR toxicity related with fish welfare and possible suggested strategies to reduce its risks.
Similar content being viewed by others
Introduction
Acrylamide (ACR), a monomer with a vinyl group, has neurotoxic effects on the nervous system in both humans and animals such as overall weakness, numbness, tingling in the limbs, or ataxia (Kopańska et al. 2022; Lakshmi et al. 2012). ACR and its components are mostly used as flocculants, and the main areas of application include extraction of inorganic minerals, purifying waste water, and food-processing manufacturing industries (Aras et al. 2017). ACR is effective in purification irrigation and drinking water (Becalski et al. 2003). The ACR applied in the purification treatments and flocculation process can pollute the ambient ecosystem by releasing its residual (Ciesarova et al. 2006). ACR does not have the ability to be adsorbed with the soil and can easily degrade into ground-water via seepage, which might lead to a high risk to public health and welfare (Croll et al. 1974). Therefore, ACR contamination in water is a threat to aquatic wildlife (Tanekhy and Mehana 2022).
The main problem with ACR pollution is the lack of a filtration procedure before its release into water streams or consumption as drinking water (Exon 2006). Besides, many regions across the world, especially in developing countries, have not performed frequent detectable analyses for ACR permissible levels in drinking water, which may threaten public health (Tepe 2015). Furthermore, there is evidence of ACR polluting surface and drinking water supplies worldwide (Tepe and Çebi 2019). Also, the release of ACR into water streams from various uses such as agriculture, herbicides, and cosmetics has been identified as the main issue for ecosystem pollution and threatens fish sustainability (Naiel et al. 2020d; Wang and Lee 2001).
According to toxicological research, ACR is quickly absorbed throughout the body and is even passed on to offspring after accumulating throughout the organism (El-Shehawi et al. 2022; Yue et al. 2021). Several recent studies have shown that ACR causes acute neurotoxicity in the developmental stage of zebrafish when exposed to nearly 1 mM ACR for 3 days (Faria et al. 2018, 2019a; Park et al. 2021). Moreover, ACR could be genotoxic to Carassius auratus at levels 50–150 mg/L, shows hemato-toxicity (such as induced anemia, red blood cell count reduction, low hemoglobin level, and decreased packed cell volume) in Clarias gariepinus at concentrations from 26.6 up to 106.4 mg/L (Ibrahim and Ibrahem 2020), and induces cardiac developmental toxicity at levels from 71 to 203 mg/L, cardiovascular toxicity at concentrations from 35.5 to 203 mg/L, retinal toxicity (71–142 mg/L), splenic toxicity (12.8 mg/kg), and oxidative stress (142 mg/L) in Danio rerio (Moura et al. 2019; Singh et al. 2019; Spencer et al. 2018). Besides, many studies have shown a lethal concentration of ACR in several fish species (Gopika et al. 2018b; Krautter et al. 1986; Larguinho et al. 2014b; Moura et al. 2019; Shanker and Seth 1986; Spencer et al. 2018). Recently, Ligina et al. (2022) exhibited that ACR at sublethal levels (13.4 µg/L) induced alteration in hematological indices and suppressed redox status in gill tissue of the A. testudineus fish. The main objective of the current literature was to shed light on the structure and resources of ACR, and its stability in the ecosystem and release into stream water. Furthermore, the main aspects of this literature are identifying the adverse effects of ACR-contaminated water on fish health. Likewise, it recommends novel appropriate and practical techniques to lower and mitigate ACR threats in the ecosystem and fish sustainability via enhanced fish immune system and antioxidant status and degrades ACR compounds through employing microbial degradation methods.
Methodology
This literature investigated all possible and available studies that were found to be related to ACR toxicity in fish and crustacean. Besides, available in vivo or in vitro reports have shown the anticipated functional relevance of some feed additives on alleviating ACR toxicity in fish. A comprehensive literature search was directed using the Scopus (2003–2021), Google Scholar, and Web of Science (1900–2021) databases. The search was done using the following search criteria for terms in the title, keywords, or abstract:
(acrylamide* OR toxic* OR hazard* OR phytochemical OR salt* OR pigment* OR herb OR *acid* OR additive* OR diet) AND (fish* OR shellfish* OR crustacean*) AND (immun* OR antioxidant* OR growth* OR oxidative AND* OR production OR meat OR bacterial OR mortality* OR disease*) AND NOT (hot AND water OR methano* OR acetone OR trace* OR metal* OR *icide* OR nano* OR toxic* OR lethal OR fungi OR mammal* OR human OR patient OR cancer OR product* OR tumor OR vaccine*) AND (acrylamide).
These searches returned a total of 183 studies from Scopus and WOS as well as other 83 studies from Google Scholar. All of the obtained results were then inspected using the method of Preferred Reporting Items for Systematic Reviews (PRISMA; Fig. 1) (Rethlefsen et al. 2021) to additionally refine the search results by reading the title, abstract, and/or material and methods. The final screening left 65 publications that met the following criteria: (1) the hazard effects of ACR on fish and shellfish production and health (49 studies); (2) the ACR sub-lethal concentration on fish rearing water (9 studies); (3) the protective potential effects of some feed additives against ACR toxicity in fish (7 research items). The main obtained results are summarized in Tables 2 and 3. The current study focuses on several safe feed additives (acids, pigments, and salts) and other phytochemical as strategies to reducing ACR toxicity in fish.
Acrylamide structure and resources
ACR (CH2 = CHC(O)NH2) is a type of organic molecule (Fig. 2). It is a white, odorless solid that is soluble in water and a variety of organic solvents (alcohol and acetone) and insoluble in benzene and heptane (Stadler and Scholz 2004). In chemistry, acrylamide is a primary amide that has been substituted with vinyl group (CONH2) (Sharma et al. 2013). Acrylamide is found to be stable at ambient temperature but may polymerize aggressively near its melting point (84.5 °C) or when exposed to ultraviolet (UV) radiation (Faroon et al. 2009).
Acrylamide is mostly present in plant-based meals that are dense in carbohydrates such as grain products, or coffee (Yu et al. 2023). ACR does not synthesize, or is formed only in low quantities in dairy, meat, or fish products (Fonger et al. 2014). ACR is more likely to accumulate when higher temperatures are used in the cooking process for longer periods of time (Lentz et al. 2008). Besides, trace levels of acrylamide contaminants could be detected in some treated drinking water from natural water streams when polyacrylamide is used to purify water (Fan et al. 2023). The permissible detectable levels of ACR found in water resources and fish flesh are displayed in Table 1. Most acrylamide exposures are derived from consumed food, with a far smaller proportion from drinking water (Jia et al. 2017). The majority of acrylamide leads to environmental pollution as a contaminant from polyacrylamide products when polyacrylamide is employed in treatments of the waste water or industrial effluents (Mehana et al. 2020; Shanker and Seth 1986).
Furthermore, ACR rapidly migrates to ambient water because of its broad range of applications and high hydrophilicity (Aras et al. 2017; Tepe and Çebi 2019). Although polyacrylamide is non-toxic, its monomer ACR has been found to be neurotoxic and carcinogenic (Exon 2006; Spencer et al. 2018). Thus, contaminated water with ACR is a threat to aquatic wildlife.
Acrylamide formation and contaminated ecosystem
ACR is a synthetic organic compound that is extremely carcinogenic, toxic, neurotoxic, and reprotoxic (Hays and Aylward 2008). ACR contamination in water occurs as a result of its release from various industrial operations such as waste water treatment, pulp and paper preparation, agriculture, oil drilling, dam construction, cement, herbicide, cosmetics, soap, chalk, skin care products, adhesives, dyes, explosives, printing inks, latex, and mining and mineral production (Tepe and Çebi 2019). Other suppliers, such as acrylamide-based grouting and waste paper recycling, may also result in the discharge of acrylamide into rivers, ultimately resulting in water contamination (Kusnin et al. 2015). Moreover, acrylamide is usually found in drinking water as a consequence of leaching during the purification process (van Dijk-Looijaard and Van Genderen 2000). Specifically, the primary source of ACR pollution in drinking water is the improper application of polyacrylamide flocculants dragging residual amounts of ACR monomer (Tepe and Çebi 2019). In overall, the maximum permitted dosage of polymer is 1 mg/L (Tepe 2015).
The International Agency for Research on Cancer (IARC) categorized ACR as a carcinogenic molecule followed by the “2A Group” (McDonald 1995). Because of their proven aquatic toxicity, oil-based ACR products are unsuitable for application in aquatic environments (WHO 2003). The lethal concentrations of ACR in various fish species are illustrated in Table 2. Acrylamide caused cancer in experimental animals when animals were exposed to extremely high doses of acrylamide (Rice 2005). Specifically in rodents, acrylamide is transformed to glycidamide, which induces DNA alterations and damage (Besaratinia and Pfeifer 2007). Besides, ACR does not form, or forms at lower levels, in dairy, meat, and fish products (Tareke et al. 2002). It has been shown that high ACR pollution in the environment leads to higher accumulation in fish flesh depending on exposure time. According to Petersen et al.’s (1985) findings, exposed fingerling rainbow trout at 0.388 and 0.710 mg L−1 ACR levels resulted in about 72% of the ACR level accumulating into tissue and the remaining 28% consumption dosage removal out of the fish body via gills, urine, and bile exert after 2 h.
The overall degradation of acrylamide has been shown to vary between 480 and over 1100 h depending on the source of water samples such as tap, river, and lake (Babuji et al. 2023). The combination of sunshine and glyphosate has been found to reduce ACR breakdown (Ver Vers 1999). Also, mechanical degradation of small amounts of ACR in water could be induced by shaking, pumping, injection, and passage water through porous media (Aktağ et al. 2022). The fundamental issue with the mechanical degradation process may be the irreversible alteration of the polymeric component (Valipour and Montazar 2012). Furthermore, the high level of ACR may be released into the aquatic environment after being used in agricultural areas, causing a risk to human health and threatening aquatic wildlife (Mekawi et al. 2023). Thus, estuarine or marine water may keep ACR stable for a substantially longer period of time compared with freshwater (Friedman et al. 1995). Moreover, the Croll et al. (1974) study on acrylamide degradation in Hackensack River, New Jersey, reported that a 10-mg L−1 ACR level required 12 days to completely degrade. While in the Thames River in England, it was illustrated that 8 g L−1 ACR pollution needs 10 days to decompose entirely (Croll et al. 1974). In addition, Shanker et al. (1990) demonstrated that ACR levels exceeding 2 mg L−1 are not easily degraded in water; thus, these levels are considered hazardous for aquatic organisms. Furthermore, it was found that the optimal water quality conditions for acrylamide biodegradation have been described as pH 6–8 and water temperature 15–30 °C (Zamora et al. 2015).
Moreover, ACR lethal concentration tests have been determined on several fish and crustacean species. The LC50 levels were estimated and identified as 160 mg L−1 for common water fleas, 110 mg L−1 for Oncorhynchus mykiss, 120 mg L−1 for Pimephales promelas, and 100 mg L−1 for Lepomis macrochirus; furthermore, opossum shrimps presented the high sensitivity to LC50 value of 78 mg L−1 (Krautter et al. 1986). The ACR LC50 values for Rasbora heteromorpha (harlequin fish) were 460 mg L−1 for 24 h, 250 mg L−1 for 48 h, and 130 mg L−1 for 96 h, and found to be 35 mg L−1 for 7 days for Poecilia reticulate (guppy) (Torres et al. 2014). In contrast, marine crustaceans are showed highly tolerant to ACR contamination and had larger LC50 values (411 mg L−1) (Larguinho et al. 2014b). Nevertheless, the highest residual ACR levels estimated in water are 5–460 times lower than ACR amounts accumulated in commonly consumed tissues (Tepe 2015).
Hazards of ACR in fish and crustacean
ACR is classified under two categories: polymeric, which is non-toxic, and monomeric, which is extremely harmful to the welfare of mammals and fish with carcinogenic, teratogenic, and neurotoxic effects (Park et al. 2021). It is well known that adult gills and embryonic yolk skin of fishes are the main organs that absorb calcium ions from the aquatic system (Lin and Hwang 2016). ACR may bind to redox-sensitive proteins (cystine) and disrupt calcium signaling genes, resulting in disruption of calcium homeostasis (Flik et al. 2009). It is hypothesized that calcium signaling disturbance might possibly trigger cancer formation through inducing hormonal alters (Pratt et al. 2020). However, the absence of actual evidence requires additional investigation toward this idea. Moreover, the neurotoxic effects of ACR have been linked to changes in the gut-brain axis signaling pathway caused by increments in oxidative stress, as well as modulates in the NrF2 signaling pathway and inflammatory mediator levels (alteration in NF-kB signaling pathway) in both the gut and the brain, which are expected to result in neurobehavioral disorders such as abnormal swimming phenotypes, including freezing, looping, and erratic movement in adult zebrafish (Singh 2020). Thus, Singh (2020) investigated that exposed adult zebrafish to several concentrations of ACR (1 mM and 2 mM) markedly induced sudden mortalities at 24 h and 48 h of exposure, while rearing zebrafish in ACR-contaminated water for 3 days at level 0.75 mM induced significant abnormalities in motor activities and oxidative stress as compared to the untreated group. Furthermore, Park et al. (2021) exhibited that ACR could induce developmental toxicity, which manifested as yolk retention, scoliosis, swim bladder deficit, and body curvature as well as reduced locomotor activity, as estimated by swimming speed and distance traversed. In addition, intoxicated zebrafish rearing water with 100 mg L−1 ACR significantly reduced the width of both the brain and spinal cord, suggesting neuronal damage (Park et al. 2021).
At the same context, exposed adult zebrafish to ACR (0.65–3.0 mM) for 72 h significantly induced severe abnormal behavior signs, which is associated with a “depression-like” phenotype (Faria et al. 2018). Besides, the downregulation of regeneration-associated genes and the upregulation of oligodendrocyte and reactive astrocyte markers, as well as changes in the expression of genes involved in presynaptic vesicle cycling, were found to be in combination with ACR exposure (Faria et al. 2018). Moreover, ACR-treated groups exhibited substantial alterations in the brain proteome and generated adducts with specific cysteine residues of particular proteins, some of which are required for presynaptic function (Faria et al. 2018).
Besides, ACR-exposed catfish for 96 h to LC50 level (133 mg L−1) considerably caused numerous aberrant behavioral indications and clinical and postmortem reactions. Furthermore, ACR-exposed catfish at the same level decreased red blood cell counts, hemoglobin level, and packed cell volume, resulting in anemic responses (Ibrahim and Ibrahem 2020). The anemic reaction may be induced by ACR’s degradation or suppression of erythrocyte formation (Ramesh et al. 2014). In the same study, malondialdehyde levels were considerably higher, in contrast with the glutathione, superoxide dismutase, and total antioxidant capacity were markedly smaller. Notably, the DNA fragmentation assay revealed a distinct laddering pattern in brain tissues affected by ACR (Ibrahim and Ibrahem 2020). Adduct formation with decreased glutathione and increased hydrogen peroxide generation results in higher levels of lipid peroxidative products and carbonyl content, and lower enzymatic and nonenzymatic antioxidants with a reduction in acetylcholinesterase (AChE) activity in the brain tissues (Petersen et al. 1985).
In comparison to the untreated group, rearing the land snail, Theba pisana, in polluted water with 1/20 LC50 ACR (ãpproximately 2.28 μg g−1) for 2 weeks substantially increased lipid peroxidation levels and the activity of catalase and glutathione-S-transferase, cell death, and hemocyanin content, while significantly decreasing DNA and reduced glutathione concentrations, phagocytic activity, lysosomal membrane stability, lectins, O2 generation, peroxidase, and phenol-oxidase levels (Radwan et al. 2019). Whereas, after 1 week of recovery, the majority of the observed markers in exposed snails were permanent and not reversible to normal values (Radwan et al. 2019), while Petersen et al. (1987) showed that exposed trout fish to 50 mg L−1 ACR for 15 days impaired swimming performance and caused rapid death. These irregular behavioral changes were discovered to be associated with ACR dose-related lesions in rainbow trout gills and the liver.
Owing to histopathological examination studies, Jia et al. (2017) investigated that acute ACR (2 mM for 36 h) exposure of zebrafish resulted in a significant decrease in motility and a loss of color-preferential swimming behavior (zebrafish preferred blue illumination to white, and white illumination to red). Hence, histopathological analysis of acrylamide-treated zebrafish eyes confirmed the results that the acrylamide exposure induced retinal damage. In addition, Haasch et al. (1992) reported that the hepatic CYP1A1 mRNA transcription increments were significantly altered by 50 ppm acrylamide monomer, whereas CYPI Al isozyme levels and EROD activity were downregulated. Sen et al. (2012) suggested that acrylamide treatment alone could have the possible isozyme selective inactivation or decreased translation of the CYP1A1 mRNA leading to reduction in isozyme levels consequently led to high transcription of the CYP1A1 gene in rainbow trout. Recently, Yue et al. (2021) reported that exposing embryos of Oryzias melastigma to various concentrations of ACR (from 0.1 to 10 mg/L) for 21 days substantially decreased hatching rate and prolonged hatching time, resulting in developmental delay, teratogenesis, and motility deficits in larvae. Transcriptome studies supported that these hazard effects might be linked to ACR-induced hypoxia and neurotoxicity. Moreover, the hazards of ACR in certain fish species are offered in Table 3.
New strategies toward the elimination of ACR hazards
ACR biodegrading process using Bacteria
Natural bacterial populations have been discovered in ecosystems to be capable of degrading existing contaminants and increasing in number under pollution environment (Mehana et al. 2020). When a contaminant is degraded, the biodegradative population decreases as a result of the contaminated element (Sharif et al. 2023). The treatment byproducts are generally nontoxic compounds such as water, carbon dioxide, and cell biomass (Abatenh et al. 2017; Farag et al. 2021). The aerobic bacteria showed a higher ability to degrade ACR in freshwater with a half-life of 55–70 h, after acclimatization for 33–50 h (Charoenpanich 2013). ACR has been revealed to be stable slightly longer in estuarine or salt than in freshwater (Peng et al. 2016). The produced biomass including aerobic bacteria, phytoplankton and zooplankton, attends as a natural feed resource for the fish (Naiel et al. 2021b). ACR is offered to totally biodegrade via microbes existing in water for 8 up to 12 days. Pseudomonas sp., which is naturally present in the ecosystem, had a higher biodegradation capacity toward ACR (4 g L−1), producing crylic acid and ammonia (Nyyssölä and Ahlgren 2019). Amidase was also exhibited to be the appropriate enzyme for the degradation of ACR and other short chain phosphoramidites such as formamide and acetamide but not on ACR derivatives, meth-acrylamide, and N, N-methylene bisacrylamide (Bao et al. 2009). Besides, Pseudomonas sp. was found to be able to produce energy and obtain carbon from the degradation of ACR (Patial et al. 2022). In addition, Pseudomonas stutzeri might be applied in waste water treatment since it has a high capacity to remove ACR at lower dosages than 440 mg L−1 under aerobic conditions (Wang and Lee 2001). It was discovered that acclimating bacteria or its byproducts (specifically, lactic acid bacteria, yeast, and cell-free extracts) to aquatic environments improves their ability to degrade ACR (Albedwawi et al. 2021). ACR hydrolysis at 10–20 ppm in river water took approximately 12 days with non-acclimated bacteria but just 2 days with acclimated bacteria (Shen et al. 2012).
Furthermore, several bacterial species isolated from natural streams such as Enterobacter aerogenes, Kluyvera georgiana, Klebsiella pneumoniae, and Enterococcus faecalis shown higher removal capacity to degrade ACR up to 5000 ppm at trophic temperatures and could degrade various aliphatic amides, particularly short- to medium-chain length, but not amide byproducts (Buranasilp and Charoenpanich 2011). While in industrial waste water treatment, E. aerogenes had a higher removal capacity against ACR and ammonia than a mixture of organisms (Charoenpanich 2013).
Recently, Ralstonia eutropha TDM-3, a denitrifying bacterium isolated from a water treatment system connected with the production of polyacrylonitrile fiber, displayed a higher capability to an ingested higher level of ACR up to 1446 mg L−1, beyond which it was not dangerous (Cha and Chambliss 2011).
In conclusion, the global interest in environmental issues is rising, as are the expectations for sustainable and controlled processes that do not substantially hazard the ecosystem. Biodegradation is a traditional technique for removing undesirable organic chemicals to undetectable quantities or below regulatory-agreed-upon limits. Consequently, it is critical to identify more useful bacterial strains capable of removing ACR from the water environment while causing no damage to aquatic welfare sustainability.
Nutritional antioxidant
Fatty acids
Oxidative stress has been associated with numerous chemical-induced cellular injuries (El-hameed et al. 2021). Oxidative stress occurs when there is an imbalance between the production and scavenging of free radicals, which is associated with neurodegenerative disorders (Naiel et al. 2020b). Absorption of ACR from the gastrointestinal tract or via the gills with reduced glutathione might produce higher levels of lipid peroxidative (LPO) and carbonyl groups, and decreased enzymic and non-enzymic antioxidant activities with reduced AChE production in the brain (Khafaga et al. 2020). Acetylcholine is an essential neurotransmitter whose activity is reliant on the creation of AChE enzyme that metabolizes it (Ismael et al. 2021). Inhibiting AChE may disrupt metabolic and neurological function, as well as cause varied membrane permeability and ionic refluxes (Naiel et al. 2020d). ACR-induced membrane sensitivity to LPO may result in a decrease in adenosine triphosphatase (ATPase) activity. Any disruption in ATPase activity affects membrane stability by inducing changes in neuronal homeostasis and electrophysiological energetics (Farag et al. 2021; Lakshmi et al. 2012). Thus, ACR accumulation causes neuronal cell death by altering the ratio of free radicals to antioxidants (Jia et al. 2017).
Fish oil, found mostly in herring and salmon, includes n-3 essential fatty acids (EFA), particularly eico-sapentaenoic acids (EPA) and docosahexaenoic acids (DHA), which are nutritional antioxidants and have been shown to be preventive elements against neurological diseases (Kinsella 1990). Fish oil has been proven to have anti-cancer, anti-inflammatory, and heart-protective properties (Al-Gabri et al. 2021; Weitz et al. 2010). Moreover, previous research has revealed that fish oil has neuroprotective effects due to its antioxidant properties (Panahi et al. 2019). For instance, Lakshmi et al. (2012) exhibited that enriched rat diets with fish oil have a neuroprotective impact on ACR-induced neurotoxicity by decreasing oxidative stress and apoptosis and upregulating the HSP27 expression. The higher omega-3 fatty acid content of fish oil promotes vitamin E incorporation into cellular membranes and prevents lipid peroxidation caused by increased membrane PUFA levels (Mabile et al. 2001). In addition, according to Saif-Elnasr et al.’s (2019) findings, administration of fish oil and/or SeNPs may be helpful in avoiding nephrotoxicity induced by cisplatin and radiation during the treatment of different tumors. As a result, it seems that fish oil improved resistance to free radical damage caused by ACR administration and increased overall antioxidant capacity (Lakshmi et al. 2012). Despite the fact that there is no available scientific data to support the preventive role of fish oil against ACR hazards in aquatic fish, further research is required to validate this important topic.
Pigments
The entire cellular antioxidant defensive mechanism is critical in the removal of numerous toxic harmful effects (Seidavi et al. 2021). Medicinal herbs are well known for their antioxidant properties and are still extensively applied as an effective therapeutic medicine resource (Farouk et al. 2021). The potential pharmacological aspects of these natural products derive from their higher contents of several bioactive compounds such as polyphenols, carotenoids, lignans, alkaloids, glycosides, cyanogenic, and terpenes (Durazzo et al. 2018; Gharib et al. 2022; Naiel et al. 2021a). Specifically, carotenoids are the main group of over 750 naturally synthesized pigments that are produced by algae, plants, and photosynthetic microorganisms (Tapiero et al. 2004). Lycopene (Ly) is an acyclic non-provitamin retinol carotenoid, as well as it is found in red-pigmented vegetables and fruits such as watermelon, tomatoes, pink guava, pink grapefruit, apricots, and pomegranate (Holick et al. 2007). It must be mentioned that Ly cannot be generated inside the body and that its bioavailability may be reduced by aging and certain pathological changes; thus, it must be supplied on a daily feed (Petyaev 2016). It is mainly found in trace quantities in the hepatocytes, adrenal gland, and brain tissues (Moran et al. 2013). Ly is strongly supposed that it might have neuroprotective effects in the central nervous system (CNS), because of its capability to permeate the blood–brain barrier (Rao and Rao 2007).
Several recent studies have indicated that lycopene-enriched fish diets, which have high antioxidant properties, effectively reduce oxidative damage induced by various toxicants or other kinds of abiotic conditions (Dawood et al. 2020). For instance, owing to the findings of Abd El-Gawad et al. (2019), supplementing the diet with Ly at a level of 400 mg/kg for 60 days might boost immunological response and sustain antioxidant defensive mechanisms in yellow perch. According to Farouk et al. (2021), oral treatment of Ly offers sufficient protection against the neurotoxicity of ACR on rat brain tissue structure and functions through regulation of oxidative and antioxidant activities. Besides, Fatma and Rabab (2019) proved that Ly attenuates the ACR-induced hepatocyte damage due to its high antioxidant properties. Owing to fish investigations, Ly helps fish stay healthy by boosting their immunological and antioxidative responses (Dawood et al. 2020). Because of its protective function via scavenging the excessive reactive oxygen species (ROS) mechanisms, fish diet inclusion with lycopene was shown to be mitigating oxidative stress (Wertz et al. 2004). It may also promote the expression of antioxidative-related genes and metabolic pathways involving phase II detoxifying enzymes (Lian and Wang 2008). Ly is a well-known, extremely effective scavenger of single t-oxygen (1O2) and other stimulated reactive oxygen molecules. During the 1O2 quenching process, the energy of generated 1O2 is transported into the lycopene molecule, converting lycopene to the energy-rich triplet form. Conversely, other generated free radicals such as OH, NO2, or peroxy-nitrite could induce oxidative degradation of the lycopene molecule. So, Ly might protect lipids, proteins, and DNA against the in vivo oxidation (Yonar 2012).
Salts
Acrylamide is a carcinogenic and neurotoxic compound produced in heat-processed starchy foods. ACR is produced during the heating process of dietary components as a byproduct of the Maillard reaction induced between decreasing sugars and amino acids (Chen et al. 2016). Generally, heat-processed commercial protein-rich foods, for instance fish, meat, and chicken, often contain less ACR levels than carbohydrate-rich meals, such as French fries, potato or tortilla chips, grains, and baked products (Açar et al. 2012). The initial reaction step is thought to involve the creation of a primary base. The reaction begins with the binding of nucleophilic asparagine to the di-carbonyl molecules partly positive carbonyl carbon, which is followed by the loss of a proton from nitrogen and the binding of a free proton to oxygen (Mottram et al. 2002). Furthermore, Becalski et al. (2003) examined whether ACR might be produced via the rearrangement of nitrogen-based compounds found in cooked meals. As a result, finding an efficient method to eliminate ACR production in heat-processed foods is an important concern in the food manufacturing industries.
It is important to note that anions as well as cations have a significant impact on ACR synthesis during food manufacturing. For instance, sodium chloride inhibits ACR production via a variety of methods. It has the ability to form complex compounds by binding amine groups with certain intermediates created from the Maillard reaction (Lindsay and Jang 2005). Recently, it was proven that positive charge ions alter the pathway of the Maillard reaction by boosting the removal of water from glucose (Ciesarova et al. 2006). Besides, Na+ ion has been discovered to binding with asparagine to prevent ACR production (Omotosho 2015). Finally, the addition of sodium chloride salt may diminish water activity, resulting in less oil absorption and therefore promoting acrolein production (Omini et al. 2019).
In recent years, many methods for decreasing acrylamide production in heated meals have been suggested, including divalent cations, such as calcium salts. Chen et al. (2016) suggested that enriched shrimp fried chips with 0.1% calcium lactate resulted in the highest reduction of ACR formation level. According to Kukurová (2015) results, calcium chloride was attained to be the most effective via removing almost 90% of the ACR content. Also, sodium acid, sodium pyro-phosphate, and potassium di-hydrogen phosphate were highly efficient in removing nearly 75% from the total acrylamide level, followed by calcium lactate, sodium chloride, and potassium chloride, which resulted in a reduction of ACR content to 40–45%, and finally sodium and potassium hydrogen carbonates were found to be effective in removing nearly 30% from ACR content. The quantity and type of calcium substitutes applied had a strong impact on the formation of ACR in fish processed chips. As previously reported by Gökmen et al. (2007a) and Gökmen et al. (2007b), the inclusion of organic acid in cookie fish recipes may increase the formation of ACR as a result of sucrose hydrolysis to decreasing sugars level. Thus, the decreasing level of sugar in shrimp chips enriched with calcium citrate was higher than that recorded in shrimp chips supplemented with other calcium salts (Chen et al. 2016). The calcium carbonate and chloride salt were found to be effective in reducing ACR production, while adding up to 0.2% calcium propionate salt for food preservation resulted in an increase in ACR production of 90%. Therefore, enhanced fish-heat processed products containing cationic or anionic salts should not be the fish processing sector’s first option for eliminating ACR generation, while avoiding high processing heat or overcooking is a more effective and safe method of preventing ACR formation.
Phytochemicals
Phytochemicals may be defined as a bioactive molecule found in many medicinal plants that is able to regulate metabolic functions and boost health status (Naiel et al. 2020a). They have a favorable impact such as antioxidant properties, promoted enzyme activity, and upregulated or downregulated specifically related genes (Naiel et al. 2020c). The antioxidant properties of herbs also played a vital role in preventing the harmful impact of ACR on fish health via improving the antioxidant enzyme activity and reducing the free radical concentration (Hassan et al. 2020). Phytochemical molecules could prevent ACR formation in fish flesh under high temperatures in several ways. The main pathway depends on activating the reduced glutathione activity, inhibiting the reactive oxygen formation, and reducing oxidative stress (Zhu et al. 2012). Also, herbs or their extracts may have an ameliorating effect due to phytochemical compounds that are simply oxidized, as well as the rate of oxidation and the oxidized products that are discovered to be reacted with asparagine (Bartoszek 2002).
Several cellular studies in animals or fish proved the potential protective role of herbs or their extracts against ACR hazards for natural phytochemical compounds such as eugenol, iso-eugenol, turmeric, polyphenol, flavonoids, N-acetylcysteine, gallate, and carnosic acid (Albalawi et al. 2018; Faria et al. 2019a; Krishnan and Kang 2019; Ning et al. 2021; Singh et al. 2019; Wang et al. 2021). Table 4 displays a collection of references that demonstrate the possible ameliorative effect of certain phytochemical substances against ACR toxicity. Besides, the phenol ion may create a powerful nucleophile, or electron giver, to the final chemical carcinogen’s electrophile. Moreover, the following essential functional groups are thought to be present in phenolics that allow them to serve as efficient electrophilic trapping agents: (1) one phenolic group must be present to react with catechol and decreased the pKa level. (2) At least one unsaturated substituent must be present in the aromatic ring in order for it to bend with reactive free oxygen. On the other hand, many studies examined the impact of different herbs and their extracts on ACR toxicity but found inconsistent results. It might depend on the antioxidants’ capacity to react with ACR intermediates, related chemicals, or ACR form itself, resulting in either decreasing or stimulating effects (Exon 2006). Thus, it is critical to conduct new experimental research on the efficiency of herbs or their extracts in reducing the toxicological risks of ACR on fish’s general health status and providing deep insights into cellular regulating activities under toxicity situations.
Conclusion
ACR is a highly water-soluble unsaturated organic polymer with a broad range of applications, including water purification, agricultural operations, paper production, and oil drilling. ACR is a neurotoxin carcinogenic, mutagenic, and reprotoxic compound for both humans and fish. The ACR could be generated in high-temperature processed food. Moreover, the ecological fate of ACR following deterioration or release into water streams has gained great attention. Because of its high solubility, ACR remains in water after treatment and is not easily absorbed by the soil. Besides, ACR is found to be biodegradation by bacteria in surface water. A contaminated ecosystem with ACR presented higher potential hazards in the behavior, reproduction, and nervous system of fish. In addition, ACR-contaminated water has deleterious effects on the early development of some fish species, for instance decreased hatching percentage, prolonged embryo hatching time, delayed larval development, and induced malformations. Transcriptome reports indicated that these harmful effects might be correlated with ACR-induced hypoxia and neurotoxicity. Though, treated fish exposed to ACR with a variety of fatty acids, salts, or phytochemical substances showed a high elimination rate against its toxicity. Meanwhile, biodegradation of ACR-contaminated environments by bacterial strains found in natural ecosystems provides an efficient solution to its ecological fate. When using fatty acids, salts, and phytochemical compounds to reduce ACR risks, both dosage and exposure duration of ACR levels should be taken into consideration. There is a need to identify the ACR threats to aquatic fish via further research efforts and to propose more applicable methods that may eliminate the risks in both fish and humans. Furthermore, this literature provides essential information on the toxicity of ACR in fish and crustaceans, which might be very useful in maintaining ACR’s aquatic environmental risk assessment.
Data availability
All related data were available under reasonable request from the corresponding author.
Code availability
Not applicable.
Abbreviations
- ACR:
-
Acrylamide
- UV:
-
Ultraviolet radiation
- IARC:
-
The International Agency for Research on Cancer
- LC50:
-
Lethal concentration 50
- NrF2:
-
Nuclear factor erythroid 2–related factor 2
- DNA:
-
Deoxyribonucleic acid
- AChE:
-
Acetylcholinesterase
- O2 :
-
Oxygen
- CYP1 A1:
-
Cholesterol side-chain cleavage enzyme
- EROD:
-
Ethoxyresorufin-O-deethylase
- LPO:
-
Lipid peroxidative
- ATPase:
-
Adenosine triphosphatase
- EFA:
-
Essential fatty acids
- EPA:
-
Eico-sapentaenoic acids
- DHA:
-
Docosahexaenoic acids
- HSP27:
-
Heat shock protein 27
- PUFA:
-
Polyunsaturated fatty acids
- SeNPs:
-
Selenium nanoparticles
- Ly:
-
Lycopene
- CNS:
-
The central nervous system
- ROS:
-
Reactive oxygen species
- 1O2 :
-
Single t-oxygen
- OH:
-
Hydroxyl group
- NO2 :
-
Nitrogen dioxide
- Na+ :
-
Sodium ion
- pKa:
-
Acid dissociation constants
- NF-κB:
-
The Nuclear factor kappa-light-chain-enhancer of activated B cells
- mM:
-
Millimolar
References
Abatenh E, Gizaw B, Tsegaye Z, Wassie M (2017) The role of microorganisms in bioremediation-a review. Open J Environ Biol 2:038–046
Abd El-Gawad EA, Wang H-P, Yao H (2019) Diet supplemented with synthetic carotenoids: effects on growth performance and biochemical and immunological parameters of yellow perch (Perca flavescens). Front Physiol 10:1056
Açar ÖÇ, Pollio M, Di Monaco R, Fogliano V, Gökmen V (2012) Effect of calcium on acrylamide level and sensory properties of cookies. Food Bioprocess Technol 5:519–526
Aktağ IG, Hamzalıoğlu A, Kocadağlı T, Gökmen V (2022) Dietary exposure to acrylamide: a critical appraisal on the conversion of disregarded intermediates into acrylamide and possible reactions during digestion. Curr Res Food Sci 5:1118–1126
Albalawi A, Alhasani RHA, Biswas L, Reilly J, Akhtar S, Shu X (2018) Carnosic acid attenuates acrylamide-induced retinal toxicity in zebrafish embryos. Exp Eye Res 175:103–114
Albedwawi AS, Turner MS, Olaimat AN, Osaili TM, Al-Nabulsi AA, Liu S-Q, Shah NP, Ayyash MM (2021) An overview of microbial mitigation strategies for acrylamide: lactic acid bacteria, yeast, and cell-free extracts. LWT 143:111159
Al-Gabri NA, Saghir SA, Al-Hashedi SA, El-Far AH, Khafaga AF, Swelum AA, Al-Wajeeh AS, Mousa SA, Abd El-Hack ME, Naiel MA (2021) Therapeutic potential of thymoquinone and its nanoformulations in pulmonary injury: a comprehensive review. Int J Nanomed 16:5117
Aras D, Cakar Z, Ozkavukcu S, Can A, Cinar O (2017) In vivo acrylamide exposure may cause severe toxicity to mouse oocytes through its metabolite glycidamide. PLoS One 12:e0172026
Babuji P, Thirumalaisamy S, Duraisamy K, Periyasamy G (2023) Human health risks due to exposure to water pollution: a review. Water 15:2532
Backe WJ, Yingling V, Johnson T (2014) The determination of acrylamide in environmental and drinking waters by large-volume injection–hydrophilic-interaction liquid chromatography and tandem mass spectrometry. J Chromatogr A 1334:72–78
Bao M, Chen Q, Li Y, Jiang G (2009) Study of biodegradation of partially hydrolyzed polyacrylamide in an oil reservoir after polymer flooding. Conference: The 32. AMOP technical seminar on environmental contamination and response, Vancouver, BC (Canada), 9-11 Jun 2009, vol 2, pp 749-758. Available from Environment Canada, Technical Seminar Coordinator, Emergencies Science and Technology Section, 335 River Road, Ottawa, Ontario, K1A 0H3 or from the Internet at www.etc-cte.ec.gc.ca/news/conferences_e.html
Bartoszek A (2002) Mutagenic, carcinogenic, and chemo-preventive compounds in foods. Chem Funct Prop Food Components, 3th edn. CRC Press, Boca Raton, pp 307–336
Becalski A, Lau BP-Y, Lewis D, Seaman SW (2003) Acrylamide in foods: occurrence, sources, and modeling. J Agric Food Chem 51:802–808
Besaratinia A, Pfeifer GP (2007) A review of mechanisms of acrylamide carcinogenicity. Carcinogenesis 28:519–528
Bolan NS, Kirkham M, Halsband C, Nugegoda D, Ok YS (2020) Particulate plastics in terrestrial and aquatic environments, 1st edn. CRC Press, Boca Raton, p 466. https://doi.org/10.1201/9781003053071
Buranasilp K, Charoenpanich J (2011) Biodegradation of acrylamide by Enterobacter aerogenes isolated from wastewater in Thailand. J Environ Sci 23:396–403
Cha M, Chambliss GH (2011) Characterization of acrylamidase isolated from a newly isolated acrylamide-utilizing bacterium, Ralstonia eutropha AUM-01. Curr Microbiol 62:671–678
Charoenpanich J (2013) Removal of acrylamide by microorganisms. Appl Bioremediation: Act Passive Approaches Book, In Tech, p 99. https://doi.org/10.5772/56150
Chen T-Y, Luo H-M, Hsu P-H, Sung W-C (2016) Effects of calcium supplements on the quality and acrylamide content of puffed shrimp chips. J Food Drug Anal 24:164–172
Ciesarova Z, Kiss E, Kolek E (2006) Study of factors affecting acrylamide levels in model systems. Czech J Food Sci 24:133
Croll B, Arkell G, Hodge R (1974) Residues of acrylamide in water. Water Resour 8:989–993
Dawood MA, Abdel-Tawwab M, Abdel-Latif HM (2020) Lycopene reduces the impacts of aquatic environmental pollutants and physical stressors in fish. Rev Aquac 12:2511–2526
Durazzo A, D’Addezio L, Camilli E, Piccinelli R, Turrini A, Marletta L, Marconi S, Lucarini M, Lisciani S, Gabrielli P (2018) From plant compounds to botanicals and back: a current snapshot. Molecules 23:1844
El-hameed A, Samah A, Negm SS, Ismael NE, Naiel MA, Soliman MM, Shukry M, Abdel-Latif HM (2021) Effects of activated charcoal on growth, immunity, oxidative stress markers, and physiological responses of Nile tilapia exposed to sub-lethal imidacloprid toxicity. Animals 11:1357
Elhassaneen YA, Abd Elhady YA (2014) Onion peel powder alleviate acrylamide-induced cytotoxicity and immunotoxicity in liver cell culture. Life Sci J 11:381–388
El-Shehawi AM, Sayed S, Hassan MM, Al-Otaibi S, Althobaiti F, Elseehy MM, Soliman M (2022) Taify pomegranate juice (TPJ) abrogates acrylamide-induced oxidative stress through the regulation of antioxidant activity, inflammation, and apoptosis-associated genes. Front Vet Sci 9:833605
EPA U (1994) U.S. Environmental protection agency. Office of pollution prevention and toxics. Chemical in the environment: acrylamide. EPA 79—06-1, Washington DC
Exon J (2006) A review of the toxicology of acrylamide. J Toxicol Environ Health Part B 9:397–412
Fallahzadeh RA, Ghaneian MT, Miri M, Dashti MM (2017) Spatial analysis and health risk assessment of heavy metals concentration in drinking water resources. Environ Sci Pollut Res 24:24790–24802
Fan M, Xu X, Lang W, Wang W, Wang X, Xin A, Zhou F, Ding Z, Ye X, Zhu B (2023) Toxicity, formation, contamination, determination and mitigation of acrylamide in thermally processed plant-based foods and herbal medicines: a review. Ecotoxicol Environ Saf 260:115059
FAO, WHO (2002) Consultation on Health Implications of Acrylamide in Food, (2002 : Geneva, Switzerland) & Food and Agriculture Organization of the United Nations & World Health Organization. Food Safety Programme. (2002). Health implications of acrylamide in food : report of a joint FAO/WHO consultation, WHO Headquarters, Geneva, Switzerland, 25-27 June 2002. Food Safety Programme, World Health Organization, Geneva
Farag MR, Alagawany M, Bilal RM, Gewida AG, Dhama K, Abdel-Latif HM, Amer MS, Rivero-Perez N, Zaragoza-Bastida A, Binnaser YS (2021) An overview on the potential hazards of pyrethroid insecticides in fish, with special emphasis on cypermethrin toxicity. Animals 11:1880
Faria M, Ziv T, Gómez-Canela C, Ben-Lulu S, Prats E, Novoa-Luna KA, Admon A, Piña B, Tauler R, Gómez-Oliván LM (2018) Acrylamide acute neurotoxicity in adult zebrafish. Sci Rep 8:1–14
Faria M, Prats E, Gómez-Canela C, Hsu C-Y, Arick MA, Bedrossiantz J, Orozco M, Garcia-Reyero N, Ziv T, Ben-Lulu S (2019a) Therapeutic potential of N-acetylcysteine in acrylamide acute neurotoxicity in adult zebrafish. Sci Rep 9:1–11
Faria M, Valls A, Prats E, Bedrossiantz J, Orozco M, Porta JM, Gómez-Oliván LM, Raldúa D (2019b) Further characterization of the zebrafish model of acrylamide acute neurotoxicity: gait abnormalities and oxidative stress. Sci Rep 9:1–7
Faroon O, Mumtaz M, Ruiz P, Wohlers D (2009) Draft toxicological profile for acrylamide. CDC Stacks 104(1):191–238
Farouk SM, Gad FA, Almeer R, Abdel-Daim MM, Emam MA (2021) Exploring the possible neuroprotective and antioxidant potency of lycopene against acrylamide-induced neurotoxicity in rats’ brain. Biomed Pharmacother 138:111458
Fatma FH, Rabab FH (2019) Ameliorative effect of lycopene on acrylamide-induced hepatotoxicity in adult albino rats. Med J Cairo Univ 87:4129–4135
Flik G, Bevelander GS, Klaren PH (2009) Regulation of calcium and magnesium handling in fishes. Essent Rev Exp Biol 1:151–181
Fonger GC, Hakkinen P, Jordan S, Publicker S (2014) The National Library of Medicine’s (NLM) Hazardous Substances Data Bank (HSDB): background, recent enhancements and future plans. Toxicology 325:209–216
Friedman MA, Dulak LH, Stedham MA (1995) A lifetime oncogenicity study in rats with acrylamide. Fundam Appl Toxicol 27:95–105
Gharib AA, Abdel-Hamid EA, Mousa MA, Naiel MA (2022) Improving water quality, growth performance, and modulating some stress physiological biomarkers in Cyprinus carpio using raw date nuclei as a zinc adsorbent agent. Appl Water Sci 12:1–9
Gökmen V, Açar ÖÇ, Köksel H, Acar J (2007a) Effects of dough formula and baking conditions on acrylamide and hydroxymethylfurfural formation in cookies. Food Chem 104:1136–1142
Gökmen V, Akbudak B, Serpen A, Acar J, Turan ZM, Eriş A (2007b) Effects of controlled atmosphere storage and low-dose irradiation on potato tuber components affecting acrylamide and color formations upon frying. Eur Food Res Technol 224:681–687
Gopika C, Sumi N, Chitra K (2018a) Involvement of reactive oxygen species in the toxicity of acrylamide in muscle tissue of the fish, Oreochromis niloticus (Linnaeus, 1758). World J Pharm Res 7:1617–1628
Gopika C, Sumi N, Chitra K (2018b) Acute neurotoxicity effect of acrylamide in Oreochromis niloticus (Linnaeus, 1758). Int J Fish Aquat Res 3:1–8
Haasch ML, Sutherland LA, Wejksnora PJ, Lech JJ (1992) Effect of acrylamide monomer on hepatic CYP1A1 monooxygenase induction in rainbow trout. Comp Biochem Physiol C Comp Pharmacol Toxicol 102:281–286
Hassan HA, El-Kholy WM, El-Sawi MR, Galal NA, Ramadan MF (2020) Myrtle (Myrtus communis) leaf extract suppresses hepatotoxicity induced by monosodium glutamate and acrylamide through obstructing apoptosis, DNA fragmentation, and cell cycle arrest. Environ Sci Pollut Res 27:23188–23198
Hays SM, Aylward LL (2008) Biomonitoring Equivalents (BE) dossier for acrylamide (AA)(CAS No. 79–06-1). Regul Toxicol Pharmacol 51:S57–S67
Holick CN, Giovannucci EL, Rosner B, Stampfer MJ, Michaud DS (2007) Prospective study of intake of fruit, vegetables, and carotenoids and the risk of adult glioma. Am J Clin Nutr 85:877–886
Huang M, Jiao J, Wang J, Xia Z, Zhang Y (2018) Exposure to acrylamide induces cardiac developmental toxicity in zebrafish during cardiogenesis. Environ Pollut 234:656–666
Huang M, Zhu F, Jiao J, Wang J, Zhang Y (2019) Exposure to acrylamide disrupts cardiomyocyte interactions during ventricular morphogenesis in zebrafish embryos. Sci Total Environ 656:1337–1345
Ibrahim MA, Ibrahem MD (2020) Acrylamide-induced hematotoxicity, oxidative stress, and DNA damage in liver, kidney, and brain of catfish (Clarias gariepinus). Environ Toxicol 35:300–308
Ismael NE, Abd El-hameed SA, Salama AM, Naiel MA, Abdel-Latif HM (2021) The effects of dietary clinoptilolite and chitosan nanoparticles on growth, body composition, haemato-biochemical parameters, immune responses, and antioxidative status of Nile tilapia exposed to imidacloprid. Environ Sci Pollut Res 28(23):29535–29550
Jia L, Raghupathy RK, Albalawi A, Zhao Z, Reilly J, Xiao Q, Shu X (2017) A colour preference technique to evaluate acrylamide-induced toxicity in zebrafish. Comp Biochem Physiol Part C: Toxicol Pharmacol 199:11–19
Khafaga AF, Naiel MA, Dawood MA, Abdel-Latif HM (2020) Dietary Origanum vulgare essential oil attenuates cypermethrin-induced biochemical changes, oxidative stress, histopathological alterations, apoptosis, and reduces DNA damage in common carp (Cyprinus carpio). Aquat Toxicol 228:105624
Kinsella JE (1990) Sources of omega-3 fatty acids in human diets. In: Book: Omega-3 fatty acids in health and disease, 1st edn. CRC Press, p 43
Komoike Y, Matsuoka M (2019) In vitro and in vivo studies of oxidative stress responses against acrylamide toxicity in zebrafish. J Hazard Mater 365:430–439
Komoike Y, Nomura-Komoike K, Matsuoka M (2020) Intake of acrylamide at the dietary relevant concentration causes splenic toxicity in adult zebrafish. Environ Res 189:109977
Kopańska M, Łagowska A, Kuduk B, Banaś-Ząbczyk A (2022) Acrylamide neurotoxicity as a possible factor responsible for inflammation in the cholinergic nervous system. Int J Mol Sci 23:2030
Krautter GR, Mast RW, Alexander HC, Wolf CH, Friedman MA, Koschier FJ, Thompson CM (1986) Acute aquatic toxicity tests with acrylamide monomer and macroinvertebrates and fish. Environ Toxicol Chem: Int J 5:373–377
Krishnan M, Kang SC (2019) Vitexin inhibits acrylamide-induced neuroinflammation and improves behavioral changes in zebrafish larvae. Neurotoxicol Teratol 74:106811
Kukurová K (2015) Effect of inorganic salts on acrylamide formation in cereal matrices. Acrylamide in Food: Analysis, Content and Potential Health Effects 27:377–392. https://doi.org/10.1016/B978-0-12-802832-2.00019-X
Kusnin N, Syed MA, Ahmad SA (2015) Toxicity, pollution and biodegradation of acrylamide –a mini review. J Biochem Microbiol Biotechnol 3:6–12
Lakshmi D, Gopinath K, Jayanthy G, Anjum S, Prakash D, Sudhandiran G (2012) Ameliorating effect of fish oil on acrylamide induced oxidative stress and neuronal apoptosis in cerebral cortex. Neurochem Res 37:1859–1867
Larguinho M, Cordeiro A, Diniz MS, Costa PM, Baptista PV (2014a) Metabolic and histopathological alterations in the marine bivalve Mytilus galloprovincialis induced by chronic exposure to acrylamide. Environ Res 135:55–62
Larguinho M, Costa PM, Sousa G, Costa MH, Diniz MS, Baptista PV (2014b) Histopathological findings on Carassius auratus hepatopancreas upon exposure to acrylamide: correlation with genotoxicity and metabolic alterations. J Appl Toxicol 34:1293–1302
Lentz R, Andrawes F, Barvenik F, Koehn A (2008) Acrylamide monomer leaching from polyacrylamide-treated irrigation furrows. J Environ Qual 37:2293–2298
Lian F, Wang XD (2008) Enzymatic metabolites of lycopene induce Nrf2-mediated expression of phase II detoxifying/antioxidant enzymes in human bronchial epithelial cells. Int J Cancer 123:1262–1268
Ligina V, Martin R, Aiswarya MV, Mashirin KR, Chitra KC (2022) Acute and sublethal effects of acrylamide on the freshwater fish Anabas testudineus (Bloch, 1792). Environ Sci Pollut Res 29:90835–90851
Lin C-H, Hwang P-P (2016) The control of calcium metabolism in zebrafish (Danio rerio). Int J Mol Sci 17:1783
Lindsay RC, Jang S (2005) Chemical intervention strategies for substantial suppression of acrylamide formation in fried potato products. In: Friedman M, Mottram D (eds) Chemistry and Safety of Acrylamide in Food. Advances in Experimental Medicine and Biology, vol 561. Springer, Boston, pp 393–404. https://doi.org/10.1007/0-387-24980-X_30
Mabile L, Piolot A, Boulet L, Fortin L-J, Doyle N, Rodriguez C, Davignon J, Blache D, Lussier-Cacan S (2001) Moderate intake of n− 3 fatty acids is associated with stable erythrocyte resistance to oxidative stress in hypertriglyceridemic subjects. Am J Clin Nutr 74:449–456
McDonald A (1995) Some industrial chemicals: IARC monographs on the evaluation of carcinogenic risks to humans. Vol 60. Occup Environ Med 52:360
Mehana E-SE, Khafaga AF, Elblehi SS, Abd El-Hack ME, Naiel MA, Bin-Jumah M, Othman SI, Allam AA (2020) Biomonitoring of heavy metal pollution using acanthocephalans parasite in ecosystem: an updated overview. Animals 10:811
Mekawi EM, Abbas MH, Mohamed I, Jahin HS, El-Ghareeb D, Al-Senani GM, AlMufarij RS, Abdelhafez AA, Mansour RR, Bassouny MA (2023) Potential hazards and health assessment associated with different water uses in the main industrial cities of Egypt. J Saudi Chem Soc 27:101587
Moran NE, Erdman JW Jr, Clinton SK (2013) Complex interactions between dietary and genetic factors impact lycopene metabolism and distribution. Arch Biochem Biophys 539:171–180
Mottram DS, Wedzicha BL, Dodson AT (2002) Acrylamide is formed in the Maillard reaction. Nature 419:448–449
Moura DS, Mota RODd, Gonçalves JF, Vasconcelos NFd, Reis MA, Grisolia CK (2019) Evaluation of the embryotoxicity in zebrafish (Danio rerio) of the flocculant and coagulant compounds used for water remediation. Acta Limnol Bras 31:1–7
Naiel MA, Ismael NE, Abd El-hameed SA, Amer MS (2020b) The antioxidative and immunity roles of chitosan nanoparticle and vitamin C-supplemented diets against imidacloprid toxicity on Oreochromis niloticus. Aquaculture 523:735219
Naiel MA, Ismael NE, Negm SS, Ayyat MS, Al-Sagheer AA (2020c) Rosemary leaf powder–supplemented diet enhances performance, antioxidant properties, immune status, and resistance against bacterial diseases in Nile Tilapia (Oreochromis niloticus). Aquaculture 526:735370
Naiel MA, Shehata AM, Negm SS, Abd El-Hack ME, Amer MS, Khafaga AF, Bin-Jumah M, Allam AA (2020d) The new aspects of using some safe feed additives on alleviated imidacloprid toxicity in farmed fish: a review. Rev Aquac 12:2250–2267
Naiel MA, Alagawany M, Patra AK, El-Kholy AI, Amer MS, Abd El-Hack ME (2021a) Beneficial impacts and health benefits of macroalgae phenolic molecules on fish production. Aquaculture 534:736186
Naiel MA, Farag MR, Gewida AG, Elnakeeb MA, Amer MS, Alagawany M (2021b) Using lactic acid bacteria as an immunostimulants in cultured shrimp with special reference to Lactobacillus spp. Aquacult Int 29:219–231
Naiel MA, Alagawany M, Patra AK, El-Kholy AI, Amer MS, Abd El-Hack ME (2020a) Beneficial impacts and health benefits of macroalgae phenolic molecules on fish production. Aquaculture 534:736186. https://doi.org/10.1016/j.aquaculture.2020.736186
Ning W, Li S, Tsering J, Ma Y, Li H, Ma Y, Ogbuehi AC, Pan H, Li H, Hu S (2021) Protective effect of Triphala against oxidative stress-induced neurotoxicity. BioMed Res Int 2021:11. https://doi.org/10.1155/2021/6674988
Nyyssölä A, Ahlgren J (2019) Microbial degradation of polyacrylamide and the deamination product polyacrylate. Int Biodeterior Biodegradation 139:24–33
Omini J, Omotosho O, Akinyomi O (2019) Sodium chloride inhibits acrylamide formation during deep fat frying of plantain. J Phys Conf Ser. 1378(4):042068. https://doi.org/10.1088/1742-6596/1378/4/042068
Omotosho OE (2015) Effect of deep-fat frying on the vitamins, proximate and mineral contents of cocoyam. Pak J Biol Sci 18(3):295–299. https://doi.org/10.3923/pjbs.2015.295.299.18:295-299
Pacetti D, Gil E, Frega NG, Álvarez L, Dueñas P, Garzón A, Lucci P (2015) Acrylamide levels in selected Colombian foods. Food Addit Contam: Part B 8:99–105
Panahi Y, Rajaee SM, Johnston TP, Sahebkar A (2019) Neuroprotective effects of antioxidants in the management of neurodegenerative disorders: a literature review. J Cell Biochem 120:2742–2748
Park J-S, Samanta P, Lee S, Lee J, Cho J-W, Chun H-S, Yoon S, Kim W-K (2021) Developmental and neurotoxicity of acrylamide to zebrafish. Int J Mol Sci 22:3518
Patial V, Kumar V, Joshi R, Gupta M, Singh D (2022) Acrylamide mitigation in foods using recombinant L-asparaginase: an extremozyme from Himalayan Pseudomonas sp. PCH182. Food Res Int 162:111936
Peng L, Xu Y, Zhou F, Sun B, Tie B, Lei M, Shao J, Gu J (2016) Enhanced removal of Cd (II) by poly (acrylamide-co-sodium acrylate) water-retaining agent incorporated nano hydrous manganese oxide. Mater Des 96:195–202
Petersen DW, Lech JJ (1987) Hepatic effects of acrylamide in rainbow trout. Toxicol Appl Pharmacol 89:249–255
Petersen DW, Kleinow KM, Kraska RC, Lech JJ (1985) Uptake, disposition, and elimination of acrylamide in rainbow trout. Toxicol Appl Pharmacol 80:58–65
Petersen DW, Cooper KR, Friedman MA, Lech JJ (1987) Behavioral and histological effects of acrylamide in rainbow trout. Toxicol Appl Pharmacol 87:177–184
Petyaev IM (2016) Lycopene deficiency in ageing and cardiovascular disease. Oxidative Med Cell Longev 2016:3218605. https://doi.org/10.1155/2016/3218605
Pratt SJ, Hernández-Ochoa E, Martin SS (2020) Calcium signaling: breast cancer’s approach to manipulation of cellular circuitry. Biophys Rev 12:1343–1359
Radwan MA, El-Gendy KS, Gad AF, Khamis AE, Eshra E-SH (2019) Ecotoxicological biomarkers as investigating tools to evaluate the impact of acrylamide on Theba pisana snails. Environ Sci Pollut Res 26:14184–14193
Ramesh M, Sankaran M, Veera-Gowtham V, Poopal RK (2014) Hematological, biochemical and enzymological responses in an Indian major carp Labeo rohita induced by sublethal concentration of waterborne selenite exposure. Chem Biol Interact 207:67–73
Rao AV, Rao LG (2007) Carotenoids and human health. Pharmacol Res 55:207–216
Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, Koffel JB (2021) PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst Rev 10:1–19
Rice JM (2005) The carcinogenicity of acrylamide. Mutat Res/Genet Toxicol Environ Mutagen 580:3–20
Saif-Elnasr M, Abdel-Aziz N, El-Batal AI (2019) Ameliorative effect of selenium nanoparticles and fish oil on cisplatin and gamma irradiation-induced nephrotoxicity in male albino rats. Drug Chem Toxicol 42:94–103
Seidavi A, Tavakoli M, Asroosh F, Scanes CG, Abd El-Hack ME, Naiel MA, Taha AE, Aleya L, El-Tarabily KA, Swelum AA (2021) Antioxidant and antimicrobial activities of phytonutrients as antibiotic substitutes in poultry feed. Environ Sci Pollut Res 29(4):5006–5031. https://doi.org/10.1007/s11356-021-17401-w
Sen A, Ozgun O, Arinç E, Arslan S (2012) Diverse action of acrylamide on cytochrome P450 and glutathione S-transferase isozyme activities, mRNA levels and protein levels in human hepatocarcinoma cells. Cell Biol Toxicol 28:175–186
Shanker R, Ramakrishna C, Seth PK (1990) Microbial degradation of acrylamide monomer. Arch Microbiol 154:192–198
Shanker R, Seth PK (1986) Toxic effects of acrylamide in a freshwater fish, Heteropneustes fossilis. Bull Environ Contam Toxicol 37(2):274–280. https://doi.org/10.1007/BF01607761
Sharif S, Hanif NQ, Ghazanfar S, Imran M, Naiel MA, Alagawany M (2023) Dominance of bacillus sp. alter microbiological and nutritional quality and improve aerobic stability of the corn silage. Rend Lincei Sci Fis Nat 34:283–293
Sharma C, Kang SC (2020) Garcinol pacifies acrylamide induced cognitive impairments, neuroinflammation and neuronal apoptosis by modulating GSK signaling and activation of pCREB by regulating cathepsin B in the brain of zebrafish larvae. Food Chem Toxicol 138:111246
Sharma K, Kaith B, Kumar V, Kumar V, Som S, Kalia S, Swart H (2013) Synthesis and properties of poly (acrylamide-aniline)-grafted gum ghatti based nanospikes. RSC Adv 3:25830–25839
Shen S-M, Wan T-J, Hwang H-Y (2012) Enhancement of degradation of acrylamide coupled with salad oil by Pseudomonas aeruginosa DS-4 using incubation periods. Biocatal Agric Biotechnol 1:110–114
Singh A (2020) Acrylamide induced Parkinson’s like neurobehavioral abnormalities in adult zebrafish via gut brain axis. Parkinsonism Relat Disord 79:e21
Singh KG, Keerthana P, Johnson S (2019) Neuroprotective effect of Mulberry (Morus nigra) leaf extract on acrylamide–induced zebrafish (Danio rerio). J Environ Res Manag 10:12–15
Sirot V, Rivière G, Leconte S, Vin K, Traore T, Jean J, Carne G, Gorecki S, Veyrand B, Marchand P (2019) French infant total diet study: dietary exposure to heat-induced compounds (acrylamide, furan and polycyclic aromatic hydrocarbons) and associated health risks. Food Chem Toxicol 130:308–316
Spencer H, Wahome J, Haasch M (2018) Toxicity evaluation of acrylamide on the early life stages of the zebrafish embryos (Danio rerio). J Environ Prot 9:1082
Stadler RH, Scholz G (2004) Acrylamide: an update on current knowledge in analysis, levels in food, mechanisms of formation, and potential strategies of control. Nutr Rev 62:449–467
Tanekhy M, Mehana E (2022) Tumorigenesis and tumor markers in fish: an updated review. Bionature 42:36–55
Tapiero H, Townsend DM, Tew KD (2004) The role of carotenoids in the prevention of human pathologies. Biomed Pharmacother 58:100–110
Tareke E, Rydberg P, Karlsson P, Eriksson S, Törnqvist M (2002) Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J Agric Food Chem 50:4998–5006
Tepe Y (2015) In book: Acrylamide in food analysis, content and potential health effects. 1st edn. Chapter: Acrylamide in Surface and Drinking Water, Publisher: Elsevier, pp 275–293. https://doi.org/10.1016/B978-0-12-802832-2.00014-0
Tepe Y, Çebi A (2019) Acrylamide in environmental water: a review on sources, exposure, and public health risks. Expo Health 11:3–12
Torres RC, Garbo AG, Walde RZML (2014) Larvicidal activity of Persea americana Mill. against Aedes aegypti. Asian Pac J Trop Med 7:S167–S170
Valipour M, Montazar AA (2012) Sensitive analysis of optimized infiltration parameters in SWDC model. Adv Environ Biol 6(9):2574–2582
van Dijk-Looijaard A, Van Genderen J (2000) Levels of exposure from drinking water. Food Chem Toxicol 38:S37–S42
Ver Vers LM (1999) Determination of acrylamide monomer in polyacrylamide degradation studies by high-performance liquid chromatography. J Chromatogr Sci 37:486–494
Wang C-C, Lee C-M (2001) Denitrification with acrylamide by pure culture of bacteria isolated from acrylonitrile–butadiene–styrene resin manufactured wastewater treatment system. Chemosphere 44:1047–1053
Wang S, Wang H, Chen Y, Liu J, He X, Huang D, Wu Y, Chen Y, Weng Z (2021) Protective effects of (-)-epigallocatechin gallate and curcumin against acrylamide toxicity. Toxicol Environ Chem 103:199–218
Weitz D, Weintraub H, Fisher E, Schwartzbard AZ (2010) Fish oil for the treatment of cardiovascular disease. Cardiol Rev 18:258
Wertz K, Siler U, Goralczyk R (2004) Lycopene: modes of action to promote prostate health. Arch Biochem Biophys 430:127–134
WHO (2003) Acrylamide in drinking-water background document for development of WHO guidelines for drinking-water quality, 2011. Available at: www.who.int/water_sanitation_ health/water-quality/guidelines. Accessed 25 Aug 2023
WHO (2011) Guidelines for drinking-water quality. WHO Chron 38:104–108
Yonar ME (2012) The effect of lycopene on oxytetracycline-induced oxidative stress and immunosuppression in rainbow trout (Oncorhynchus mykiss, W.). Fish Shellfish Immunol 32:994–1001
Yu WZ, Shen P, Lim I, Shi RRS, Cai M, Chin YS, Tay AJ, Ang WM, Er JC, Lim GS (2023) Occurrence and dietary exposure to acrylamide from foods consumed within and outside main meals in Singapore. Foods 12:3022
Yue Z, Tian E, Chen Y, Luo L, Yang L, He L, Li L, Wang J (2021) The adverse effects of acrylamide exposure on the early development of marine medaka (Oryzias melastigma) and its mechanisms. Mar Pollut Bull 163:111875
Zamora R, Delgado RM, Hidalgo FJ (2015) Use of nucleophilic compounds, and their combination, for acrylamide removal. In: Gökmen V (ed.) Acrylamide in Food: Analysis, Content and Potential Health Effects. Academic Press, New York, pp 297–307
Zhu W, Jia Q, Wang Y, Zhang Y, Xia M (2012) The anthocyanin cyanidin-3-O-β-glucoside, a flavonoid, increases hepatic glutathione synthesis and protects hepatocytes against reactive oxygen species during hyperglycemia: Involvement of a cAMP–PKA-dependent signaling pathway. Free Radical Biol Med 52:314–327
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by MN, SN, SG, AF, and MS. The first draft of the manuscript was written by MN and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
All the authors equally participated to prepare the manuscript in all stages.
Consent for publication
Before the work is submitted to Environmental Science and Pollution Research Journal, all authors agreed with the content of the literature and given permission to submit by the corresponding author.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Acrylamide (ACR) is extensively applied in a broad range of industrial and water treatment applications.
• ACR degradation directly threatens the health of humans and aquatic wildlife.
• Organic or inorganic compounds might be able to eliminate ACR toxicity in fish.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Naiel, M.A.E., Negm, S.S., Ghazanfar, S. et al. Acrylamide toxicity in aquatic animals and its mitigation approaches: an updated overview. Environ Sci Pollut Res 30, 113297–113312 (2023). https://doi.org/10.1007/s11356-023-30437-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30437-4