Abstract
Purpose
This research studies the alleviation potential of N- or/and P- deprived Coccomyxa chodatii SAG 216–2 extracts as biostimulants on mercury stress (10 and 30 mg L−1) of wheat seedlings.
Materials
The study includes the interactive effect of mercury and biostimulants on growth, reactive nitrogen and oxygen species, membrane stability, and antioxidant activity in wheat seedlings.
Results
The imposed toxic effects of Hg-stress on the studied parameters were to a great extent less noticeable under different algal extracts, and the magnitude of augmentation was P-deprived extract > P-&N-deprived extract > N-deprived extract > Normal algal extract. Higher Hg-tolerance modulated by algal extracts, especially P-deprived extract, was associated with high antioxidant capacity and ferric reducing power. These activities could instigate the antioxidant system (enzymatic and non-enzymatic) under Hg-stress. Furthermore, the algal extracts broadly alleviated wheat chelating mechanism deterioration by Hg-stress via enhancing phytochelatins, reduced glutathione, and metallothioneins. Thus, the applied algal extracts retarded Hg accumulation in wheat tissues exposed to Hg stress. In addition, the nitrosative stress induced by Hg-stress in terms of high nitric oxide content was minimized by various algal extracts. All these regulations by algal extracts are reflected in high membrane stability as denoted by the reduction of lipid peroxidation, lipoxygenase, and methylglyoxal as a sign of reducing oxidative damage and reactive oxygen species (ROS).
Conclusion
Thus, we recommended using the macronutrient-deprived algal extracts of Coccomyxa chodatii SAG 216–2 as potential biostimulants of wheat growth under Hg-stress and may be under other stresses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to human activities, toxic trace element accumulation is a serious problem in many ecosystems, including air, water, and soil (El-Mahdy et al. 2021; Shahid et al. 2020; Seleiman et al. 2020a; ALHaithloul et al. 2022). The biological impact harmful or beneficial effects of these trace elements varied potentially on plants (Seleiman and Kheir 2018; Abu-Shahba et al. 2022). Mercury (Hg) is considered one of the most poisonous heavy metals. Hg accumulation is also a major source of heavy metal pollution (Han et al. 2018), where its accumulation in tissues has a lengthy half-life time. Ahmad et al. (2018) reported that the distribution of Hg as a contaminant in agricultural soils and hence to plants ascribed to its use as a seed disinfectant, herbicide, fertilizer, and blending agent during silver and gold extraction. Furthermore, industrialization and urbanization have resulted in the release of Hg into ecosystems all over the globe (Yang et al. 2018; Seleiman et al. 2020b). Mercury chloride (HgCl2), as a main source of Hg, is a significant chemical that has been utilized in skin-lightening lotions intended to eradicate freckles and skin blemishes (Chen et al. 2020).
It has been found that Hg at lower concentrations (1 mg L−1 Hg) severely hindered the development of garlic seedlings (Zhao et al. 2013). Also, Sahu et al. (2012) reported the phytotoxic effects of Hg (0.0, 2.5, 5.0, 10, and 25 μM) on wheat plants. Various concentrations of Hg (25 to 500 μM) adversely affected growth, chlorophyll, membrane stability, proline, and relative water content (Gontia-Mishra et al. 2016). Mercury causes an oxidative burst and influences the antioxidant system's defensive mechanism, even in low quantities (Dawood et al. 2021a). Also, Hg stress has a harmful influence on reactive oxygen species-metabolizing enzymes in garlic plants (Hu et al. 2020). Hg lowers photosynthetic capacity, respiration, and nutritional intake while also causing peroxidation of lipids and membrane degradation (Lima et al. 2019; Zhang et al. 2017). Hg interacts with biomolecule sulfhydryl groups, changing cellular structures and interfering with signaling patterns, causing mineral shortages by substituting Hg with various essential elements, hence increasing mineral deficiencies (Chen et al. 2014). For example, Hg impedes growth by interfering with nitrogen metabolism and changing root growth (Mondal et al. 2015). Plants, on the other hand, defend themselves against the damaging effects of heavy metals via a range of protective mechanisms, including immobilization, chelation, exclusion, osmotic control, compartmentalization, and increased antioxidant systems (Dawood and Azooz 2019, 2020; Hou et al. 2019; Moustafa-Farag et al. 2020; Ali et al. 2020; Rasheed et al. 2018).
As the toxicity of mercury is increasing globally, it is critical to take serious action to limit its pollution and prevent its accumulation in highly consumed crops as wheat. Although several approaches, such as phytoremediation, soil remediation, and heavy-metal-resistant cultivars, may help to reduce heavy metal deposition in crops, their effectiveness is limited (Rizwan et al. 2012; Badawy et al. 2022). Hu et al. (2020) found that synthesized or natural-sourced elicitors are highly effective approaches to minimizing heavy metal toxicity in plants. Many studies used products that have biological origins for more sustainable and environmentally friendly ways to upgrade agricultural productivity, such as microalgae, fungi, and bacteria. These biological materials have been identified as beneficial pools having biofertilizer and bio-stimulating potential to instigate crop production and protection (Chiaiese et al. 2018; Pan et al. 2019; Pathak et al. 2018; Singh et al. 2017). Biostimulants are compounds applied to seeds, plants, and soil and are either natural or synthetic. These products alter critical and structural processes to impact plant development and boost tolerant mechanisms for improving abiotic stress tolerance (Du Jardin 2015). These biostimulants may be composed of various raw ingredients, like hormones, algal extracts, humic acids, and plant growth-promoting microorganisms (Amer et al. 2021; Ashry et al. 2018). Algal biomass has been widely used in agriculture for thousands of years (Dmytryk and Chojnacka 2018; Górka et al. 2018). Still, in the twentieth century, numerous products derived from algal extracts have piqued the interest of farmers all over the globe (Dmytryk and Chojnacka 2018), such as seaweed extracts or their derived-active chemicals like alginates, carrageenans, laminarin, or their breakdown products have previously been used effectively in agriculture (Du Jardin 2015). On the other hand, microalgae have received less attention as a possible source of plant biostimulants. Many physiologically active substances, such as terpenoids, phenols, polysaccharides, carotenoids, phytohormones, and free fatty acids, have been isolated from cyanobacteria and microalgae in the literature and have potential benefits on crop productivity (Pan et al. 2019; Singh et al. 2017) which are found in microalgae and cyanobacteria (Pan et al. 2019). Both biomass and extracts from cyanobacteria and microalgae are commercially accessible, owing to their potential advantages for establishing sustainable agriculture (Górka et al. 2018). These algal metabolites impact plants via three mechanisms: (i) soil purification and fertilization; (ii) plant protection against abiotic and biotic stress factors, and (iii) plant growth (Górka et al. 2018; Ronga et al. 2019). The use of Ulva-algal extracts boosted the total chlorophyll and antioxidant content and the fresh weight, yield components, and grain weight of wheat cultivated on saline soils (Latique et al. 2021). Rathore et al. (2009) observed that an algal extract applied to Glycine max (L.) increased seed yield. Exposure of microalgae to nutrients deprivation such as nitrogen (N) or phosphorous (P), alters the physiological condition of algae by increasing carbohydrates, lipids, amino acids, and proteins (Abdel-Kader et al. 2022; Dean et al. 2010; Jakob et al. 2007; Palmucci et al. 2011). Thus, the extract of these microalgae may be good biostimulants for plant growth.
However, the use of Coccomyxa chodatii is scarce to be studied as a biostimulant agent on plants. It has been reported that various environmentally-friendly methods were applied to extract the bioactive compounds from algae as boiling or priming with distilled water which produces various bioactive compounds beneficial for crop growth (Godlewska et al. 2016). Furthermore, little is known about the regulatory functions of Coccomyxa extracts, especially macronutrient-deprived extracts under mercury stress. The current study proposed that macronutrient deficiency may induce some metabolic products that may be greater than that of normally growing algae. Thus, in the present work, the antioxidative potential and other metabolic products of the used extracts have been screened, which may exert a beneficial effect in regulating the oxidative status of the Hg-stressed plants. In addition, the FTIR spectrum of algal biomass under different treatments was evaluated to describe the main functional groups associated with the biostimulant effect of various extracts. Thus, the present work was conducted to evaluate the osmoprotectants and bioactive components of the sonicated and boiled Coocomyxa grown under sufficient nutrient or macromolecules-deprived ones to study their potentiality as biostimulants against Hg-stress. Keeping in view the importance of wheat as an important grain crop and its susceptibility to Hg-stress, the current search was designed to test the efficacy of macronutrient-deprived algal extract to ameliorate the phytotoxicity of Hg stress on wheat seedlings during the germination stage. The study focus on the mechanistic role of macronutrient deprived Coccomyxa chodatii extracts on alleviating Hg stress of wheat seedling through the studying some physiological parameters such as photosynthetic pigments, water status, primary and secondary metabolism, oxidative damage, chelation mechanism, antioxidant system, and Hg accumulation.
Materials and methods
Coccomyxa chodatii growth and extract preparation
Algal growth conditions
Coccomyxa chodatii SAG 216–2 has been kindly offered by SAG (Güttingen University, Germany); it was cultivated under sanitized conditions in 200 mL BG11 modified medium (Rippka 1992), at pH of 7.5, a temperature of 25 ± 2 °C, and received illumination from white fluorescent lamps (48.4 μmole m−2 s−1). The cultures were agitated by an orbital shaker for 15 days at 150 rpm. Coccomyxa chodatii was cultivated using the following treatments: (1) the control treatment, in which the culture was treated with a sufficient nutrient supply throughout the experimental period (normal algal extract; NAE), (2) the P-deficient treatment, in which the culture was treated with all nutrients as in the control treatment but without KH2PO4 (phosphorous-deprived algal extract; PDAE), (3) N-deficient treatment, in which the culture was supplied with all nutrients as in the control treatment without the addition of NaNO3 (nitrogen-deprived algal extract; NDAE), and (4) the P- and N-deficient treatment, in which the culture was treated with all nutrients as in the control treatment but without KH2PO4 and NaNO3 (phosphorous & nitrogen-deprived algal extract; PNDAE). The growth of Coccomyxa chodatii cultures was conducted for 15 days, and then the different algal extracts were prepared.
Biostimulant algal extract preparation
At the end of the growth period, 200 mL algal cultures were boiled in the water bath for two hours. After cooling, the cultures were centrifuged for 30 min at 5000 rpm. The precipitate was dried and used for FTIR analysis. The supernatant was collected for their application as biostimulants on wheat grain germination. In addition, 20 mL from the different extracts were conducted for further metabolic analysis. Proteins, amino acids, reducing sugars contents, phenolic compounds, total flavonoid, total antioxidants, and ferric reducing power were estimated (Lodge 2016; Lowry et al. 1951; Miller 1959; Moore and Stein 1948; Oyaizu 1986; Prieto et al. 1999; Zou et al. 2004).
Fourier transform infrared (FTIR)
The Fourier transform infrared (FTIR) spectra for Coccomyxa chodatii biomass prepared previously were recorded using a Nicolet iS10 (Thermo Scientific, USA) with 1 cm−1 resolution and a range of 400–4000 cm−1. Furthermore, the KBr–Wafer method was used.
Germination conditions
The grains of wheat (Triticum aestivium L.) El-karm cultivar (provided kindly from Saudi Arabia) were surface sterilized by commercial sodium hypochlorite (3%) for 10 min and then washed several times with double-distilled water. The grains were placed in Petri dishes (10 grains/petri dish) and then subjected to three levels of Hg (HgCl2; Sigma-Aldrich) dissolved in distilled water; 0, 10, and 30 mg L−1 (Further concentrations of Hg completely inhibited grains germination). The Petri dishes were subdivided into five groups: Reference control group (grains soaked in different levels of Hg dissolved in distilled water and control without Hg+2), normal algal extract (grains soaked in different levels of Hg dissolved in normal algal extract), N-deprived algal extract (grains soaked in different levels of Hg dissolved in N-deprived algal extract), P-deprived algal extract (grains soaked in different levels of Hg dissolved in N-deprived algal extract), N- & P-deprived algal extract (grains soaked in different levels of Hg dissolved in N- & P-deprived algal extract). Each treatment included 5 Petri dishes (each Petri dish received 10 ml of different extracts containing the corresponding concentration of Hg+2). All Petri dishes were kept in the dark for 7 days. Then the Petri dishes were opened and exposed to laboratory conditions for 8 days (photoperiod of 10 h and day/night temperature of 19 ± 3/12 ± 3 °C). The Hoagland nutrient solutions (Hoagland and Arnon 1950) were used throughout the study for the growth of the seedlings. After 15 days from sowing, the provided seedlings were harvested for the following morphological and physiological measurements. After 15 days of germination, the plants exposed to Hg + 2 showed some toxic effects of the metal and a reduction in the growth and germination which could be improved by using N- &/or P-deprived algal extract.
Morphological achievements and water status
The lengths of shoot and root of the developed samples under different treatments were recorded in cm. The fresh weight of samples was immediately weighted, then oven-dried for 2 days at 80 °C.
Photosynthetic pigments measurements
Fresh leaves were prepared to extract chlorophyll a, b, and carotenoids using ethyl alcohol (95%) overnight, and then the absorption of the extract was read at 663, 644, and 452 with the methods and formulae of Lichtenthaler and Wellburn (1983).
Metabolites assessment
The fresh leaves were homogenized in distilled water, boiled for 2 h, and then centrifuged. The extract was mixed with an anthrone-sulphuric acid reagent and then boiled for 7 min for soluble carbohydrate detection based on Fales (1951) using glucose as a standard material. For monitoring the foliar content of soluble proteins, Lowry et al. (1951) devised a technique by mixing the extract with the alkaline reagent and utilizing folin reagent where blue color was measured at 720 nm. Moore and Stein (1948) technique used stannous chloride and ninhydrin reagent to measure free amino acids. The water extract was mixed with stannous chloride reagent (stannous chloride + ninhydrin reagent/ethanol reagent + citrate buffer) and boiled in the water bath for 20 min. After cooling, a diluent reagent (ethanol 50%) was added to the last mixture, where the absorbance of violet color was done at 570 nm.
Reactive oxygen species detection
Mukherjee and Choudhuri (1983), Elstner and Heupel (1976), and Halliwell et al. (1987) used spectrophotometry to determine the shoot level of hydrogen peroxide (H2O2) using sulfuric acid-titanium dioxide reagent mixed with cooled acetone extract where yellow color was monitored at 420 nm, superoxide anion (O2•−) utilizing NaNO2 as a standard, and hydroxyl radical (•OH) using deoxyribose in the estimation mixture. In detail, for superoxide anion detection, the shoots of wheat seedings were extracted in K-P buffer (65 mM, pH 7.8). The extract was then incubated with hydroxylamine hydrochloride and naphthylamine (Sigma-Aldrich Pvt, Ltd. Bengaluru, India), where the developed color was monitored at 520 nm. Hydroxyl radical was detected in fresh shoots by incubation in K-P buffer containing 2-deoxy-d-ribose at 37 °C for 2 h. The suspended solution was incubated in glacial acetic acid and thiobarbituric acid (TBA, Sigma-Aldrich Pvt, Ltd. Bengaluru, India) dissolved in sodium hydroxide and then boiled in the water bath for 10 min. The malondialdehyde-formed absorbance was done at 532 nm, and the calculation was performed using extinction coefficient 155,000 mM−1 cm−1.
Oxidative stress markers
The thiobarbituric acid reaction was used to assess lipid peroxidation in fresh shoots by measuring malondialdehyde generation, as detailed by Rao and Sresty (2000). The fresh shoots were macerated in trichloroacetic acid (TCA) containing thiobarbituric acid (TBA) and then boiled at 95 °C for 30 min. The absorbance of the clean chromophore was monitored at 532 nm.
Minguez-Mosquera et al. (1993) approaches were used to monitor lipoxygenase activity in shoots extract. A reaction mixture of K-phosphate buffer and linoleic acid was used, and the increase in absorbance was monitored at 234 nm. Gilbert and Brandt (1975) approach was used to calculate methylglyoxal (MG) of shoots where the dinitrophenylhydrazine (Sigma-Aldrich Pvt, Ltd. Bengaluru, India)-HCl reagent was used, and the absorbance reading was measured after 45 min at 432 nm.
Non-enzymatic antioxidants
Phenolic compounds: Using the Folin-Ciocalteu reagent with sodium carbonate on a methanolic extract of wheat shoots and a standard curve of gallic acid, phenolic compounds (mg/g FW) were determined using the Dihazi et al. (2003) technique. Ascorbic acid (AsA): Fresh wheat shoots were homogenized in TCA solution (5%), and the supernatant was mixed with TCA (10%), which was then diluted with distilled water and used to measure AsA using diluted Folin-Ciocalteu reagent where blue color was monitored at 760 nm as reported by Jagota and Dani (1982) technique. α-Tocopherol: Kivçak and Mert (2001) methodology where the fresh shoots were crushed in chloroform, and the supernatant was mixed with dipyridyl reagent. Then, ferric chloride was added, and the produced color was read at 522 nm using a spectrophotometer within 50 s.
Chelation molecules
Reduced glutathione (GSH), phytochelatins (PCs), and metallothioneins were studied based on the methods of Ellman (1959), Nahar et al. (2016), and Cataldo et al. (2011), respectively. To extract GSH and non-protein thiol, TCA and sulfosalicylic acid were used as an extraction solution added to Ellman’s reaction mixture. PCs are determined by subtracting from non-protein thiols and GSH, as cited by Nahar et al. (2016). The shoots' metallothionein was extracted in homogenization buffer (sucrose, Tris–HCl buffer, and mercaptoethanol), then centrifugation was done; chilled 90% ethanol: chloroform was added to the supernatant. Then three levels of cold ethanol were added to the resulting supernatant and kept at -20 °C for 1 h, and then centrifugated to obtain metallothionein pellets. Ethanol: chloroform: homogenization buffer (87:1:12) was added then the pellets were left to air dry and remixed with Tris–HCl and EDTA. Then, the produced mixture was incubated with 5,5-dithiobis nitrobenzoic acid in phosphate buffer for 30 min at room temperature, and then absorbance was read at 412 nm.
Nitric oxide and Nitrate reductase
The nitric oxide (NO) content was determined using the technique described by Adams et al. (1997). The shoots were homogenized in glacial acetic acid, and then the supernatant was mixed with Griess reagent were left 30 min at room temperature. The optical density of samples was done at 560 nm. Nitrate reductase (NR) activity was measured in fresh shoots using Downs et al. (1993) technique. Shoots were grounded in phosphate buffer and KNO3 and incubated in the dark for 1 h. Then sulfanilamide and 1-Naphthyl-ethylene diamine dihydrochloride (Sigma-Aldrich Pvt, Ltd. Bengaluru, India) were added, and the absorbance was measured at 542 nm using NaNO2 as a standard solution.
Antioxidant Enzymes
As previously mentioned by Dawood et al. (2021b), the enzyme extract was prepared in cold conditions by macerating the shoots in a K-P extraction buffer containing ethylene diamine tetra acetic acid (EDTA) and polyvinyl pyrrolidone. Superoxide dismutase (SOD) activity was measured in this extract using epinephrine autoxidation of media containing sodium carbonate buffer (pH 10.2), EDTA, enzyme extract, and epinephrine, applying Misra and Fridovich (1972) method of epinephrine at 480 nm, the change in absorbance was measured. The H2O2–decomposing enzyme catalase (CAT) was detected by measuring H2O2 consumption and recording the reduction in absorbance at 240 nm (Aebi 1984). The activity of ascorbate peroxidase (APX) was determined in phosphate buffer extract by measuring the oxidation of ascorbate as substrate at 290 nm in the presence of ascorbic acid, EDTA, and H2O2 (Nakano and Asada 1981). Polyphenol oxidase (PPO) activity was evaluated using Kumar and Khan (1982) procedure by detecting purpurogallin production at 495 nm. The phosphate buffer and catechol assay mixture were left for 5 min at 25 °C. Then the reaction was terminated by adding H2SO4, and the optical density was read at 495 nm. The phenylalanine ammonia-lyase (PAL) activity was determined using the protocol of Havir and Hanson (1973) by incubating the shoot extract in borate buffer and the substrate (phenylalanine) and then stopping the reaction by HCl, where the trans-cinnamic acid formed was followed at 290 nm. The activities of ionic and soluble peroxidase (SPO and IPO) were determined after the enzymes were extracted from the shoots by following the procedures of Ghanati et al. (2002) technique. The reaction mixture was composed of K-P buffer, guaiacol, and H2O2, where the increase in absorbance was measured at 470 nm utilizing the extinction coefficient of 26.6 mM−1 cm−1 for calculations.
Hg-determination
Estimation of Hg contents in wheat was done using the protocol of Cargnelutti et al. (2006). The powdered wheat seedlings were digested with a mixture of sulfuric and hydrochloric acid in the ratio (6:1 v/v) at 85 °C. The digested mixture was used for the determination of Hg using atomic absorption spectrophotometry (AAS) provided with a hollow cathode lamp (Perkin-Elmer Analyst 100, Waltham, MA, USA) with have reduction reactor composed of a cold vapor chamber (Perkin-Elmer MHS-20) and sodium borohydride (NaBH4).
Statistical analysis
For each trait, three replicates were utilized to represent the means and standard errors. In the SPSS 28.0 program, the data were subjected to one-way analysis of variance. Tukey's multiple range test was used to compare among means at the level of 5%.
Experimental Results
Coccomyxa chodatii extracts’ biochemical analysis
For declaring some biochemical characters of the different Coccomyxa chodatii extracts applied, Table 1 represents some traits. Soluble protein contents were found to be increased under PDAE and decreased under nitrogen NDAE and PNDAE. Amino acids were also enhanced by using PDAE and PNDAE, while they decreased under NDAE compared to NAE. Reducing sugars were higher for phosphorus deficiency (PDAE) and combined deficiency of PNDAE than the control (NAE), while under nitrogen deficiency (NDAE), slightly higher than those of the NAE. When compared to their reference control (NAE), various extracts stimulated phenolic compounds, flavonoids, total antioxidants, and ferric reducing power activity, but with high values for PDAE, PNDAE, and NDAE.
Characterization of the prepared Algal extracts
The impacts of N-deficient, P-deficient, and P- and N-deficient treatment on the characteristics of Coccomyxa chodatii and their functional groups were investigated using FTIR analysis Table 2 and Fig. 1).
The FTIR spectra (Fig. 1 and Table 2) from Coccomyxa chodatii SAG 216–2 showed the main functional groups (nitro, carboxyl, amino, sulfide, phosphoryl, and hydroxyl) from untreated algal cells. The strong peak at 3304.83 cm−1 belongs to O–H and N–H stretching vibrations. The peaks at 2923 and 2852 cm−1 are recorded for C-H stretching, while the 1655 cm−1 peak is linked to C = O stretching and N–H deformation (amide II region). Also, the peaks of the carboxyl functional group were recorded at 1540 and 1454 cm−1, and that of phosphate were recognized at 1239 and 604 cm−1. The polysaccharide functional groups as C–C, C–O–C, C = C, and C–O–P were assigned at 1058 cm−1. The peaks that appeared at 560 and 445 cm−1 were recognized for two bi-phenyl rings “conjugated and Si–O stretching”, respectively (Table 2 and Fig. 1).
In the PDAE, NDAE, and PNDAE treatments, the FTIR spectra denoted some differences where some peaks shifted, new peaks were recognized, and others vanished (Table 2 and Fig. 1). This was possible due to the alignment of P and N deficient in the active functional group causing various changes under the vibration frequency range. In addition, this could be associated with the correlation of P and N deficiency to active functional groups, which causes a smaller vibration frequency.
Coccomyxa chodatii various extracts as a biostimulant for germinating wheat seedlings under mercury stress
Seedling's Growth under mercury stress and the applied biostimulants
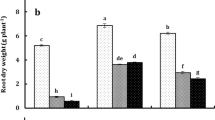
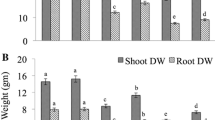
The data presented in Fig. 2a-e revealed that mercury's application to germination solution drastically affected the lengths and weights of the produced seedlings. The most damaging impact of Hg on seedlings was inhibition of roots development even at the lower dose applied (Fig. 2). However, the co-application of Hg with the extract of NAE, NDAE, PDAE, and PNDAE differentially ameliorated the damaging impact of Hg on the phenology of wheat seedlings. The treatments of P-deprived algal extract can reduce the damaging impact of Hg on roots where the roots are well-developed (Fig. 2e). Timidly beginning of root emergence was denoted under P-deprived algal extract for both levels, and PNDAE induced root initiation at the lower level of Hg tested (10 mg L−1). Fresh and dry weights were also improved by the application of algal extract, especially in P-deprived algal extract (PDAE) and N-&P-deprived algal extract (PNDAE).
a-e: The fresh (a) and dry (b) weights as well as a shoot (c) and roots (d) lengths of wheat seedlings, and (e) over all phenology of seedlings grown under three levels of Hg-stress (0, 10, and 30 mg L.−1) using different soaking solutions, distilled water (NC = Negative control), normal algal extract (NAE), nitrogen-deprived algal extract (NDAE), phosphorous-deprived algal extract (PDAE), and phosphorous & nitrogen-deprived algal extract (PNDAE). The values are represented as means ± SE (n = 3), according to Duncan's test, different letters on the same bars show significant differences at p < 0.05
Hg content of wheat seedlings under mercury stress and the applied biostimulants
Mercury has been progressively accumulated in wheat tissues, especially at the level of 30 mg L−1 Hg. The application of various algal biostimulants extensively retarded the entry of Hg into the plant tissues, where the lowest content of Hg was reported for PDAE-treated plants (Fig. 3).
Hg content of wheat seedlings grown under three levels of Hg-stress (0, 10, and 30 mg L.−1) using different soaking solutions, distilled water (NC = Negative control), normal algal extract (NAE), nitrogen-deprived algal extract (NDAE), phosphorous-deprived algal extract (PDAE), and phosphorous & nitrogen-deprived algal extract (PNDAE). The values are represented as means ± SE (n = 3), according to Duncan's test, different letters on the same bars show significant differences at p < 0.05
Metabolic products of wheat seedlings under mercury stress and the applied biostimulants
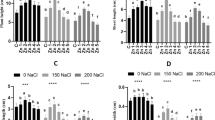
Chlorophyll a, b contents, and carotenoids were found to be reduced by Hg stress. While using algal extracts improved the content of photosynthetic pigments compared to the stressed plants only. Also, PDAE and PNDAE enhanced the values of chlorophyll a, b contents, and carotenoids comparable to control (Fig. 4a-c).
a-c: Chlorophyll a (a), chlorophyll b (b), and carotenoids (c) of wheat seedlings grown under three levels of Hg-stress (0, 10, and 30 mg L.−1) using different soaking solutions, distilled water (NC = Negative control), normal algal extract (NAE), nitrogen-deprived algal extract (NDAE), phosphorous -deprived algal extract (PDAE), and phosphorous- & nitrogen-deprived algal extract (PNDAE). The values are represented as means ± SE (n = 3), according to Duncan's test, different letters on the same bars show significant differences at p < 0.05
The major primary metabolites of the plant body were found to be adversely affected by Hg stress. In this sense, soluble proteins, soluble carbohydrates, and free amino acids were decreased by Hg stress. On the other hand, the vigorous seedlings produced from the interactive effect of Hg and algal and macronutrient-deprived algal extracts have higher metabolic products. The effect of various extracts mainly recovered from being comparable to control for seedlings experienced 10 mg L−1 Hg and higher than the corresponding stressed plants for 30 mg L−1 Hg (Fig. 5a-c). The most pronounced effect was denoted for PDAE.
a-c: Soluble proteins (a), soluble sugars (b), and amino acids (c) of wheat seedlings grown under three levels of Hg-stress (0, 10, and 30 mg L.−1) using different soaking solutions, distilled water (NC = Negative control), normal algal extract (NAE), nitrogen-deprived algal extract (NDAE), phosphorous-deprived algal extract (PDAE), and phosphorous- & nitrogen-deprived algal extract (PNDAE). The values are represented as means ± SE (n = 3), according to Duncan's test, different letters on the same bars show significant differences at p < 0.05
Oxidative markers of wheat seedlings under mercury stress and the applied biostimulants
Induction of oxidative stress by Hg stress was denoted by exacerbation of ROS in terms of superoxide anion (O2•−), hydrogen peroxide (H2O2), and hydroxyl radical (•OH). At the 30 mg L−1, Hg level, the percent increment of H2O2, O2•−, and •OH were 147, 212, and 163%, respectively, over the control (Fig. 6a-c). Interestingly, the co-existence of the algal extract and their macronutrient deprived ones with Hg attenuated the oxidative damage by lessening the contents of H2O2, O2•−, and •OH compared to their stressed plants. The pronounced positive effect of the applied extracts was denoted for PDAE, followed by PNDAE (Fig. 6).
a-c: Hydrogen peroxide; H2O2 (a), hydroxyl radical; ─●OH (b), and superoxide anion; O2●─ (c) grown under three levels of Hg-stress (0, 10, and 30 mg L.−1) using different soaking solutions, distilled water (NC = Negative control), normal algal extract (NAE), nitrogen-deprived algal extract (NDAE), phosphorous-deprived algal extract (PDAE), and phosphorous & nitrogen-deprived algal extract (PNDAE). The values are represented as means ± SE (n = 3), according to Duncan's test, different letters on the same bars show significant differences at p < 0.05
There was a positive correlation between membrane damage criteria and Hg stress. In this regard, lipoxygenase activity enhances progressively even at the lower level of Hg stress with increment percentages of 63 and 178% at the levels of 10 and 30 mg L−1 Hg, respectively, over the control (Fig. 7a). The application of different algal extracts retarded the lipid peroxidation process due to Hg stress, where the increment of MDA level reached 12 and 47% over the control plants under P-deprived algal extract (Fig. 7b). The lipoxygenase activity was also immensely increased with the application of Hg, with an increasing percent of 73 and 259% over the control plants. Using various algal extracts highly significantly reduced lipoxygenase activity to different degrees, reaching 174, 129, 95, and 53% for NAE, NDAE, PNDAE, and PDAE, respectively, at the level of 30 mg1 Hg. Furthermore, the applied algal extracts had a profound differential effect on reducing methylglyoxal (Fig. 7c) in wheat tissues exposed to 30 mg L−1 Hg stress with percent increments reaching 325, 258, 178, and 133 for NAE, NDAE, PNDAE, and PDAE, respectively compared to 373% of stressed plants only (Fig. 7c). The data of NO and methylglyoxal increased in response to Hg-stress by about 257 and 227% over the control at the level of 30 mg L−1 Hg. Different algal extracts reduced NO and methylglyoxal lower than the corresponding treatment, with the highest reduction denoted for PDAE (Fig. 7d).
a-d: Lipoxygenase activity; LOX (a), lipid peroxidation; MDA (b) methylglyoxal; MG (c), and nitric oxide; NO (d) of wheat seedlings grown under three levels of Hg-stress (0, 10, and 30 mg L−1) using different soaking solutions, distilled water (NC = Negative control), normal algal extract (NAE), nitrogen-deprived algal extract (NDAE), phosphorous deprived algal extract (PDAE), and phosphorous- & nitrogen-deprived algal extract (PNDAE). The values are represented as means ± SE (n = 3), according to Duncan's test, different letters on the same bars show significant differences at p < 0.05
Non-enzymatic antioxidants, secondary metabolites, and chelation mechanisms of wheat seedlings under mercury stress and the applied biostimulants
The non-enzymatic antioxidants were also included in the current study. Ascorbic acid (AsA) is adversely impacted by Hg stress which reduced to 73 and 47% of control plants for 10 and 30 mg L−1, Hg, respectively. However, algal extracts differentially improved AsA content of Hg-stressed plants. Except for PDAE and PNDAE, partial recovery of AsA content was found, with values greater than Hg-stressed plants but still lower than the control (Fig. 8a). α-Tocopherol showed a significant reduction by about 15% at the level of 30 mg L−1 Hg, while raised by 18% at the level of 10 mg L−1 Hg. Further, the accumulation of α-tocopherol was denoted whatever the extract applied, but much more so for PDAE and PNDAE (Fig. 8b).
a-c: Ascorbic acid; AsA (a), α-tocopherol (b), and phenolic compounds (c) of wheat seedlings grown under three levels of Hg-stress (0, 10, and 30 mg L.−1) using different soaking solutions, distilled water (NC = Negative control), normal algal extract (NAE), nitrogen-deprived algal extract (NDAE), phosphorous-deprived algal extract (PDAE), and phosphorous & nitrogen-deprived algal extract (PNDAE). The values are represented as means ± SE (n = 3), according to Duncan's test, different letters on the same bars show significant differences at p < 0.05
The phenolic content was reduced to 60% of control plants at the level of 30 mg L−1 Hg. Applying normal algal extracts or their macronutrient-deprived ones to Hg-stressed plants significantly alleviated the damaging impacts of Hg on phenolic compounds. The best results were denoted under the interactive effect of Hg with PDAE or PNDAE, where phenolic compounds mainly enhanced more than 2- and 1.6- folds, respectively . On the other hand, the lowest alleviating results were generally recorded for NAE where phenolics recovered to the control values at 10 mg L−1 Hg and at 30 mg L−1 Hg, with phenolics still lower than control, recording 73% (Fig. 8c).
In the current investigation, the chelation agents of heavy metals in plant cells included various molecules such as reduced glutathione (GSH), metallothioneins, and phytochelatins (PCs). The data clearly denoted that Hg stress breaks down the major chelation mechanisms in wheat seedlings with significantly reduced GSH, metallothionein, and phytochelatins even at the lower level of Hg applied. On the other hand, the applied algal extract restrained the reduction of these compounds to be generally higher than the corresponding treatments. Generally, NAE and NDAE have the lowest positive effect on GSH, metallothionein, and PCs at 30 mg L−1 Hg, where the values are just higher than Hg-stressed plants. On the other hand, PDAE and PNDAE provided the plants with higher accumulation values of these chelation compounds than control values, especially for PNDAE (Fig. 9a-c).
a-c: Reduced glutathione; GSH (a), Phytochelatins; PCs (b), and metallotheionins (c) of wheat seedlings grown under three levels of Hg-stress (0, 10, and 30 mg L.−1) using different soaking solutions, distilled water (NC = Negative control), normal algal extract (NAE), nitrogen-deprived algal extract (NDAE), phosphorous-deprived algal extract (PDAE), and phosphorous & nitrogen-deprived algal extract (PNDAE). The values are represented as means ± SE (n = 3), according to Duncan's test, different letters on the same bars show significant differences at p < 0.05
Enzymatic antioxidants and nitrate reductase activity of wheat seedlings under mercury stress and the applied biostimulants
The plants withstand oxidative stress through the inherent antioxidative system compromised through a wide array of non-enzymatic and enzymatic systems and plant tolerance to environmental stress associated with the activation of the defense system before the commencement of damage. Unlikely, the stressed wheat seedlings encountered progressive accumulation of reactive oxygen species (ROS) levels, which negatively impacted the antioxidant enzymes (CAT, SOD, and APX). At the level of 30 mg L−1 Hg, the activities of SOD, CAT, and APX reached 32, 55, and 65%, respectively, of control plants. Thus, the regulatory role of the applied extracts was denoted by a highly significant recovery of the SOD, CAT, and APX activities, but much more so for plants that received PDAE and PNDAE, whatever the level of Hg supplied (Fig. 10).
a-h: Superoxide dismutase; SOD (a), catalase; CAT (b), ionic peroxidase; IPO (c), soluble peroxidase; SPO (d), ascorbate peroxidase; APX (e), phenylalanine ammonia-lyase; PAL (f), polyphenol oxidase; PPO (g), and nitrate reductase (h) of wheat seedlings grown under three levels of Hg-stress (0, 10, and 30 mg L.−1) using different soaking solutions, distilled water (NC = Negative control), normal algal extract (NAE), nitrogen-deprived algal extract (NDAE), phosphorous-deprived algal extract (PDAE), and phosphorous- & nitrogen-deprived algal extract (PNDAE). The values are represented as means ± SE (n = 3), according to Duncan's test, different letters on the same bars show significant differences at p < 0.05
Intriguingly, ionic and soluble peroxidase (IPO and SPO) were stimulated by Hg stress to be reached 338 and 107%, respectively, over the control values at the level of 30 mg L−1 Hg. Thus, the applied protectants were able to slow down the activities of IPO and SPO, where the most reducing extract was denoted for seedlings supplied with PDAE and PNDAE (Fig. 9). In the same line, the polyphenol oxidase (PPO) activity data was also enhanced by Hg stress. However, the activity of PPO was minimized, whatever the type of algal extracts used (Fig. 10).
The phenylalanine ammonia-lyase (PAL) data was affected differentially by Hg stress, where the PAL activity rose by 27% over the control at 10 mg L−1 Hg level and reduced to 72% of control activity at the level of 30 mg L−1 Hg. Algal extracts exacerbated the PAL activity higher than their corresponding stressed level only for NAE and NDAE and folded several times compared to control seedlings for PDAE and PNDAE. However, a highly significant reduction of nitrate reductase (NR) was also potentially impacted by Hg stress, with a significantly reduced activity even at the lower Hg level. However, the algal extracts instigated the PAL and NR activity of stressed seedlings, especially PDAE and PNDAE (Fig. 10).
Discussion
Macronutrient-deprived algal extracts, especially PDAE and PNDAE ones, have been plentiful in several bioactive products that might act on plants, instigating multiple positive morphological and physiological responses, such as improved seedling’s weight and lengths and modulating stress resistance. The research is concerned more with the antioxidant properties of the algal or macronutrient-deprived algal extracts. In addition, using the FTIR spectrum for identifying functional groups related to most algal constituents. Thus, the rationale behind these extracts may be linked to their antioxidant potential, osmoprotectants, or growth regulating molecules which may induce plants' antioxidant and defense system, hence raising Hg stress tolerance. For this reason, they can be involved in agricultural systems to improve crop healthiness and vitality. Microalgal extracts as biostimulants have been applied to several species, providing general stimulatory effects (Behera et al. 2021; Chiaiese et al. 2018; Martini et al. 2021; Mutale-Joan et al. 2020; Ronga et al. 2019). The present study describes various functional groups for algal biomass under different conditions, such as nitro, carboxyl, amino, sulfide, phosphoryl, and hydroxyl groups. This could be the key behind their bio-stimulatory effects on wheat plants. Herein, the change of these functional groups under different conditions could aid in Hg-chelation and compartment, thus improving the growth and metabolism of the Hg-stressed plants.
The used microalga extract and macro-nutrient deprived extracts improved the shoot system of the non-stressed seedlings and ameliorated the reduction of the shoot system of Hg-stressed plants, especially that of the lowest Hg-level. With regard to roots, PDAE, PNDAE, and NDAE succeed in the reappearance of the root system of Hg-stressed plants (completely disappeared under Hg-stress), but not NAE. Interestingly, only PDAE and PNDAE stimulated the lateral root production, which is vastly seen for non-stressed seedlings. These responses could be associated with the richness of algae and macronutrient-deprived algal extracts with sustainable plant growth-promoting compounds, antioxidants, osmoprotectants, and secondary metabolites that benefit wheat seedling's growth, hence Hg-tolerance. Many literatures have recommended that different algal extracts are rich in phytohormone-like plant-growth molecules, which boost the production and growth (Sofy et al. 2022; Ghaderiardakani et al. 2019; Yalçın et al. 2019). Of these molecules, Martini et al. (2021) reported that the physical treatment of algal cells as a bio stimulator agent ascribed to the liberation of specific peptides or proteins which has bio-stimulant properties. This response concomitant with our findings where characteristic amide groups were reported in FTIR spectrum at wave lengths 1654–1658 cm–1 which distinguishable for polypeptides and peptide sequences as reported by Cobb et al. (2020). Furthermore, Rachidi et al. (2021) found that polysaccharides secreted or present in microalgal biomass were found to instigate the biostimulant effect on plants. In the present work, various peaks have been detected related to polysaccharides from 900–1200 nm. These polysaccharides in algal extracts could boost germination and root (Mohamed et al. 2021) due to their biological activities as a stimulator of defense responses in plants (Dawood et al. 2022a, b). Moreover, the different extracts of the present work nearly showed characteristic bands of indoles viz., 3296–3405, 1538–1541, 1654–1658, 1454–1455, and 720 cm−1. As has been documented by Trivedi et al. (2015) and Vazquez-Vuelvas et al. (2011), the FTIR spectrum of indole is characterized by peaks at 3406 cm−1 (related to the N–H), 3022 and 3049 cm−1 (symmetric and asymmetric C-H group), and that at 1508 and 1577 cm−1 were distinguishable for aromatic C = C strong stretching. The peaks found at 1616 and 1456 cm−1 were ascribed to C–C stretching. Also, 1336 and 1352 cm−1 were observed for C-H bending modes of symmetric and asymmetric methyl groups, and that at 609, 731, and 744 cm−1 were characteristic for = C-H bending peaks. Thus, auxins could be the basic constituents of the used extracts that aided in the reestablishment of lateral roots and enhanced wheat seedling morphogenesis under Hg-stress. Thus, the present study data revealed that PDAE and PNDAE could have strong biostimulant properties compared to the rest of the algal extract. These results were confirmed by low accumulation of Hg in wheat tissues by using different algal extracts and the lowest accumulation was denoted for PDAE. In this sense, the extracts were rich in sugars, proteins, and amino acids, which may stimulate tolerance against various stresses. The effects of amino acids on anion fluxes through membranes have been vastly documented, with most having a positive effect on reducing stress-induced potassium efflux (Cuin and Shabala 2007). Thus, the presence of amino acids and proteins in algal extracts may benefit the Hg-stressed plants. In this regard, it can be suggested that Hg tends to bind to the cell wall-extracted molecules of the alga and therefore reduces Hg entry to wheat seedlings. This is further documented from FTIR spectra where several functional groups such as CH2, C–H, C = O, N–O, C–N, –OH, PO4, and –NH2 are associated with algae biosorption capacity. These groups are characterized by their binding sites for metal ions which could aid in ion exchange, electrostatic forces, and complexation (El-Naggar et al. 2021).
The severity of Hg stress is commonly associated with photosynthetic pigment catabolism; hence Chl a, Chl b, and carotenoids retarded for Hg-stressed wheat seedlings. This could be associated with the negative effect of HM on chlorophyll and has been linked to the activation of Chl-degradative enzymes and the suppression of enzymes responsible for Chl biosynthesis (Parmar et al. 2013; Sofy et al. 2021a). In this sense, decreasing sugar content under mercury stress could be associated with the deterioration of primary photosynthetic pigments revealing high metabolic perturbations. These deteriorations were concomitant also with the reduction of proteins and amino acids biosynthesis. This could be due to Hg binding to cysteine-rich regions in proteins causing protein misfolding, structural disruption, substitution of critical co-factor ions, and suppression of enzymatic activity, disturbing the redox equilibrium of energy-generating cellular activities, photosynthesis, and respiration (Mostofa et al. 2015). On the other hand, the beneficial effect of algal extracts especially that of PNDAE and PDAE, is documented chiefly by chlorophyll restoration even under the high level of Hg, which could be an elegant mechanism to trigger wheat tolerance against Hg-stress. In this line, the result of the present study denoted that PDAE, NDAE, and NAE up-regulated the primary metabolism of Hg-stressed plants as well as non-stressed plants in terms of proteins, carbohydrates, and amino acids with most relevant effects for PDAE. Furthermore, applying algal extracts in high efficiency increased free amino acids and soluble proteins parallel to the increment of nitrate reductase activity and vice versa under Hg stress. These results demonstrated the effectuality of algal extracts on wheat seedlings' growth, most probably through triggering the carbon and nitrogen metabolism.
An imbalance either in the disintegration or formation of chlorophyll and high accumulation of Hg in wheat tissues have been shown to result in oxidative stress. This is represented mainly by a change in the cellular redox balance toward a more oxidative state of the Hg-stressed wheat tissues, as seen by excessive generation of H2O2, •OH, and O2•–. Comparatively, the wheat seedlings treated with various algal extracts showed a significant increase in up-regulated chlorophyll moiety, implying that the eliciting compounds of algal extracts reduced the oxidative stress on the tested seedling. In the present study, it was found that the H2O2, •OH, and O2•– levels at 10 mg L–1 were comparable to control plants under the interactive effect of Hg and PDAE or PNDAE, indicating that both extracts confer complete protection from oxidative damage at this level and the oxidative damaging impacts at 30 mg L–1 were decreased partially with the used extracts (especially PDAE and PNDAE). Thus, nutrient deprivation of the algae induces compensatory pathways that aid plant development under stressed conditions. Such response could be linked to the presence of high antioxidative properties of algal or macronutrient-deprived extract as phenolics, flavonoids, and total antioxidants that may play a potential role in scavenging ROS similar to that reported in the other studies (Aremu et al. 2015; Jayshree et al. 2016; Safafar et al. 2015; Smerilli et al. 2019). Interestingly, the used extracts were rich in ferric reducing power, especially that of macronutrient-deprived extracts, also participate in enhancing the antioxidant property of the used extracts. Additionally, the existence of soluble metabolites such as carbohydrates and amino acids aided also in further antioxidant properties of the used extracts, especially PDAE, which is considered the richest extract with various antioxidants. In addition, algae contain a variety of binding groups that aid in the biosorption of metal ions, such as carboxyl and phosphoryl groups. These binding groups can be found in the cytoplasm and cell surface, particularly in vacuoles (Salama et al. 2019).
Photo-oxidative stress caused by ROS generated in the chloroplast causes lipid peroxidation damage to the membrane lipids, impacting the PSII reaction center (Bashandy et al. 2020; Dawood et al. 2021a). The high increment in the MDA level recommended this suggestion, indicating severe membrane deterioration of wheat seedlings induced by Hg stress. Furthermore, increased LOX activity might have been linked to oxidative damage caused by peroxidizing membrane lipids. Otherwise, the membrane integrity status was reported to be more stable for Hg-stressed plants supplied with algal extracts compared to the stressed plants. In this regard, different algal extracts attenuated lipid peroxidation and LOX activity levels to a large extent for P-deprived extract and, to a lesser extent, for normal algal extract treatment. Many studies showed that various algal extracts had the ability to reduce lipid peroxidation (Coulombier et al. 2021; Natrah et al. 2007). Also, Chaudhuri et al. (2014) reported that Euglena tuba is capable of attenuating lipid peroxidation and buffering the superoxide radical.
Plant resistance to abiotic stressors, such as heavy metals, mainly depends on NO exerting beneficial or harmful effects depending on its quantity (Siddiqui et al. 2011). In the present study, the data recommended that ROS (H2O2, O2•−, and •OH) and NO were highly triggered under Hg toxicity, which could indicate a nitrosative stress, besides the previously mentioned oxidative stress. Similarly, nitrosative and oxidative stresses were reported under alkalinity and/or aniline stresses (Dawood et al. 2021b), collectively known as secondary stress, i.e., “nitro-oxidative stress” (Corpas and Barroso 2013). Thus, herein, NO could be a potential agent in Hg-mediated ROS production and, subsequently, oxidative perturbations, as recommended by the study of Wu et al. (2019). Contrary to this, the application of algal extracts vastly reduced both ROS and NO; consequently, high tolerance to Hg stress was the result. Similar nitric oxide scavenging capacity for algal extracts was reported in previous studies (Azizan et al. 2018; Lee et al. 2010). Thus, the applied extracts induced a balance between NO and ROS production. This adjustment is critical for heavy metal tolerance and acclimatization to abiotic stimuli (Dawood and Azooz 2020; Farnese et al. 2016; Pető et al. 2013). It is worth mentioning that under the interactive effect of Hg and PDAE or PNDAE, the NO level was reduced to a large extent compared to Hg-stressed plants, but still higher than control. Under these conditions, the fine balance in NO content has a function in combating heavy-metal-induced ROS via scavenging ROS or boosting the antioxidant defense system of plants (Mengel et al. 2013). Importantly, during marigold adventitious root formation, NO may act as an upstream signaling molecule for H2O2 in the auxin signal transduction system (Arc et al. 2013). Based on this finding, the high reduction of NO under the interaction of Hg and PDAE or PNDAE could be reached a beneficial level that modulates the H2O2 level and adjusts auxin levels in the wheat cells that highly efficiently trigger the reappearance of roots which blocked under Hg-stress.
Methylglyoxal (MG), a cytotoxic compound, is a byproduct of different metabolic activities and is a potential participant in increasing ROS in plants (Dawood et al. 2021c). Parallel to increment of Hg content, H2O2, •OH, and O2•– in wheat seedlings, methylglyoxal, herein, was found to be amplified under Hg-stress causing oxidative burst (Dawood et al. 2021c, 2022a, b). Furthermore, higher generation of MG suppressed germinability, glycation of proteins, cell proliferation, denature of the antioxidant pool, and other metabolic dysfunctions (Hoque et al. 2012, 2016). Mano et al. (2009) added that MG induced retardation of root and shoot growth due to photosynthesis depletion via disabling CO2-photoreduction. This situation could express the damaging role of enhanced MG levels under Hg stress on wheat roots and shoots. Thus, in the present study, by reducing MG content in wheat seedlings treated with algal extracts under Hg stress, a noticeable reduction in ROS production, decrement of MDA, higher chlorophyll content, and stimulation of antioxidants pool was resultants. It is worth mentioning that the application of macronutrients deprived algal extract for Hg-stressed plants had the highest reduction capacity on methylglyoxal, thereby the blocking of roots by Hg-stress was reduced to a large extent, and a high increase of shoots was also observed.
Within cells, plants instigate various chelating compounds under heavy metals stress as organic acids, amino acids, reduced glutathione (GSH), or heavy metals-linking ligands such as phytochelatins (PCs) and metallothioneins (MTs). It has been proposed that GSH has fruitful multifunctional roles in various physiological debates, as detoxification of ROS, MG, and heavy metals concerns (viz., uptake, chelation, detoxification, and translocation) (Meharg 1993; Ahmad et al. 2021). Metallothioneins, low molecular weight polypeptides with Cys-rich residues that use their thiol functional group to attach to metals, have been reported to maintain homeostasis of ion transport across the plant, heavy metal sequestration, and protection against ROS-induced intracellular damage (Ghori et al. 2019; Hossain et al. 2012). In addition, the balance between ROS/NO is affected by PCs, influencing metal accumulation ability (Kováčik et al. 2018). Thus, the depletion of GSH, PCs, and metallothioneins triggered an imbalance in ROS/NO and reduced Hg-sequestration ability, hence increasing the accumulation of Hg in wheat tissues. On the other hand, the ability of algal extracts to accumulate GSH could be linked to differential alleviation abilities of the applied extracts to the chelation agents (GSH, PCs, and metallothioneins) to mitigate the negative impacts of Hg stress on wheat seedlings. In this regard, PDAE and PNDAE enhanced GSH, PCs, and metallothioneins over the non-stressed plants, while NAE alleviated the reduction to be higher than the stressed plants. This high accumulation of GSH content activates the phytochelatin productions, which activate the linkage of heavy metal and phytochelatins and then their sequestration into the vacuole (Hasanuzzaman et al. 2012; Nahar et al. 2016) and further protection was conferred by the ability of metallothioneins to link heavy metals to their thiol group. Thus, the accumulation of Hg was retarded by various algal extracts, especially that of PDAE and PNDAE.
The activity of the antioxidant system was tested in the present work to tolerate Hg-induced oxidative stress, where the up-regulation of ROS-scavenging enzymes allows plants to withstand metal toxicity (Rizvi and Khan 2018). In addition, the rationale behind this study was exposing Coccomyxa chodatii to macronutrient deprivation, enhancing the extracts' antioxidative potential. This reflected the high antioxidant potential of extracts that enriched the stressed or non-stressed wheat plants with a high antioxidantive quenching ability. The abridge of algal extracts in membrane damage traits largely for PDAE or PNDAE, and to a lesser extent, NAE may be due to their antioxidants capability in terms of phenolic compounds, total antioxidants, and ferric reducing activity to maintain less ROS energized cells, hence less oxidative burst and membrane perturbations. The used algal extracts elicited over-production of α-tocopherol as well as specifically curtailed the reduction in carotenoids and AsA to be larger than control plants in most cases. In this sense, carotenoids are responsible for scavenging ROS, preventing peroxidation of lipid membranes, and mitigating oxidative bursts, hence saving the photosynthetic apparatus (Younes et al. 2020). α-Tocopherol is soundly reported to aid in stabilizing the redox state in chloroplast during stresses and implicit in the deactivation of membrane lipids peroxidation by lessening the malondialdehyde content, thereby protecting the bio-membranes rigidity (Abdel Latef et al. 2020; El-Beltagi et al. 2019). AsA can directly quench H2O2, O2•−, 1O2, and •OH and regenerate α-tocopherol from α-chromanoxyl radical, thereby protecting membranes (El-Sheshtawy et al. 2022; Dawood et al. 2021c; Ghonaim et al. 2021). Thus, the Hg-stress was significantly accompanied by the impairment of various defending non-enzymatic antioxidants. The inclination of different algal extracts to co-opt plant defense metabolites against Hg-stress was delegated by induction of secondary metabolites such as phenolics, alleviated relative to that of Hg-stressed plants. In addition to their antioxidant role through their hydrogen donating (antioxidant) potential, phenolics act as radical scavengers and reduce the toxic effects of oxidative stress on metabolism and cells (Abdel Latef et al. 2020; Sofy et al. 2021b).
Plant responses to environmental stressors may be measured using ROS-scavenging enzymes, which are sensitive biochemical parameters (Fouda and Sofy 2022; Singh et al. 2020). In the present work, wheat plants demonstrated a poor response of enzymatic antioxidants to Hg-induced oxidative stress. This ineffectiveness might be attributed to a high level of reactive oxygen molecules that exceeds the scavenging capabilities of enzyme antioxidants (Agha et al. 2021). Although SOD activity in plants is regarded as an essential oxidative stress indicator and first-line defense against superoxide radicals, it was not activated in the present research under Hg-stress, which might be due to its inhibition by a high increment of H2O2. In addition, Hg stress attenuated the CAT and APX activities to scavenge H2O2 in wheat plants, revealing that Hg stress might have retarded the limit of the ability of wheat to synthesize CAT and APX enzymes. The decrement of APX activity is consistent with reducing AsA and GSH contents. When the APX/GSH pools are depleted, the free radicals generated by oxidative stress might finally overpower the antioxidant defense mechanism, similar to that reported by El-Sheshtawy et al. (2021). Hence ROS-enzymes have been overused or inhibited, resulting in membrane lipid peroxidation and, finally, cell death (Hossain et al. 2012; Maksoud et al. 2022). It is worth mentioning that SOD, APX, and CAT activities were stimulated under the interactive effect of Hg stress and P- or PN-deprived algal extract relative to that of other treatments, which indicates increased detoxification. The high level of SOD activity induced by algal extracts might be sufficient to scavenge O2•– and reduce its toxicity under Hg-stress. In addition, a profound induction of APX and CAT activities under the interactive effect of Hg-stressed seedlings and different algal extracts trapped H2O2 and attenuated its accumulation, which implied that different algal extracts, especially P- or PN-deprived extracts exert protection against O2•– and H2O2. This showed that antioxidant system involvement seems to prevent damage of the membrane peroxidation and methylglyoxal level in the shoots, although to a larger degree for PDAE or PNDAE than the other extracts. As reported in extracts analysis, PDAE or PNDAE had the highest contents of total antioxidants and ferric reducing algal extracts compared to NAE or NDAE.
Foliar PAL activity was higher in plants treated with different algal extracts than that of control, and the lowest values were reported for Hg-stressed seedlings. This up-regulation of PAL under metal-deprived extracts and Hg-stress could be mirrored by the accumulation of phenolic compounds that function as the strong non-enzymatic antioxidant and quench free radicals in cells. The increased PAL activity suggested that the activated phenylpropanoid pathway synthesizes secondary metabolites as phenolic compounds that help quench oxidative damage. According to many studies, PAL has a crucial role in accumulating phenolics and flavonoids, which detoxify ROS, decreasing lipid peroxidation, and enhancing the cellular defense responses (Dawood et al. 2021b; Younes et al. 2019). Conversely, the enhancement of PPO activity under stress could be a stress marker reported in previous studies (Bashandy et al. 2020; Dawood and Azooz 2020). Thus, the algal extracts reduced the PPO activities under Hg stress.
The effect of Hg exposure on peroxidase activity in terms of soluble peroxidase and ionic peroxidases (SPO and IPO) showed an accumulation trend with raising Hg concentrations compared to the control. However, this accumulation did not exert gain to seedlings that were severely compromised with oxidative stress. Thus, the accumulation of IPO and SPO is a stress marker parallel to PPO triggering under Hg stress. On the other hand, the used algal extract, especially P- or PN-deprived ones, vastly reduced IPO and SPO to a large extent compared to Hg-stress only. This indicates the role of APX, CAT, and SOD and the accompanied non-enzymatic antioxidants alleviated by the algal extracts in encountering oxidative stress mediated by Hg stress. Thus, the present investigation suggests the importance of the antioxidant potential of N- and/or P-deprived extracts as multi-bio stimulating agents against Hg stress on wheat plants. Further studies should be done on other stress agents, and more details on the composition of the algal extract should be evaluated. A diagram of the mechanism of normal and macronutrient-deprived algal extracts on modulation Hg-stress tolerance in wheat seedlings was represented in Fig. 11. The mechanisms of algae are extracellular precipitation, biosorption to cell walls, decreased uptake, or increased efflux, which all serve to lower the concentration of metal entering the cell. Additionally, Hg is intracellularly chelated by producing amino acids, GSH, or HM-binding ligands like phytochelatins (PCs), and up-regulation of the antioxidant defense to combat the harmful effects brought on by ROS.
The mechanism of algae and macronutrient-deprived algal effect on modulation of Hg-stress tolerance in wheat. ↑ = increase, ↑↑↑ = high increase, ↓ = reduction, ↓↓↓ = high reduction. NAE = normal algal extract, NDAE = Nitrogen deprived algal extract, PDAE = Nitrogen deprived algal extract, PNDAE = Phosphorous and Nitrogen deprived algal extract, Chl a, b = chlorophyll a and b, NR = nitrate reductase, AA = amino acids, pro = proteins, Car = carbohydrates, ROS/NOS = Reactive oxygen species/reactive nitrogen species, H2O2= Hydrogen peroxide, •OH = Hydroxyl radical, O2•– = superoxide anion, NO = Nitric oxide, MDA = Malondialdehyde, MG = Methylglyoxal, LOX = lipoxygenase enzyme, Phen = Phenolics, PAL = Phenylalanine ammonialyase, GSH = reduced glutathione, PCS = Phytochelatins, MET = metallothioneins, AsA = Ascorbic acid, Toc = Tocopherol, SOD = Superoxide dismutase, CAT = Catalase, APX = Ascorbate peroxidase, IPO = ionic peroxidase, SPO = soluble peroxidase, PPO = Polyphenol oxidase
Conclusion
The result of the present investigation denoted a highly significant improvement of seedling's status under the interactive effect of various algal extract and Hg imposition with different degrees concomitant to low accumulation of Hg level in the wheat tissues. The applied N- or/and P-deprived extracts are characterized by higher metabolic products, amino acids, proteins, sugars, flavonoids, phenolics, total antioxidant capacity, and ferric reducing capacity than normally growing algal extract. The FTIR recommended the presence of active growth regulator substances as auxins, peptides, and polysaccharides, which have a biostimulation effect on plants. The augmentation capacity of algal extracts to Hg-damaging impacts related to i) reduction of Hg accumulation in wheat tissues; ii) induction of antioxidant machinery (enzymatic and non-enzymatic antioxidants); iii) improvement of chelation mechanism (GSH, PCs, and metallotheinins); iv) reduction of oxidative stress and membrane damage traits (MG, MDA, and LOX); v) modulation of photosynthetic pigments, primary and secondary metabolism. All these up-regulation mainly reflected on the prevention of wheat seedlings inhibition and allowed the seedlings to cope better with Hg-stress.
References
Abdel-Kader HA, Abdel-Basset R, Danial AW (2022) Yeast and enzymatic hydrolysis in converting Chlorella biomass into hydrogen gas by Rhodobacter sp. and Rhodopseudomonas palustris. Int J Hydrogen Energy 47:1516–1528. https://doi.org/10.1016/j.ijhydene.2021.10.126
Abdel Latef AAH, Dawood MF, Hassanpour H, Rezayian M, Younes NA (2020) Impact of the Static Magnetic Field on Growth, Pigments, Osmolytes, Nitric Oxide, Hydrogen Sulfide, Phenylalanine Ammonia-Lyase Activity, Antioxidant Defense System, and Yield in Lettuce. Biology 9:172. https://doi.org/10.3390/biology9070172
Abu-Shahba MS, Mansour MM, Mohamed HI, Sofy MR (2022) Effect of biosorptive removal of cadmium ions from hydroponic solution containing indigenous garlic peel and mercerized garlic peel on lettuce productivity. Sci Hortic 293:110727. https://doi.org/10.1016/j.scienta.2021.110727
Adams L, Dinauer M, Morgenstern D, Krahenbuhl J (1997) Comparison of the roles of reactive oxygen and nitrogen intermediates in the host response to Mycobacterium tuberculosis using transgenic mice. Tuber Lung Dis 78:237–246. https://doi.org/10.1016/S0962-8479(97)90004-6
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
ALHaithloul HAS, Khan MI, Musa A, Ghoneim MM, ALrashidi AA, Khan I, Azab E, Gobouri AA, Sofy MR, El-Sherbiny M (2022) Phytotoxic effects of Acacia saligna dry leachates on germination, seedling growth, photosynthetic performance, and gene expression of economically important crops. PeerJ 10:e13623
Ali S, Abbas Z, Seleiman MF, Rizwan M, YavaŞ İ, Alhammad BA, Shami A, Hasanuzzaman M, Kalderis D (2020) Glycine betaine accumulation, significance and interests for heavy metal tolerance in plants. Plants 9:896. https://doi.org/10.3390/plants9070896
Agha MS, Abbas MA, Sofy MR, Haroun SA, Mowafy AM (2021) Dual inoculation of Bradyrhizobium and Enterobacter alleviates the adverse effect of salinity on Glycine max seedling. Not Bot Horti Agro Cluj-Napoca 49:12461. https://doi.org/10.15835/nbha49312461
Ahmad P, Ahanger MA, Egamberdieva D, Alam P, Alyemeni MN, Ashraf M (2018) Modification of osmolytes and antioxidant enzymes by 24-epibrassinolide in chickpea seedlings under mercury (Hg) toxicity. J Plant Growth Regul 37:309–322. https://doi.org/10.1007/s00344-017-9730-6
Ahmad G, Khan AA, Mohamed HI (2021) Impact of the low and high concentrations of fly ash amended soil on growth, physiological response and yield of pumpkin (Cucurbita moschata Duch. Ex Poiret L.). Environ Sci Pollut Res 28:17068–17083. https://doi.org/10.1007/s11356-020-12029-8
Amer A, Ghoneim M, Shoala T, Mohamed HI (2021) Comparative studies of eco-friendly compounds like humic acid, salicylic, and glycyrrhizic acids and their nanocomposites on French basil (Ocimum basilicum L. cv. Grand verde). Environ Sci Poll Res 1–17. https://doi.org/10.1007/s11356-021-14022-1
Arc E, Galland M, Godin B, Cueff G, Rajjou L (2013) Nitric oxide implication in the control of seed dormancy and germination. Front Plant Sci 4:346. https://doi.org/10.3389/fpls.2013.00346
Aremu AO, Neményi M, Stirk WA, Ördög V, van Staden J (2015) Manipulation of nitrogen levels and mode of cultivation are viable methods to improve the lipid, fatty acids, phytochemical content, and bioactivities in Chlorella minutissima. J Phycol 51:659–669. https://doi.org/10.1111/jpy.12308
Ashry NA, Ghonaim MM, Mohamed HI, Mogazy AM (2018) Physiological and molecular genetic studies on two elicitors for improving the tolerance of six Egyptian soybean cultivars to cotton leaf worm. Plant Physiol Biochem 130:224–234
Azizan A, Ahamad Bustamam MS, Maulidiani M, Shaari K, Ismail IS, Nagao N, Abas F (2018) Metabolite profiling of the microalgal diatom Chaetoceros calcitrans and correlation with antioxidant and nitric oxide inhibitory activities via 1H NMR-based metabolomics. Mar Drugs 16:154. https://doi.org/10.3390/md16050154
Badawy IH, Hmed AA, Sofy MR, Al-Mokadem AZ (2022) Alleviation of Cadmium and Nickel Toxicity and Phyto-Stimulation of Tomato Plant L. by Endophytic Micrococcus luteus and Enterobacter cloacae. Plants 11:2018. https://doi.org/10.3390/plants11152018
Bashandy SR, Abd-Alla MH, Dawood MF (2020) Alleviation of the toxicity of oily wastewater to canola plants by the N2-fixing, aromatic hydrocarbon biodegrading bacterium Stenotrophomonas maltophilia-SR1. Appl Soil Ecol 154:103654. https://doi.org/10.1016/j.apsoil.2020.103654
Behera B, Supraja KV, Paramasivan B (2021) Integrated microalgal biorefinery for the production and application of biostimulants in circular bioeconomy. Bioresour Technol 339:125588. https://doi.org/10.1016/j.biortech.2021.125588
Cargnelutti D, Tabaldi LA, Spanevello RM, de Oliveira JG, Battisti V, Redin M, Linares CEB, Dressler VL, de Moraes Flores EM, Nicoloso FT (2006) Mercury toxicity induces oxidative stress in growing cucumber seedlings. Chemosphere 65:999–1006
Cataldo RJ, Hidalgo LM, Neaman A, Gaete OH (2011) Use of molecular biomarkers in Eisenia foetida to assess copper toxicity in agricultural soils affected by mining activities. J Soil Sci Plant Nutr 11:57–70. https://doi.org/10.4067/S0718-95162011000300005
Chaudhuri D, Ghate NB, Deb S, Panja S, Sarkar R, Rout J, Mandal N (2014) Assessment of the phytochemical constituents and antioxidant activity of a bloom forming microalgae Euglena tuba. Biol Res 47:1–11. https://doi.org/10.1186/0717-6287-47-24
Chen J, Ye Y, Ran M, Li Q, Ruan Z, Jin N (2020) Inhibition of tyrosinase by mercury chloride: spectroscopic and docking studies. Front Pharmacol 11:81. https://doi.org/10.3389/fphar.2020.00081
Chen Y-A, Chi W-C, Trinh NN, Huang L-Y, Chen Y-C, Cheng K-T, Huang T-L, Lin C-Y, Huang H-J (2014) Transcriptome profiling and physiological studies reveal a major role for aromatic amino acids in mercury stress tolerance in rice seedlings. PLoS One 9:e95163. https://doi.org/10.1371/journal.pone.0095163
Chiaiese P, Corrado G, Colla G, Kyriacou MC, Rouphael Y (2018) Renewable sources of plant biostimulation: microalgae as a sustainable means to improve crop performance. Front Plant Sci 9:1782. https://doi.org/10.3389/fpls.2018.01782
Cobb JS, Zai-Rose V, Correia JJ, Janorkar AV (2020) FT-IR spectroscopic analysis of the secondary structures present during the desiccation induced aggregation of elastin-like polypeptide on silica. ACS Omega 5:8403–8413. https://doi.org/10.1021/acsomega.0c00271
Corpas FJ, Barroso JB (2013) Nitro-oxidative stress vs oxidative or nitrosative stress in higher plants. New Phytol 199:633–635
Coulombier N, Blanchier P, Le Dean L, Barthelemy V, Lebouvier N, Jauffrais T (2021) The effects of CO2-induced acidification on Tetraselmis biomass production, photophysiology and antioxidant activity: A comparison using batch and continuous culture. J Biotechnol 325:312–324. https://doi.org/10.1016/j.jbiotec.2020.10.005
Cuin TA, Shabala S (2007) Amino acids regulate salinity-induced potassium efflux in barley root epidermis. Planta 225:753–761. https://doi.org/10.1007/s00425-006-0386-x
Dawood MF, Sofy MR, Mohamed HI, Sofy AR, Abdel-kader HA (2022a) Hydrogen Sulfide Modulates Salinity Stress in Common Bean Plants by Maintaining Osmolytes and Regulating Nitric Oxide Levels and Antioxidant Enzyme Expression. J Soil Sci Plant Nutr 1-19. https://doi.org/10.1007/s42729-022-00921-w
Dawood MF, Abu-Elsaoud AM, Sofy MR, Mohamed HI, Soliman MH (2022b) Appraisal of kinetin spraying strategy to alleviate the harmful effects of UVC stress on tomato plants. Environ Sci Poll Res 1-21. https://doi.org/10.1007/s11356-022-19378-6
Dawood MF, Azooz MM (2019) Concentration-dependent effects of tungstate on germination, growth, lignification-related enzymes, antioxidants, and reactive oxygen species in broccoli (Brassica oleracea var. italica L.). Environ Sci Pollut Res 26:36441–36457. https://doi.org/10.1007/s11356-019-06603-y
Dawood MF, Azooz MM (2020) Insights into the oxidative status and antioxidative responses of germinating broccoli (Brassica oleracea var. italica L.) seeds in tungstate contaminated water. Chemosphere 261:127585. https://doi.org/10.1016/j.chemosphere.2020.127585
Dawood MF, Mourad AM, Alomari DZ, Latef AAHA (2021a) Insights into the enzymatic antioxidants and their genetic expressions responses of plants to heavy metals. Organic Solutes, Oxidative stress, and antioxidant enzymes under abiotic stressors. CRC Press 1st Ed: 36. eBook
Dawood MF, Sohag AAM, Tahjib-Ul-Arif M, Latef AAHA (2021b) Hydrogen sulfide priming can enhance the tolerance of artichoke seedlings to individual and combined saline-alkaline and aniline stresses. Plant Physiol Biochem 159:347–362. https://doi.org/10.1016/j.plaphy.2020.12.034
Dawood MF, Tahjib-Ul-Arif M, Sohag AAM, Abdel Latef AAH, Ragaey MM (2021c) Mechanistic insight of allantoin in protecting tomato plants against ultraviolet C stress. Plants 10:11. https://doi.org/10.3390/plants10010011
Dean AP, Sigee DC, Estrada B, Pittman JK (2010) Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae. Biores Technology 101:4499–4507. https://doi.org/10.1016/j.biortech.2010.01.065
Dihazi A, Jaiti F, El Hadrami I, El Hassni M, Zouine J (2003) Effect of salicylic acid on phenolic compounds related to date palm resistance to Fusarium oxysporum f. sp. albedinis. Phytopathol Mediterr 42:9–16
Dmytryk A, Chojnacka K (2018) Algae as fertilizers, biostimulants, and regulators of plant growth. In: K Chojnacka, PP Wieczorek, G Schroeder, I Michalak (eds) Algae biomass: characteristics and applications: towards algae-based products. Springer International Publishing, Cham 8:115–122. https://doi.org/10.1007/978-3-319-74703-3_10
Downs MR, Nadelhoffer KJ, Melillo JM, Aber JD (1993) Foliar and fine root nitrate reductase activity in seedlings of four forest tree species in relation to nitrogen availability. Trees 7:233–236. https://doi.org/10.1007/BF00202079
Du Jardin P (2015) Plant biostimulants: definition, concept, main categories and regulation. Sci Hortic 196:3–14. https://doi.org/10.1016/j.scienta.2015.09.021
El-Beltagi HS, Mohamed HI, Abdelazeem AS, Youssef R, Safwat G (2019) GC-MS analysis, antioxidant, antimicrobial and anticancer activities of extracts from Ficus sycomorus fruits and leaves. Notulae Bot Horti Agrobot Cluj-Napoca 47(2):493–505. https://doi.org/10.15835/NBHA47211405
El-Mahdy OM, Mohamed HI, Mogazy AM (2021) Biosorption effect of Aspergillus niger and Penicillium chrysosporium for Cd-and Pb-contaminated soil and their physiological effects on Vicia faba L. Environ Sci Pollut Res 28:67608–67631. https://doi.org/10.1007/s11356-021-15382-4
El-Naggar NE-A, Hamouda RA, Abuelmagd MA, Abdelgalil SA (2021) Bioprocess development for biosorption of cobalt ions and Congo red from aquatic mixture using Enteromorpha intestinalis biomass as sustainable biosorbent. Sci Rep 11:1–21. https://doi.org/10.1038/s41598-021-94026-6
El-Sheshtawy HS, Sofy MR, Ghareeb DA, Yacout GA, Eldemellawy MA, Ibrahim BM (2021) Eco-friendly polyurethane acrylate (PUA)/natural filler-based composite as an antifouling product for marine coating. Appl Microbiol Biotechnol 105:7023–7034. https://doi.org/10.1007/s00253-021-11501-w
El-Sheshtawy HS, Mahdy HM, Sofy AR, Sofy MR (2022) Production of biosurfactant by Bacillus megaterium and its correlation with lipid peroxidation of Lactuca sativa. Egypt J Petrol 31(2):1–6. https://doi.org/10.1016/j.ejpe.2022.03.001
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Elstner EF, Heupel A (1976) Formation of hydrogen peroxide by isolated cell walls from horseradish (Armoracia lapathifolia Gilib.). Planta 130:175–180. https://doi.org/10.1007/BF00384416
Fales FW (1951) The assimilation and degradation of carbohydrates by yeast cells. J Biol Chem 193:113–124. https://doi.org/10.1016/S0021-9258(19)52433-4
Farnese FS, Menezes-Silva PE, Gusman GS, Oliveira JA (2016) When bad guys become good ones: the key role of reactive oxygen species and nitric oxide in the plant responses to abiotic stress. Front Plant Sci 7:471. https://doi.org/10.3389/fpls.2016.00471
Fouda HM, Sofy MR (2022) Effect of biological synthesis of nanoparticles from Penicillium chrysogenum as well as traditional salt and chemical nanoparticles of zinc on canola plant oil productivity and metabolic activity. Egypt J Chem 65:507–516. https://doi.org/10.21608/ejchem.2021.95120.4469.
Ghaderiardakani F, Collas E, Damiano DK, Tagg K, Graham NS, Coates JC (2019) Effects of green seaweed extract on Arabidopsis early development suggest roles for hormone signalling in plant responses to algal fertilisers. Scie Rep 9:1–13. https://doi.org/10.1038/s41598-018-38093-2
Ghanati F, Morita A, Yokota H (2002) Induction of suberin and increase of lignin content by excess boron in tobacco cells. Soil Sci Plant Nutr 48:357–364. https://doi.org/10.1080/00380768.2002.10409212
Ghonaim MM, Mohamed HI, Omran AAA (2021) Evaluation of wheat salt stress tolerance using physiological parameters and retrotransposon-based markers. Genet Resour Crop Evol 68:227–242
Ghori N-H, Ghori T, Hayat M, Imadi S, Gul A, Altay V, Ozturk M (2019) Heavy metal stress and responses in plants. Int J Environ Sci Technol 16:1807–1828. https://doi.org/10.1007/s13762-019-02215-
Gilbert RP, Brandt RB (1975) Spectrophotometric determination of methyl glyoxal with 2, 4-dinitrophenylhydrazine. Anal Chem 47:2418–2422. https://doi.org/10.1021/ac60364a003
Godlewska K, Michalak I, Tuhy Ł, Chojnacka K (2016) Plant growth biostimulants based on different methods of seaweed extraction with water. BioMed Res Int 2016. https://doi.org/10.1155/2016/5973760
Gontia-Mishra I, Sapre S, Sharma A, Tiwari S (2016) Alleviation of mercury toxicity in wheat by the interaction of mercury-tolerant plant growth-promoting rhizobacteria. J Plant Growth Regul 35:1000–1012. https://doi.org/10.1007/s00344-016-9598-x
Górka B, Korzeniowska K, Lipok J, Wieczorek PP (2018) The biomass of algae and algal extracts in agricultural production. In: K Chojnacka, PP Wieczorek, G Schroeder, I Michalak (eds) Algae biomass: characteristics and applications: towards algae-based products. springer international publishing, Cham. 8: 103-114. https://doi.org/10.1007/978-3-319-74703-3_9
Halliwell B, Gutteridge JM, Aruoma OI (1987) The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 165:215–219. https://doi.org/10.1016/0003-2697(87)90222-3
Han Y, Ni Z, Li S, Qu M, Tang F, Mo R, Ye C, Liu Y (2018) Distribution, relationship, and risk assessment of toxic heavy metals in walnuts and growth soil. Environ Sci Pollut Res 25:17434–17443. https://doi.org/10.1007/s11356-018-1896-3
Hasanuzzaman M, Hossain MA, Fujita M (2012) Exogenous selenium pretreatment protects rapeseed seedlings from cadmium-induced oxidative stress by upregulating antioxidant defense and methylglyoxal detoxification systems. Biol Trace Element Res 149:248–261. https://doi.org/10.1007/s12011-012-9419-4
Havir EA, Hanson KR (1973) L-phenylalanine ammonia-lyase (maize and potato). Evidence that the enzyme is composed of four subunits. Biochemistry 12:1583–1591. https://doi.org/10.1021/bi00732a019
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circular (2nd edit). California agricultural experiment station 347:23–32
Hoque MA, Uraji M, Torii A, Banu MNA, Mori IC, Nakamura Y, Murata Y (2012) Methylglyoxal inhibition of cytosolic ascorbate peroxidase from Nicotiana tabacum. J Biochem Mol Toxicol 26:315–321. https://doi.org/10.1002/jbt.21423
Hoque TS, Hossain MA, Mostofa MG, Burritt DJ, Fujita M, Tran L-SP (2016) Methylglyoxal: an emerging signaling molecule in plant abiotic stress responses and tolerance. Fron Plant Sci 7:1341. https://doi.org/10.3389/fpls.2016.01341
Hossain MA, Piyatida P, da Silva JAT, Fujita M (2012) Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J Bot 2012. https://doi.org/10.1155/2012/872875
Hou M, Li M, Yang X, Pan R (2019) Responses of nonprotein thiols to stress of vanadium and mercury in maize (Zea mays L.) seedlings. Bull Environ Contamin Toxicol 102:425–431. https://doi.org/10.1007/s00128-019-02553-w
Hu Y, Wang Y, Liang Y, Guo J, Gong H, Xu Z (2020) Silicon alleviates mercury toxicity in garlic plants. J Plant Nutr 43:2508–2517. https://doi.org/10.1080/01904167.2020.1783302
Jagota S, Dani H (1982) A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal Biochem 127:178–182. https://doi.org/10.1016/0003-2697(82)90162-2
Jakob T, Wagner H, Stehfest K, Wilhelm C (2007) A complete energy balance from photons to new biomass reveals a light-and nutrient-dependent variability in the metabolic costs of carbon assimilation. J Exp Bot 58:2101–2112
Jayshree A, Jayashree S, Thangaraju N (2016) Chlorella vulgaris and Chlamydomonas reinhardtii: effective antioxidant, antibacterial and anticancer mediators. Indian J Pharm Sci 78:575–581. https://doi.org/10.4172/pharmaceutical-sciences.1000155
Kivçak B, Mert T (2001) Quantitative determination of α-tocopherol in Arbutus unedo by TLC-densitometry and colorimetry. Fitoterapia 72:656–661. https://doi.org/10.1016/S0367-326X(01)00305-7
Kováčik J, Dresler S, Peterková V, Babula P (2018) Metal-induced oxidative stress in terrestrial macrolichens. Chemosphere 203:402–409. https://doi.org/10.1016/j.chemosphere.2018.03.112
Kumar K, Khan P (1982) Peroxidase and polyphenol oxidase in excised ragi (Eleusine corocana cv PR 202) leaves during senescence. Indian J Exp Biol 20:412–416
Latique S, Mrid RB, Kabach I, Kchikich A, Sammama H, Yasri A, Nhiri M, El Kaoua M, Douira A, Selmaoui K (2021) Foliar Application of Ulva rigida Water Extracts Improves Salinity Tolerance in Wheat (Triticum durum L.). Agronomy 11:265. https://doi.org/10.3390/agronomy11020265
Lee S-H, Lee J-B, Lee K-W, Jeon Y-J (2010) Antioxidant properties of tidal pool microalgae, Halochlorococcum porphyrae and Oltamannsiellopsis unicellularis from Jeju Island, Korea. Algae 25:45–56. https://doi.org/10.4490/algae.2010.25.1.045
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Portland Press Ltd 591–592. https://doi.org/10.1042/bst0110591
Lima F, Martins G, Silva A, Vasques I, Engelhardt M, Cândido G, Pereira P, Reis R, Carvalho G, Windmöller C (2019) Critical mercury concentration in tropical soils: impact on plants and soil biological attributes. Sci Total Environ 666:472–479. https://doi.org/10.1016/j.scitotenv.2019.02.216
Lodge TE (2016) The Everglades handbook: understanding the ecosystem 4th Ed. CRC Press 464. https://doi.org/10.1201/9781315369037
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275. https://doi.org/10.1016/S0021-9258(19)52451-6
Maksoud MA, Bekhit M, El-Sherif DM, Sofy AR, Sofy MR (2022) Gamma radiation-induced synthesis of a novel chitosan/silver/Mn-Mg ferrite nanocomposite and its impact on cadmium accumulation and translocation in brassica plant growth. Int J Biol Macromol 194:306–316. https://doi.org/10.1016/j.ijbiomac.2021.11.197
Mano Ji, Miyatake F, Hiraoka E, Tamoi M (2009) Evaluation of the toxicity of stress-related aldehydes to photosynthesis in chloroplasts. Planta 230:639–648. https://doi.org/10.1007/s00425-009-0964-9
Martini F, Beghini G, Zanin L, Varanini Z, Zamboni A, Ballottari M (2021) The potential use of Chlamydomonas reinhardtii and Chlorella sorokiniana as biostimulants on maize plants. Algal Res 60:102515. https://doi.org/10.1016/j.algal.2021.102515
Meharg AA (1993) The role of the plasmalemma in metal tolerance in angiosperms. Physiol Plant 88:191–198. https://doi.org/10.1034/j.1399-3054.1993.880126.x
Mengel A, Chaki M, Shekariesfahlan A, Lindermayr C (2013) Effect of nitric oxide on gene transcription–S-nitrosylation of nuclear proteins. Front Plant Sci 4:293. https://doi.org/10.3389/fpls.2013.00293
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Minguez-Mosquera M, Jaren-Galan M, Garrido-Fernandez J (1993) Lipoxygenase activity during pepper ripening and processing of paprika. Phytochemistry 32:1103–1108. https://doi.org/10.1016/S0031-9422(00)95073-8
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175. https://doi.org/10.1016/S0021-9258(19)45228-9
Mohamed HI, Abd-Elsalam KA, Tmam AM, Sofy MR (2021) Silver-based nanomaterials for plant diseases management: Today and future perspectives. Silver Nanomaterials for Agri-Food Applications. Elsevier. https://doi.org/10.1016/B978-0-12-823528-7.00031-7
Mondal NK, Das C, Datta JK (2015) Effect of mercury on seedling growth, nodulation and ultrastructural deformation of Vigna radiata (L) Wilczek. Environ Monito Assess 187:1–14. https://doi.org/10.1007/s10661-015-4484-8
Moore S, Stein WH (1948) Photometric ninhydrin method for use in the chromatography of amino acids. J Biol Chem 176:367–388. https://doi.org/10.1007/s00709-014-0691-3
Mostofa MG, Hossain MA, Fujita M (2015) Trehalose pretreatment induces salt tolerance in rice (Oryza sativa L.) seedlings: oxidative damage and co-induction of antioxidant defense and glyoxalase systems. Protoplasma 252:461–475. https://doi.org/10.1007/s00709-014-0691-3
Moustafa-Farag M, Mohamed HI, Mahmoud A, Elkelish A, Misra AN, Guy KM, Kamran M, Ai S, Zhang M (2020) Salicylic acid stimulates antioxidant defense and osmolyte metabolism to alleviate oxidative stress in watermelons under excess boron. Plants 9:724. https://doi.org/10.3390/plants9060724
Mukherjee S, Choudhuri M (1983) Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plantar 58:166–170. https://doi.org/10.1111/j.1399-3054.1983.tb04162.x
Mutale-Joan C, Redouane B, Najib E, Yassine K, Lyamlouli K, Laila S, Zeroual Y, Hicham EA (2020) Screening of microalgae liquid extracts for their bio stimulant properties on plant growth, nutrient uptake and metabolite profile of Solanum lycopersicum L. Sci Rep 10:1–12. https://doi.org/10.1038/s41598-020-59840-4
Nahar K, Hasanuzzaman M, Alam MM, Rahman A, Suzuki T, Fujita M (2016) Polyamine and nitric oxide crosstalk: antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotoxicol Environ Saf 126:245–255. https://doi.org/10.1016/j.ecoenv.2015.12.026
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Natrah F, Yusoff F, Shariff M, Abas F, Mariana N (2007) Screening of Malaysian indigenous microalgae for antioxidant properties and nutritional value. J Appl Phycol 19:711–718. https://doi.org/10.1007/s10811-007-9192-5
Oyaizu M (1986) Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr Diet 44:307–315. https://doi.org/10.5264/eiyogakuzashi.44.307
Palmucci M, Ratti S, Giordano M (2011) Ecological and evolutionary implications of carbon allocation in marine phytoplankton as a function of nitrogen availability: a fourier transform infrared spectroscopy approach 1. J Phycol 47:313–323. https://doi.org/10.1111/j.1529-8817.2011.00963.x
Pan S, Jeevanandam J, Danquah MK (2019) Benefits of Algal Extracts in Sustainable Agriculture. In: A Hallmann, PH Rampelotto (eds) Grand Challenges in Algae Biotechnology. Springer International Publishing, Cham, pp 501–534. https://doi.org/10.1007/978-3-030-25233-5_14
Parmar P, Kumari N, Sharma V (2013) Structural and functional alterations in photosynthetic apparatus of plants under cadmium stress. Bot Stud 54:1–6. https://doi.org/10.1186/1999-3110-54-45
Pathak J, Maurya PK, Singh SP, Häder D-P, Sinha RP (2018) Cyanobacterial farming for environment friendly sustainable agriculture practices: innovations and perspectives. Front Environ Sci 6:7
Pető A, Lehotai N, Feigl G, Tugyi N, Ördög A, Gémes K, Tari I, Erdei L, Kolbert Z (2013) Nitric oxide contributes to copper tolerance by influencing ROS metabolism in Arabidopsis. Plant Cell Rep 32:1913–1923
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269:337–341. https://doi.org/10.1006/abio.1999.4019
Rachidi F, Benhima R, Kasmi Y, Sbabou L, Arroussi HE (2021) Evaluation of microalgae polysaccharides as biostimulants of tomato plant defense using metabolomics and biochemical approaches. Sci Rep 11:1–16. https://doi.org/10.1038/s41598-020-78820-2
Rao KM, Sresty T (2000) Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci 157:113–128. https://doi.org/10.1016/S0168-9452(00)00273-9
Rasheed R, Ashraf MA, Kamran S, Iqbal M, Hussain I (2018) Menadione sodium bisulphite mediated growth, secondary metabolism, nutrient uptake and oxidative defense in okra (Abelmoschus esculentus Moench) under cadmium stress. J Hazar Materials 360:604–614
Rathore S, Chaudhary D, Boricha G, Ghosh A, Bhatt B, Zodape S, Patolia J (2009) Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. S Afr J Bot 75:351–355. https://doi.org/10.1016/j.sajb.2008.10.009
Rippka R (1992) Pasteur culture collection of cyanobacterial strains in axenic culture. Catalogue and taxonomic handbook, catalogue of strains 1992/1993. Institut Pasteur 1:1–103
Rizvi A, Khan MS (2018) Heavy metal induced oxidative damage and root morphology alterations of maize (Zea mays L.) plants and stress mitigation by metal tolerant nitrogen fixing Azotobacter chroococcum. Ecotoxicol Environ Saf 157:9–20. https://doi.org/10.1016/j.ecoenv.2018.03.063
Rizwan M, Meunier J-D, Miche H, Keller C (2012) Effect of silicon on reducing cadmium toxicity in durum wheat (Triticum turgidum L. cv. Claudio W.) grown in a soil with aged contamination. J Hazard Mater 209:326–334. https://doi.org/10.1016/j.jhazmat.2012.01.033
Ronga D, Biazzi E, Parati K, Carminati D, Carminati E, Tava A (2019) Microalgal biostimulants and biofertilisers in crop productions. Agronomy 9:192
Safafar H, Van Wagenen J, Møller P, Jacobsen C (2015) Carotenoids, phenolic compounds and tocopherols contribute to the antioxidative properties of some microalgae species grown on industrial wastewater. Mar Drugs 13:7339–7356
Sahu GK, Upadhyay S, Sahoo BB (2012) Mercury induced phytotoxicity and oxidative stress in wheat (Triticum aestivum L.) plants. Physiol Mol Biol Plants 18:21–31. https://doi.org/10.1007/s12298-011-0090-6
Salama ES, Roh HS, Dev S, Khan MA, Abou-Shanab RA, Chang SW, Jeon BH (2019) Algae as a green technology for heavy metals removal from various wastewater. World J Microbiol Biotechnol 35(5):1–9. https://doi.org/10.1007/s11274-019-2648-3
Seleiman MF, Alotaibi MA, Alhammad BA, Alharbi BM, Refay Y, Badawy SA (2020a) Effects of ZnO nanoparticles and biochar of rice straw and cow manure on characteristics of contaminated soil and sunflower productivity, oil quality, and heavy metals uptake. Agronomy 10:790. https://doi.org/10.3390/agronomy10060790
Seleiman MF, Kheir AM (2018) Maize productivity, heavy metals uptake and their availability in contaminated clay and sandy alkaline soils as affected by inorganic and organic amendments. Chemosphere 204:514–522. https://doi.org/10.1016/j.chemosphere.2018.04.073
Seleiman MF, Santanen A, Mäkelä PS (2020b) Recycling sludge on cropland as fertilizer–Advantages and risks. Resour Conserv Recycl 155:104647. https://doi.org/10.1016/j.resconrec.2019.104647
Shahid MJ, Ali S, Shabir G, Siddique M, Rizwan M, Seleiman MF, Afzal M (2020) Comparing the performance of four macrophytes in bacterial assisted floating treatment wetlands for the removal of trace metals (Fe, Mn, Ni, Pb, and Cr) from polluted river water. Chemosphere 243:125353. https://doi.org/10.1016/j.chemosphere.2019.125353
Siddiqui MH, Al-Whaibi MH, Basalah MO (2011) Role of nitric oxide in tolerance of plants to abiotic stress. Protoplasma 248:447–455. https://doi.org/10.1007/s00709-010-0206-9
Singh H, Kumar D, Soni V (2020) Copper and mercury induced oxidative stresses and antioxidant responses of Spirodela polyrhiza (L.) Schleid. Biochem Biophys Rep 23:100781. https://doi.org/10.1016/j.bbrep.2020.100781
Singh R, Parihar P, Singh M, Bajguz A, Kumar J, Singh S, Singh VP, Prasad SM (2017) Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: current status and future prospects. Front Microbiol 8:515
Smerilli A, Balzano S, Maselli M, Blasio M, Orefice I, Galasso C, Sansone C, Brunet C (2019) Antioxidant and photoprotection networking in the coastal diatom Skeletonema marinoi. Antioxidants 8:154. https://doi.org/10.3390/antiox8060154
Sofy MR, Mancy AG, Alnaggar AEAM, Refaey EE, Mohamed HI, Elnosary ME, Sofy AR (2022) A polishing the harmful effects of Broad Bean Mottle Virus infecting broad bean plants by enhancing the immunity using different potassium concentrations. Not Bot Hortic Agro Cluj-Nap 50:12654. https://doi.org/10.15835/nbha50112654
Sofy AR, Sofy MR, Hmed AA, Dawoud RA, Refaey EE, Mohamed HI, El-Dougdoug NK (2021a) Molecular characterization of the Alfalfa mosaic virus infecting Solanum melongena in Egypt and the control of its deleterious effects with melatonin and salicylic acid. Plants 10:459. https://doi.org/10.3390/plants10030459
Sofy M, Mohamed H, Dawood M, Abu-Elsaoud A, Soliman M (2021b) Integrated usage of Trichoderma harzianum and biochar to ameliorate salt stress on spinach plants. Arch Agron Soil Sci 1-22. https://doi.org/10.1080/03650340.2021.1949709
Trivedi M, Tallapragada RM, Branton A, Trivedi D, Nayak G, Mishra RK, Jana S (2015) Biofield treatment: A potential strategy for modification of physical and thermal properties of indole. Environ Anal Chem 4. https://doi.org/10.4172/2380-2391.1000152
Vázquez-Vuelvas OF, Hernández-Madrigal JV, Gaviño R, Tlenkopatchev MA, Morales-Morales D, Germán-Acacio JM, Gomez-Sandoval Z, Garcias-Morales C, Ariza-Castolo A, Pineda-Contreras A (2011) X-ray, DFT, FTIR and NMR structural study of 2, 3-dihydro-2-(R-phenylacylidene)-1, 3, 3-trimethyl-1H-indole. J Mol Struct 987:106–118. https://doi.org/10.1016/j.molstruc.2010.12.001
Wu J, Zhang Y, Hao R, Cao Y, Shan X, Jing Y (2019) Nitric oxide enhances cytotoxicity of lead by modulating the generation of reactive oxygen species and is involved in the regulation of Pb2+ and Ca2+ fluxes in tobacco BY-2 cells. Plants 8:403. https://doi.org/10.3390/plants8100403
Yalçın S, Şükran Okudan E, Karakaş Ö, Önem AN, Sözgen Başkan K (2019) Identification and quantification of some phytohormones in seaweeds using UPLC-MS/MS. J Liq Chromatogr Relat Technol 42:475–484. https://doi.org/10.1080/10826076.2019.1625374
Yang J, Li G, Bishopp A, Heenatigala P, Hu S, Chen Y, Wu Z, Kumar S, Duan P, Yao L (2018) A comparison of growth on mercuric chloride for three Lemnaceae species reveals differences in growth dynamics that effect their suitability for use in either monitoring or remediating ecosystems contaminated with mercury. Front Chem 6:112. https://doi.org/10.3389/fchem.2018.00112
Younes N, Dawood MF, Wardany A (2019) Biosafety assessment of graphene nanosheets on leaf ultrastructure, physiological and yield traits of Capsicum annuum L. and Solanum melongena L. Chemosphere 228:318–327. https://doi.org/10.1016/j.chemosphere.2019.04.097
Younes NA, Dawood MF, Wardany AA (2020) The phyto-impact of fluazinam fungicide on cellular structure, agro-physiological, and yield traits of pepper and eggplant crops. Environ Sci Pollut Res 27. https://doi.org/10.1007/s11356-020-08289-z
Zhang T, Lu Q, Su C, Yang Y, Hu D, Xu Q (2017) Mercury induced oxidative stress, DNA damage, and activation of antioxidative system and Hsp70 induction in duckweed (Lemna minor). Ecotoxicol Environ Saf 143:46–56
Zhao J, Gao Y, Li Y-F, Hu Y, Peng X, Dong Y, Li B, Chen C, Chai Z (2013) Selenium inhibits the phytotoxicity of mercury in garlic (Allium sativum). Environ Res 125:75–81. https://doi.org/10.1016/j.envres.2013.01.010
Zou Y, Lu Y, Wei D (2004) Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J Agric Food Chem 52:5032–5039. https://doi.org/10.1021/jf049571r
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Mona Dawood, and Huwida Abdel-kader; Conceptualization, Methodology, Data curation, Formal analysis, Investigation, Writing -review & editing. Mona Dawood, Mahmoud Sofy, Heba Mohamed, Ahmed Sofy, Huwida Abdel-Kader; Conceptualization, Writing—review & editing. Mona Dawood, Mahmoud Sofy, Heba Mohamed, Ahmed Sofy, Huwida Abdel-Kader; Software, Validation, Methodology, Formal analysis, Investigation.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Juan Barcelo.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dawood, M.F.A., Sofy, M.R., Mohamed, H.I. et al. N- or/and P-deprived Coccomyxa chodatii SAG 216–2 extracts instigated mercury tolerance of germinated wheat seedlings. Plant Soil 483, 225–253 (2023). https://doi.org/10.1007/s11104-022-05732-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05732-7