Abstract
Tracheary elements (TEs), including vessels and tracheids, occur as a product of xylogenesis and are highly adapted for the transportation of water and solutes. Xylogenesis or wood formation encompasses various stages of cellular development, which requires stringent temporal and spatial regulation. To further complicate matters, TEs are polymorphous and associated with other complex tissues. These complexities have necessitated the development of in vitro culture systems that are capable of synchronously inducing TEs on demand. In this review, we cover the challenges associated with inducing TEs in vitro and how this has been overcome using mesophyll and callus culture systems in herbaceous plants, yielding transdifferentiation efficiencies of up to 76% and 90%, respectively. We postulate that when equipped with such information, a great opportunity exists to optimise these culture systems in commercially valuable woody genera that currently display lower efficiencies in the range of 15.8–65%. Although both the mesophyll and callus induction cultures have proven essential for uncovering the fundamental processes associated with secondary growth, the mesophyll-based systems have recently become much less prominent (2.8x) in the literature compared to the callus-based systems. This is largely due to ease of application of the callus system to other plant species, paving the way for applications ranging from fundamental research in economically valuable woody genera to the 3D-printing of biomaterial products in vitro.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants are one of the most dominant life forms, accounting for 80% of the biomass found on Earth, of which 70% is invested in the stem in the form of wood (Houghton and Hole 2008; Bar-On et al. 2018). Considering the value of wood as a sustainable source of biofuel and biomaterial products, it is imperative to understand the underlying mechanisms that drive xylogenesis (wood formation) in order to improve the quality and quantity of wood (Turner et al. 2007; Bollhöner et al. 2012; Oda and Fukuda 2012). Plants accumulate biomass in two ways, one of which is through primary growth that occurs in a longitudinal direction from the apical meristem, while the other originates from the lateral meristem (i.e. cambium) to increase the stem girth (Shi et al. 2019). Growth in a radial direction arises from the bidirectional proliferation of the cambial zone, which produces two tissue types, namely xylem on the inner side and phloem towards the outer side of the stem (Fischer et al. 2019). These tissues produced by the cambium, and including the cambium, are collectively termed vascular tissues (De Rybel et al. 2014). The vascular tissues form structures that are essential for transportation of water, nutrients and other assimilates, all of which contribute to the accumulation of biomass (Bar-On et al. 2018).
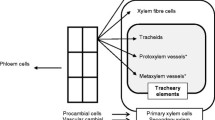
In the context of xylem, xylogenesis is initiated by the division of the cambium to form xylem mother cells (XMCs) that subsequently differentiate by cellular expansion, secondary cell wall (SCW) thickening and programmed cell death (PCD) to form various tissue types, including tracheary elements (TEs) and xylem fibres (Fig. 1, Růžička et al. 2015). Although performing different functions, TEs follow the same developmental process as fibers, requiring PCD to become functional (Funada et al. 2016). TE is an umbrella term for two cell types, namely vessel elements, found in angiosperms, and tracheids, more often occurring in gymnosperms (Demura 2014). Upon maturity, vessels and tracheids have a rigid outer secondary cell wall with a hollow lumen, enabling efficient water conductance throughout the stem (Kamon and Ohtani 2021). Tracheids generally develop a smaller lumen area with a thicker cell wall compared to vessels, this enables these to assist the fibers to provide structural support to the stem, but with the drawback that they are often less efficient at water transport than vessels (Bollhöner et al. 2012; Aloni 2015). Despite these differences, all TEs originate in the same zone, often tightly interspersed with fibers and parenchyma (Fig. 1), making it difficult to access the TEs for study (Kondo 2018). Typically, complex histological protocols are applied to generate microsections from hypocotyl or stem tissues (Fig. 1) that are analysed to study wood dynamics (Von Arx et al. 2016). Unfortunately, these preparations merely provide a snapshot of the final developmental state of the vasculature and exclude the stages preceding the terminal development that determine the final cell fate (Kondo 2018).
Cross-section of Eucalyptus grandis stem tissue, observed with a light microscope under 40x objective lens. The figure demonstrates the complexities of tissue structure associated with the plant stem. The arrows demarcate the diverse tissue populations within the stem, such as the phloem parenchyma (PP), phloem fibers (PF), sieve elements (SE), cambial initials (CI), xylem fibres (XF), xylem vessels (XV), incipient vessels (IV), ray (RP) and xylem parenchyma (XP)
To increase the accessibility of TEs for the study of developmental dynamics, culture systems have been developed that enable the process of xylogenesis to be emulated in vitro (Devillard and Walter 2014). These systems incorporate a series of experimental stages to induce the formation of TEs in vitro, such as culture initiation, maintenance and, lastly, differentiation into TEs. Two types of culture systems are used, firstly those that are established with leaf mesophyll cells, such as the Zinnia elegans culture system (Fukuda and Komamine 1980; Roberts and McCann 2000; Twumasi et al. 2009), and secondly those that require callus to initiate in vitro development, as seen in various species that include Arabidopsis thaliana (Oda et al. 2005; Pesquet et al. 2010), Pinus radiata, P. sylvestris, P. contorta (Savidge 1983; Ramsden and Northcote 1987; Möller et al. 2006), Populus tremula x P. tremuloides (Ohlsson et al. 2006), Pseudotsuga menziesii (Pillai et al. 2011) and Cupressus sempervirens (Havel et al. 1997). Due to the highly synchronous differentiation that occurs in vitro, a large proportion of TEs can be isolated at precise stages of development, which provides an accurate means of investigating the cellular gene expression, biochemistry, metabolome, or proteome at any given stage of xylogenesis (Demura et al. 2002; Milioni et al. 2002; Kubo et al. 2005; Möller et al. 2006). The model systems developed for Zinnia elegans and Arabidopsis thaliana have proven instrumental in establishing an improved understanding of the processes involved in xylogenesis (Iakimova and Woltering 2017; Kondo 2018).
Considering that wood formation is a highly complex process, simplification and control of wood development ex situ has grown in popularity and amounted to many breakthroughs in the topic of xylogenic research (Iakimova and Woltering 2017). This review covers the crucial developments of the xylogenic mesophyll (Fukuda and Komamine 1980) and callus (Kubo et al. 2005; Oda et al. 2005) induction systems. Furthermore, we explore how these systems have been optimised in Z. elegans and A. thaliana, but focus also on progress made in angiosperm and gymnosperm species, pointing out the latest applications in each of these systems and why the adoption of such in vitro xylogenesis systems for woody tree species is of vital importance for tree breeding and improvement programs.
Zinnia elegans mesophyll culture
Introduction of a technique to explore TE development
In 1980, the Zinnia elegans xylogenic cell culture was introduced as a technique to explore the process of TE development in vitro, which succeeded in obtaining a differentiation rate of 30% (Fukuda and Komamine 1980). Studies by Twumasi et al. (2009) successfully improved the differentiation rate and reproducibility of in vitro-formed TEs to 76%, attributing this feat to four factors, including the conditioning of the mesophyll culture, utilizing a cell density of 105 cells.mL− 1 or higher, selection of a cell viability of 60% or above, and the correct phytohormonal balance. The processes required to produce TEs from Z. elegans mesophyll cells are comprised of three broad stages, namely (I) dedifferentiation of mesophyll cells, (II) transdifferentiation of procambial-like cells and (III) PCD with lignification, each of which is associated with disparate morphological, physiological, biochemical, and molecular events (Pesquet et al. 2013).
Establishment of xylogenic mesophyll cell cultures
Zinnia cultures are initiated via the mechanical isolation of mesophyll cells from the first true leaves of seedlings, typically at 14-d old (Kärkönen et al. 2011). During the isolation procedure, the leaves are removed, surface-sterilised with 0.05% sodium hypochlorite (NaOCl), with or without 0.01% Triton X-100, and macerated in a blender, prior to transfer into either conditioning or differentiation medium (López-Serrano et al. 2004; Twumasi et al. 2010; Pesquet and Tuominen 2011). Conditioning medium, as described in Twumasi et al. (2009), which contains no plant growth regulators (PGRs), is considered an essential component for successful induction of mesophyll cells into TEs and is conducted for a brief culture period prior to transfer into medium containing PGRs. The mechanical wounding incurred during isolation is essential, as this triggers the onset of stage I, where mesophyll cells lose their photosynthetic ability, dedifferentiate and increase in responsiveness to auxin and cytokinin (Fukuda 1997, 2010). Once established in vitro, the dedifferentiated mesophyll cells comprise of a homogenous cell type and possess the potential for synchronous transdifferentiation into TEs in an orderly sequence of events (Iakimova and Woltering 2017).
Fukuda-Komamine’s medium (FKM), is commonly adopted to induce TEs directly from mesophyll cells (Fukuda and Komamine 1980; Twumasi et al. 2009; Pesquet and Tuominen 2011). After conditioning, PGRs such as NAA (auxin) and BAP (cytokinin) are generally supplemented into FKM in concentrations that range from 0.1 mg.L− 1 to 2 mg.L− 1 to induce TEs (Roberts et al. 1992; Church 1993; Ye and Varner 1996; Groover and Jones 1999; Twumasi et al. 2010; Takeuchi et al. 2013; Devillard and Walter 2014). These exogenously-supplied auxins and cytokinins stimulate the onset of stage II or transdifferentiation, where the formation of procambium-like cells, synthesis of SCW components, initial depositions of the SCW and the development of TE precursors occur ( Fig. 2; Fukuda 2010). The expression of the gene tracheary element differentiation 4 (TED4) is one of the markers associated the onset of stage II during the differentiation process (Pesquet and Tuominen 2011).
Processes and timeline associated with in vitro xylogenesis. During the early stages of tracheary element stimulation, the cells will initially (a) expand via turgor pressure and begin the accumulation of hydrolytic enzymes in the vacuole. During cellular expansion, (b) the precursors necessary for the secondary cell wall (SCW) are synthesized and SCW deposition begins. Once the SCW has reached the optimal thickness, and turgor driven pressures cannot lead to further expansion due to the rigidity of the SCW, the (c) vacuole tonoplast is degraded, which releases the hydrolytic enzymes into the cytoplasm. These hydrolytic enzymes (d) degrade all the cellular components, organelles, and nucleic acids. Lastly, (e) lignin is deposited onto the SCW, and the perforation plates are formed at the polar regions of the cell, leading to the formation of a functional dead hollow conduit. Timeline adapted from Twumasi et al. (2009)
The late processes of TE maturation, referred to as stage III, involves the continuation of SCW deposition accompanied by PCD, which includes vacuole enlargement and subsequent rupture of the tonoplast to release various proteases, endonucleases and other hydrolytic enzymes that lyse the cellular components and fragment DNA ( Fig. 2; Twumasi et al. 2009). This stage can be distinguished via quantitation of the marker gene Zinnia elegans serine protease (ZeSP), which is specific to stage III (Pesquet et al. 2005). Iakimova and Woltering (2017) suggest the inclusion of a fourth stage of development, involving lignification of the SCW post mortem, in a non-autonomous fashion via the delivery of substances from neighbouring living cells ( Fig. 2; Serk et al. 2015). By the end of differentiation, the mesophyll cells, generally 50 μm in length and 25 μm in width, differentiate over 72-h into TE displaying a length of 70–100 μm and a width of 30–60 μm (Fukuda and Komamine 1980).
Evidently, many studies that utilize Zinnia elegans mesophyll cell cultures to investigate TE differentiation are based off the initial findings of Fukuda and Komamine (1980, 1982), with minor alterations to the procedure and protocol (López-Serrano et al. 2004; Twumasi et al. 2009; Kákošová et al. 2013; Pesquet et al. 2013). Although providing a wealth of knowledge regarding the processes associated with TE differentiation, the application of the Z. elegans mesophyll system has been declining of late, as evidenced by the reduction in the number of published articles in the literature (Fig. 3, Online Resource 1). This factor is in part due to the lack of molecular markers and mutant lines in Z. elegans, and additionally failure to adapt this method into other plant species, possibly due to recalcitrance of mesophyll cells to form TEs in these other species (Iakimova and Woltering 2017).
The number of tracheary element (TE) induction articles published since 1986 until 2021, that make use of either the mesophyll, callus, or other induction strategies. Google scholar was utilized as a survey platform, via the search query “Tracheary element in vitro induction culture” and broken up into four-year increments, starting from 1986. The first twenty articles with reference to TE induction were selected and broken up into the demarcated categories, with review papers excluded
Arabidopsis thaliana culture
TE system in a model plant
In 1969, Fosket and Torrey (1969) developed a system for inducing TEs from soybean callus via the combination of the growth hormones kinetin and NAA. This early finding paved the way for xylogenic research in the model plant species A. thaliana, of which liquid suspension callus cultures were used to induce TEs (Kubo et al. 2005; Oda et al. 2005). Establishment of the A. thaliana culture system would prove to be highly valuable due to the relatively small genome size of 132 megabase pairs (Mbp) and the abundance of molecular tools available for this species (Woodward and Bartel 2018).
The various stages involved in the transdifferentiation of A. thaliana callus cultures follows a similar route to that of Z. elegans (Fukuda 2004; Oda et al. 2005). However, instead of dedifferentiation of mesophyll cells and subsequent differentiation of procambial cells into TEs, as is the case for the Z. elegans system, this system initially requires callus induction, from which parenchyma cells are formed and subsequently stimulated to produce TEs (Fukuda 2010; Mira et al. 2019). The stages by which the parenchyma cells form mature TEs are analogous to the mesophyll system, where events such as cellular elongation, deposition of cellulose, PCD and lignification occur sequentially ( Fig. 2; Devillard and Walter 2014, Pesquet et al. 2019).
Optimisation of Arabidopsis TE induction cultures
Xylogenic cell cultures for the A. thaliana callus system are typically initiated from Columbia-0 (Col-0) cell lines, with the origin of Col-0 described by Mathur et al. (1998). Furthermore, alternative cell lines grown on hormone-free medium have been established by Pesquet et al. (2010) from the root calli of 2-week-old Col-0 seedlings, generated via the transfer of root explants onto a callus-induction medium containing the hormones 2,4-D and BAP. Those authors obtained 40% TE differentiation and later demonstrated that, through overexpression of the gene coding for microtubule-associated protein – 1 (MAP70-1), they were able to enhance the TE differentiation efficiency to values ranging from 54.8 − 60.1% (Pesquet et al. 2010). Hormonal treatments with 1 mg.L− 1 BAP, 6 mg.L− 1 NAA and 4–5 µM (1.923–2.403 mg.L− 1) brassinolide have been implemented to induce xylogenesis in in vitro callus culture (Derbyshire et al. 2015; Mira et al. 2019). Oda et al. (2005) found that the auxin 2,4-D caused an inhibitory effect on TE differentiation and that 30% TE differentiation could only be achieved with the omission of 2,4-D, and treating transgenic A. thaliana Col-0 cell lines with 1 µM (0.480 mg.L− 1) brassinolide. Improvement of this treatment was obtained by a combination of 1 µM (0.480 mg.L− 1) brassinolide with 10 mM (0.6183 g.L− 1) boric acid, achieving 50% TE formation (Kubo et al. 2005).
These xylogenic culture systems have enabled the functional characterization of numerous regulators of xylogenesis, significantly improving our understanding of the Vascular-Related NAC-Domain (VND) transcription factors and tracheary element differentiation inhibitory factor (TDIF) – TDIF receptor (TDR, i.e. TDIF-TDR) signalling pathway (Fukuda and Ohashi-Ito 2019; Kamon and Ohtani 2021). Exploiting this recently found knowledge has enabled the development of the VND, KDB (kinetin, 2,4-D, and brassinolide) and VISUAL (Vascular cell Induction culture System Using Arabidopsis Leaves) TE induction systems (Tan et al. 2019).
VNDs, which comprise of seven members (VND1-VND7), are a family of transcription factors (TFs) regarded as the master regulators of xylogenesis (Ohashi-Ito et al. 2018). These are expressed in association with developing TEs, each of which displays a degree of spatiotemporal difference in gene expression (Yamaguchi et al. 2008; Zhou et al. 2014). VND6 and VND7 have been shown to function as transcriptional switches for meta- and proto- xylem formation respectively (Kubo et al. 2005; Li et al. 2016). Consequently, due to the important regulatory function played by these master TFs, these have been the target for improvement of in vitro TE systems (Tan et al. 2019). For instance, TE differentiation in the range of 80–90% has been reported with the construction of transgenic A. thaliana or Nicotiana tabacum lines, either harbouring an oestrogen-inducible VND6 gene insert or a glucocorticoid-inducible VND7 insert, respectively (Oda et al. 2010; Yamaguchi et al. 2010; Oda 2017). The signalling cascade triggered by the VND7 system has been exploited to unveil numerous molecular and cellular processes associated with in vitro TE formation (Kawabe et al. 2018; Noguchi et al. 2018; Ohtani et al. 2018; Takenaka et al. 2018; Watanabe et al. 2018). In particular, the functioning of the proteome (Arae et al. 2022), gene regulatory networks (Tong et al. 2021), calcium signalling (Kamon et al. 2021) and cellular stress responses (Hirai et al. 2022) in developing TE have been elucidated via the stimulation of the VND7 insert.
Our understanding of the TDIF-TDR signalling pathway has improved vastly with the introduction of the callus in vitro culture system and has since enabled the development of KDB and VISUAL induction systems (Fukuda and Ohashi-Ito 2019). TDIF-TDR signalling is responsible for the maintenance of vascular stem cells or the procambium, at the expense of xylem formation, and manipulations of this have enabled researchers to enhance TE formation in vitro (Hirakawa et al. 2008). The TDIF peptide molecule is encoded by the CLE41 (CLAVATA3/embryo surrounding region-related 41) and CLE44 genes from the phloem ( Fig. 4a; Fletcher 2020). TDIF migrates from the phloem and binds to the polyonymous TDR or PHLOEM INTERCALATED WITH XYLEM (PXY) receptor, located in the plasma membrane of procambial cells ( Fig. 4b; Hirakawa et al. 2010, Sorce et al. 2013). Once bound, this complex emits two signals, one that enhances the proliferation of procambial cells and a second one that prevents their differentiation into xylem ( Fig. 4c; Fisher and Turner 2007, Hirakawa et al. 2008). The procambial proliferation signal is carried out by the gene WUSCHEL-related HOMEOBOX gene 4 (WOX4) that encodes a developmental transcription factor (Hirakawa et al. 2010). In contrast, glycogen synthase kinase 3s (GSK3s) are activated at the plasma membrane via the TDIF-TDR signalling complex and are responsible for the inhibition of xylem development via targeting the BRI1-EMS SUPPRESSOR 1 (BES1) TF, that plays a major role in brassinosteroid signalling (Kondo et al. 2014). Furthermore, treatment of Arabidopsis cultures with TDIF has been shown to strongly reduce VND6 expression, which suggests an additional mode by which GSK3s can supress xylogenesis (Kondo et al. 2015).
The TDIF-TDR signalling pathway is initiated in the phloem where the (a) TDIF-peptide is synthesized. Subsequently, this (b) peptide migrates to the cambium where it (c) recognizes and binds to the TDR-receptor. This creates a complex that triggers a cascade of events that induces the proliferation of the cambium via the induction of WOX4 and hinders the proliferation of the xylem through the action of GSK3s. The above figure was constructed via information gathered from Kondo et al. ( 2015), Hirakawa et al. (2008), Lucas et al. (2013), Hirakawa et al. (2010) and Sorce et al. (2013)
KDB and VISUAL Arabidopsis systems
In the KDB and VISUAL system, TEs are directly induced from mesophyll or epidermal cells, following a similar route to the Z. elegans system (Kondo et al. 2015; Tan et al. 2018). The premise behind these systems is to stimulate TE differentiation by enhancing brassinosteriod signalling, or alleviating its suppression (Tan et al. 2019). In the case of the KDB system, differentiation is stimulated from A. thaliana cotyledons by directly adding brassinolide to the culture medium, with the addition of kinetin and 2,4-D (Tan et al. 2018). On the other hand, in the VISUAL induction system, A. thaliana leaf disks are treated with bikinin (4-[(5-bromo-2-pyridinyl) amino]-4-oxobutanoic acid) to alleviate the suppression of brassinosteroid signalling, and thereby produce TEs (Kondo et al. 2015, 2016). Bikinin, is known to compete with ATP (Adenosine triphosphate) for binding to GSK3s, and in doing so this competitive inhibition of the “ATP-binding pocket” has been shown to reduce the kinase activity of GSK3 (De Rybel et al. 2009; Kondo 2018). Therefore, the inhibition of GSK3 by bikinin is proposed to enhance xylem differentiation, in vitro, by alleviating the suppression of VNDs, BES1, as well as brassinosteroid signalling (Kondo et al. 2015). In contrast to the KDB system that strictly produces TEs, the VISUAL system has been shown to produce phloem sieve-like cells amidst the TEs (Kondo et al. 2016; Tan et al. 2018). These observations open the door for interesting prospects, where xylem- and phloem-specific tissues can be isolated from these induction cultures for comparative studies, such as performing a differential expression analysis to uncover genes that are specifically associated with xylem or phloem differentiation.
Although the VISUAL and KDB systems have been proven to produce TEs, the final differentiation frequency of the ectopic TEs are illustrated in figures and have yet to be precisely reported in the text, hence we cannot accurately extrapolate their efficiencies at this point (Kondo et al. 2015; Tan et al. 2018). Nevertheless, it is clear that the development of in vitro induction systems in the herbaceous plant species Z. elegans and A. thaliana have proven themselves as suitable models for TE differentiation (Kondo et al. 2015; Iakimova and Woltering 2017). However, as herbaceous, and woody-plant species rarely share similar characteristics in relation to their wood anatomy (Spicer and Groover 2010), application of these systems in these various woody angio- and gymnosperm species may enable comparative studies to determine the molecular basis for such differences in wood anatomy.
Wood-forming gymnosperm and angiosperm species
Adoption of callus-based TE systems in trees
Although the development of an in vitro TE system in herbaceous plant species has contributed substantially to uncovering various aspects associated with xylogenesis, it is important to also model TE formation in woody tree species as the xylogenic processes may vary between woody and herbaceous plants (Devillard and Walter 2014). In consideration of the mesophyll and callus induction systems, it is evident that the latter presents a greater degree of versatility since callus can be induced from a host of plant types and tissues. Although the callus system has been applied to woody tree species in the past, it is clear that the TE transdifferentiation potentials of these are generally lagging behind Z. elegans mesophyll and A. thaliana callus systems (Table 1). Only recently, since the sequencing of the first tree genome in Populus during 2006, and subsequently in Pinus and Eucalyptus in 2014, has the molecular information and tools surrounding trees provided incentive to improve these systems (Tuskan et al. 2006; Myburg et al. 2014; Zimin et al. 2014). In this section, we explore some of the early advancements in the application of the callus induction system on trees and how techniques applied in the A. thaliana system can be adapted to improving these.
Initiation of TE cultures in woody plants
The review by Möller (2006) outlines some of the systems which have been previously established to induce TEs in the genera Cupressus, Cryptomeria, Ginkgo, Pinus, Picea, Pseudostuga and Sequoia. Populus is one of the first woody tree species that has been used as a model to investigate the process of xylogenesis (Mellerowicz et al. 2001). The advantage of this genus as a model is due to its relatively small genome size of 550 Mbp, which is approximately 5 times larger than that of A. thaliana (Pillai et al. 2011; Woodward and Bartel 2018). In addition, Populus is amenable to genetic transformation with the use of A. tumefaciens-mediated gene transfer methods (Yang et al. 2017). Xylogenic suspension cultures have been developed for the hybrid aspen, Populus tremula x P. tremuloides, to analyse the function and activity of enzymes believed to be associated with the synthesis of the cell wall (Ohlsson et al. 2006). Furthermore, Yamagishi et al. (2013) successfully obtained 15.8% TE differentiation from the hybrid, Populus sieboldii x P. grandidentata, following 10-d in culture medium supplemented with 1 µM (0.480 mg.L− 1) brassinolide.
The initiation of xylogenic cultures in woody-plant species, as with the A. thaliana system, typically involves the induction of callus from tissues such as cambial strips, stem cuttings, leaves and petioles ( Fig. 5; Ohlsson et al. 2006, Pillai et al. 2011, Yamagishi et al. 2013, 2015). The processes involved in callus induction have been extensively reviewed (Ikeuchi et al. 2013). Callogenesis is generally conducted on Murashige and Skoog (MS) basal medium containing exogenously-supplied auxins and cytokinins at concentrations ranging from 0.5 mg.L− 1 to 3 mg.L− 1 (Pillai et al. 2011; Cai et al. 2015; Yamagishi et al. 2021; Shwe et al. 2022). Fresh callus is essential for the efficient formation of TEs, and consequently callus clumps are sub-cultured onto fresh medium every 7–28 days, depending on the system (Ohlsson et al. 2006; Yamagishi et al. 2015).
Optimising culture conditions for TE development
To improve TE differentiation efficiencies in woody-plant callus cultures there are various factors to consider, namely; the concentration of sucrose and nutrients in the medium, light and temperature, age of the callus cultures and, lastly, the phytohormone type or concentration (Möller 2006). Sucrose serves as an important source of carbon for in vitro cultures, and naturally will promote vascular differentiation by meeting the energy requirements of the cells (Aloni 1980, 1987; Yaseen et al. 2013). Hypocotyl-derived callus cultures from Pinus gerardiana, have been shown to produce “tracheid-like cells” upon an increase of sucrose concentration from 1 to 5% in the medium (Konar 1974). On the other hand, Washer et al. (1977), reported that nodules from P. radiata callus cultures contained parenchymatous cells with secondary cell walls upon the reduction of sucrose from 3 to 1.5%. Alternative sugars or carbohydrate sources such as myo-inositol have more recently been supplemented into TE induction medium (Dziedzic and McDonald 2012; Yamagishi et al. 2015). TE formation was reportedly enhanced in P. contorta callus cultures with an increase in boric acid concentration in the medium from trace amounts to 124 mg.L− 1, a similar finding was obtained in the A. thaliana xylogenic cultures where boric acid was required for TE formation (Kubo et al. 2005; Möller 2006). In cultures of Picea glauca, nitrogen sources such as ammonium nitrate (NH4NO3) and ammonium chloride (NH4Cl) were reported to be essential factors to induce TEs, compared to glutamine (Möller 2006). Yamagishi et al. (2012) utilized potassium nitrate (KNO3) at 3.4 g.L− 1 as a source of nitrogen to induce TE formation in Torreya nucifera, whereas the authors of the same study supplemented the induction medium with 800 mg.L− 1 of NH4NO3 and 2400 mg.L− 1 of L-glutamine to elicit xylogenesis in Cryptomeria japonica.
Xylogenesis of in vitro callus cultures of Pinus radiata was influenced by exposure to light, where cultures were shown to increase the production of TEs from 20% to 45% upon increased exposure to continuous low light (Möller et al. 2006). Furthermore, in the same study, those authors demonstrated that the emergence of TEs occurred within 48-h when callus cultures were exposed to light, and only at 120-h when cultured in the dark. Despite these findings, many studies report the induction of TEs in darkness (Pillai et al. 2011; Yamagishi et al. 2015), whilst some use a combination of both light and dark conditions (Ohlsson et al. 2006). The temperature typically implemented for inducing xylogenic cultures ranges from 23 to 25 °C (Möller et al. 2003, 2006; Ohlsson et al. 2006; Dziedzic and McDonald 2012, 2015; Yamagishi et al. 2013).
The totipotent nature of callus is pre-determined and possesses the ability, through cell “memory”, to be maintained through successive generations of subculture in vitro (Szechynska-Hebda et al. 2012; Hua Su et al. 2020). However, after numerous subcultures these totipotent callus derivatives can potentially lose their regeneration capacity in comparison to the initial, morphologically younger, cultures (Wedzony et al. 2014). The age of the callus cultures thus strongly influences the amenability of these cultures to produce TEs, where older cultures tend to display recalcitrance to differentiation (Möller 2006). Mehra and Anand (1979) demonstrated that 2-month-old callus cultures displayed 34% TE differentiation, as opposed to 18 month-old cultures that displayed 2–3% TE formation. Similarly, once maintained in vitro for a period of one year, the liquid callus cultures of Cryptomeria japonica were unable to develop into TEs, despite subculture onto fresh medium every 4-weeks and implementing methods previously shown to produce TEs (Yamagishi et al. 2015).
PGRs are essential components for in vitro culture application, as these chemical messengers regulate growth and development of various plant tissues (reviewed by Phillips and Garda, 2019). Different hybrids of Populus, namely P. sieboldii x P. grandidentata and P. tremula x P. tremuloides, have been shown to produce tracheids in vitro when subject to treatments with 1 µM (0.480 mg.L− 1) brassinolide, or with a combination of 1 mg.L− 1 2,4-D and 0.02 mg.L− 1 kinetin (Ohlsson et al. 2006; Yamagishi et al. 2013). Furthermore, the desiccation of cells prior to transfer onto 1 µM (0.480 mg.L− 1) brassinolide induction medium was found to further enhance the differentiation of calli into TEs from 9% to 27%, a threefold increase (Yamagishi et al. 2017). Pillai et al. (2011) demonstrated that optimal TE formation of 65% was obtained when the medium was supplemented with only 2 mg.L− 1 2,4-D, whereas treatments with 2 mg.L− 1 BAP or NAA, under the same conditions, failed to form TEs, indicating that 2,4-D is a critical component for TE formation in Douglas-fir (Pseudostuga menziesii). Similarly, Douglas fir has been shown to produce TEs in response to a combination of PGRs that include BAP and 2,4-D at an equivalent concentration of 2 mg.L− 1 (Dziedzic and Mcdonald 2015). On the other hand, hormone-free medium, containing 5 g.L− 1 activated charcoal (AC) was found to be sufficient for inducing TEs from calli of Torreya nucifera and Cryptomeria japonica (Yamagishi et al. 2012).
It is evident that the variability in treatment requirements highlights the fact that different varietal and genotypic factors can equate to vastly different in vitro responses in trees (Keret et al. 2021), implying that optimisation per genotype or species is often a requirement to enhance in vitro performance. A. thaliana culture systems, to date, are far superior in terms of TE differentiation efficiencies, in comparison to the tree systems (Table 1). This is in part due to the migration away from traditional hormonal induction systems to molecular-based transgenic inducible systems, such as the VND-inducible Arabidopsis cell lines, that can predictably produce high numbers of TEs (Tan et al. 2019). Transgenic Populus tremula x tremuloides, harbouring glucocorticoid-inducible VND6 and VND7 inserts, have been shown to produce TEs and we believe that the next stage in the evolution of TE systems in trees is to create transgenic callus cultures, capable of producing 80–90% TE differentiation, as seen in certain herbaceous plants (Oda et al. 2010; Yamaguchi et al. 2010; Yang et al. 2017; Tan et al. 2019). Achieving such efficient induction systems in trees will enable scientists to comprehensively explore the molecular cascades of events that are triggered by VNDs, and determine whether these cascades reflect those reported in model or well-studied plants (Kim et al. 2022; Xu et al. 2022). Fundamentally, this empirical information will add another dimension to models that aim to accurately predict tree growth and wood dynamics.
Future perspectives on TE induction systems
Cross-application of Z. elegans, A. thaliana and woody induction systems
The application of TE induction systems in trees will aid in unlocking many of the mysteries associated with secondary growth, and since vascular tissues in trees constitute a major portion of the world’s biomass, this may prove instrumental in ensuring global renewable bioenergy and product security (Pleguezuelo et al. 2015; Turley and Etchells 2022). However, it is clear that directly applying the induction methods optimised in Z. elegans and A. thaliana is not so straightforward, since trees may possess different regulatory pathways to induce TEs.
For instance, supplementing brassinolide into culture was found to induce 50% TE differentiation in A. thaliana, whereas its application in Populus was found to induce a rather low 15.8% (Kubo et al. 2005; Yamagishi et al. 2013). Additionally, with respect to A. thaliana, the auxin 2,4-D was shown to inhibit TEs from developing in culture, whereas this same hormone was found to be a critical component for producing TEs in Douglas fir and in Populus hybrids (Ohlsson et al. 2006; Pillai et al. 2011; Dziedzic and Mcdonald 2015). These conflicting results may suggest the possibility that herbaceous plants rely mostly on brassinosteroid signals for secondary growth, whereas woody plants rely mainly on auxin signals. Evidently, these results open the door for some interesting comparative studies to be conducted between herbaceous and woody plant species, to determine what regulatory factors are responsible for such contrasting responses to PGRs.
Despite these differences, certain similarities do exist, and these have enabled large strides to be made in the development of TE systems in trees. For example, both Z. elegans and P. sieboldii x P. grandidentata required a wounding or stress response, in the form of mechanical isolation or desiccation, for optimal induction of TEs (Fukuda 2010; Yamagishi et al. 2017). This phenomenon can be used as a tool to answer questions pertaining to the stress response in plants, and whether this is a significant driver of plant cell differentiation. More critically, comparisons can be drawn between herbaceous and woody plant species, to determine if stress responses are conserved and why this ultimately leads to the production of TEs rather than other cell types.
Inducing specific TE structures in vitro
The application of in vitro TE induction systems in various species has undoubtedly developed rapidly in recent years (Iakimova and Woltering 2017). However, it is clear that the element lacking in these systems is not only the inefficient TE transdifferentiation in woody plants, but the inability to mimic the cellular structure and organisational levels found in naturally-occurring xylem tissues, with these features often forming sporadically in culture (Pesquet et al. 2019). Recently, a critical cell structure, namely the perforation plates, was shown to be present in the TEs induced from the calli of Aesculus turbinata (Yamagishi et al. 2021). Perforation plates are indispensable to water flow in plants, as these enable the individual monomeric units that build up vessels and tracheids to form a single interconnected TE strand (Ruonala et al. 2017). Furthermore, Shwe et al. (2022) have released the first report of an induction system capable of producing organised TE strands in Eucalyptus bosistoana. In that report, those authors observed both parallel and polar linkages of TEs to form xylem strand-like structures, presumed to be connected at end walls and possessing pit pairs between the parallel TE formations (Shwe et al. 2022). These findings are critical, as the callus systems of Aesculus turbinata and Eucalyptus bosistoana may now serve as models to improve our understanding of the mechanisms that govern the formation of perforations and strands in TEs.
Biomaterial production
In addition to improving our fundamental understanding of wood formation, TE systems have also been proposed as a tool for ex planta biomaterial production, with the first proof-of-concept studies being conducted recently (Beckwith et al. 2021). Applying the Z. elegans model, 3D-printable and tuneable plant materials are produced in culture, and offer the potential to increase targeted biomaterial yields while simultaneously reducing the waste and environmental implications associated with harvesting full live plants (Beckwith et al. 2022). Although still rather premature, we propose that this system could be adapted to operate in woody plant species, using callus rather than mesophyll cells, where various PGR combinations can stimulate the production of TEs with properties suited to any given biomedical or structural application.
Conclusion
Clearly, TE systems offer boundless potentials, both in research and practical applications. Researching xylogenesis in situ is difficult, and generally extracting plant material from the differentiation zone of the stem will yield cell mixtures at different stages of development, and hence can only be utilized to answer broad scale questions about wood anatomy and xylogenesis. On the other hand, in vitro systems have been proven capable of synchronously inducing not only TEs, but also phloem-like cells, which will enable more targeted questions to be asked about xylogenesis. Answering these targeted questions will enable us to unlock the full power of xylogenic differentiation, thus enabling us to apply this knowledge practically to produce biomaterial products, and alleviate some of the strain placed on natural forest ecosystems (Beckwith et al. 2022).
However, since in vitro xylogenic systems have only been optimized in very few plant species such as Z. elegans and A. thaliana, this leaves room for improvement in woody tree genera, of which the properties and biomass of the secondary growth tissues are immensely valuable. Establishment of an optimized protocol for these valuable forest trees can improve our fundamental understanding of wood development in ring and diffuse porous trees to create future predictive models of wood formation to ultimately improve wood yield and quality.
Data availability
All data generated or analysed during this study are included in this published article as an Online Resource (Online Resource 1).
Abbreviations
- XMCs:
-
Xylem mother cells
- SCW:
-
Secondary cell wall
- PCD:
-
Programmed cell death
- TEs:
-
Tracheary elements
- NaOCL:
-
Sodium hypochlorite
- PGRs:
-
Plant Growth Regulators
- FKM:
-
Fukuda-Komamine’s medium
- NAA:
-
1-Naphthaleneacetic acid
- BAP:
-
6-Benzylaminopurine
- TED4 :
-
Tracheary element differentiation 4
- ZeSP :
-
Zinnia elegans serine protease
- 2,4-D:
-
2,4-Dichlorophenoxyacetic Acid
- Col-0:
-
Columbia-0
- MAP70-1:
-
Microtubule-associated protein-1
- VND :
-
Vascular related NAC-Domain
- TDIF :
-
Tracheary element differentiation inhibitory factor
- TDR :
-
Tracheary element differentiation receptor
- KDB:
-
Kinetin, 2,4-D, Brassinolide
- VISUAL:
-
Vascular cell induction culture system using Arabidopsis leaves
- TFs:
-
Transcription factors
- CLE:
-
CLAVATA3/embryo surrounding region-related
- PXY:
-
Phloem intercalated with xylem
- WOX4 :
-
WUSCHEL-related HOMEOBOX gene 4
- GSK3s:
-
Glycogen synthase kinase 3s
- BES1:
-
BRI1-EMS SUPPRESSOR 1
- ATP:
-
Adenosine triphosphate
- MS:
-
Murashige and Skoog
- NH4NO3 :
-
Ammonium nitrate
- NH4Cl:
-
Ammonium chloride
- KNO3 :
-
Potassium nitrate
- KH2PO4 :
-
Monopotassium phosphate
- AC:
-
Activated charcoal
- LS:
-
Linsmaier and Skoog medium
- PVPP:
-
Polyvinylpolypyrrolidone
References
Aloni R (1980) Role of auxin and sucrose in the differentiation of sieve and tracheary elements in plant tissue cultures. Planta 150:255–263
Aloni R (1987) Differentiation of vascular tissues. Annu Rev Plant Physiol 38:179–204. https://doi.org/10.1146/annurev.pp.38.060187.001143
Aloni R (2015) Ecophysiological implications of vascular differentiation and plant evolution. Trees - Struct Funct 29:1–16. https://doi.org/10.1007/s00468-014-1070-6
Arae T, Nakakoji M, Noguchi M et al (2022) Plant secondary cell wall proteome analysis with an inducible system for xylem vessel cell differentiation. Dev Growth Differ 64:5–15. https://doi.org/10.1111/dgd.12767
Bar-On YM, Phillips R, Milo R (2018) The biomass distribution on Earth. Proc Natl Acad Sci U S A 115:6506–6511. https://doi.org/10.1073/pnas.1711842115
Beckwith AL, Borenstein JT, Velásquez-García LF (2021) Tunable plant-based materials via in vitro cell culture using a Zinnia elegans model. J Clean Prod 288. https://doi.org/10.1016/j.jclepro.2020.125571
Beckwith AL, Borenstein JT, Velásquez-García LF (2022) Physical, mechanical, and microstructural characterization of novel, 3D-printed, tunable, lab-grown plant materials generated from Zinnia elegans cell cultures. Mater Today 54:27–41. https://doi.org/10.1016/j.mattod.2022.02.012
Bollhöner B, Prestele J, Tuominen H (2012) Xylem cell death: emerging understanding of regulation and function. J Exp Bot 63:1081–1094. https://doi.org/10.1093/jxb/err438
Cai Z, Jing X, Tian X et al (2015) Direct and indirect in vitro plant regeneration and the effect of brassinolide on callus differentiation of Populus euphratica Oliv. S Afr J Bot 97:143–148. https://doi.org/10.1016/J.SAJB.2015.01.006
Church DL (1993) Tracheary element differentiation in Zinnia mesophyll cell cultures. Plant Growth Regul 12:179–188. https://doi.org/10.1007/BF00027197
De Rybel B, Audenaert D, Vert G et al (2009) Chemical inhibition of a subset of Arabidopsis thaliana GSK3-like kinases activates brassinosteroid signaling. Chem Biol 16:594–604. https://doi.org/10.1016/j.chembiol.2009.04.008
De Rybel B, Adibi M, Breda AS et al (2014) Integration of growth and patterning during vascular tissue formation in Arabidopsis. Science 345:1255215. https://doi.org/10.1126/science.1255215
Demura T, Tashiro G, Horiguchi G et al (2002) Visualization by comprehensive microarray analysis of gene expression programs during transdifferentiation of mesophyll cells into xylem cells. Proc Natl Acad Sci U S A 99:15794–15799. https://doi.org/10.1073/pnas.232590499
Demura T (2014) Tracheary element differentiation. Plant Biotechnol Rep 8:17–21. https://doi.org/10.1007/s11816-013-0293-0
Derbyshire P, Ménard D, Green P et al (2015) Proteomic analysis of microtubule interacting proteins over the course of xylem tracheary element formation in Arabidopsis. Plant Cell 27:2709–2726. https://doi.org/10.1105/tpc.15.00314
Devillard C, Walter C (2014) Formation of plant tracheary elements in vitro – a review. New Zeal J For Sci 44:1–14. https://doi.org/10.1186/s40490-014-0022-7
Dziedzic JA, McDonald AG (2012) A comparative survey of proteins from recalcitrant tissues of a non-model gymnosperm, Douglas-fir. Electrophoresis 33:1102–1112. https://doi.org/10.1002/elps.201100526
Dziedzic JA, Mcdonald AG (2015) in vitro protein profiles in the early and late stages of Douglas-fir xylogenesis. Electrophoresis 36:2035–2045. https://doi.org/10.1002/elps.201400561
Fischer U, Kucukoglu M, Helariutta Y, Bhalerao RP (2019) The dynamics of cambial stem cell activity. Annu Rev Plant Biol 70:293–319. https://doi.org/10.1146/annurev-arplant-050718-100402
Fisher K, Turner S (2007) PXY, a receptor-like kinase essential for maintaining polarity during plant vascular-tissue development. Curr Biol 17:1061–1066. https://doi.org/10.1016/j.cub.2007.05.049
Fletcher JC (2020) Recent sdvances in Arabidopsis CLE peptide signaling. Trends Plant Sci 25:1005–1016. https://doi.org/10.1016/j.tplants.2020.04.014
Fosket DE, Torrey JG (1969) Hormonal control of cell proliferation and xylem differentiation in cultured tissues of Glycine max var. Biloxi. Plant Physiol 44:871–880. https://doi.org/10.1104/pp.44.6.871
Fukuda H, Komamine A (1980) Establishment of an experimental system for the study of tracheary element differentiation from single cells isolated from the mesophyll of Zinnia elegans. Plant Physiol 65:57–60. https://doi.org/10.1104/pp.65.1.57
Fukuda H, Komamine A (1982) Lignin synthesis and its related enzymes as markers of tracheary-element differentiation in single cells isolated from the mesophyll of Zinnia elegans. Planta 155:423–430
Fukuda H (1997) Tracheary element differentiation. Plant Cell 9:1147–1156. https://doi.org/10.1007/s11816-013-0293-0
Fukuda H (2004) Signals that control plant vascular cell differentiation. Nat Rev Mol Cell Biol 5:379–391. https://doi.org/10.1038/nrm1364
Fukuda H (2010) Plant tracheary elements. Encycl Life Sci. https://doi.org/10.1002/9780470015902.a0001814.pub2
Fukuda H, Ohashi-Ito K (2019) Vascular tissue development in plants. Curr Top Dev Biol 131:141–160. https://doi.org/10.1016/bs.ctdb.2018.10.005
Funada R, Yamagishi Y, Begum S et al (2016) Xylogenesis in trees: from cambial cell division to cell death. Second Xylem Biol Orig Funct Appl 25–43. https://doi.org/10.1016/B978-0-12-802185-9.00002-4
Groover A, Jones AM (1999) Tracheary element differentiation uses a novel mechanism coordinating programmed cell death and secondary cell wall synthesis. Plant Physiol 119:375–384. https://doi.org/10.1104/PP.119.2.375
Havel L, Scarano M, Durzan D (1997) Xylogenesis in Cupressus callus involves apoptosis. Adv Hortic Sci 11:37–40
Hirai R, Wang S, Demura T, Ohtani M (2022) Histone deacetylation controls xylem vessel cell differentiation via transcriptionalregulation of a transcription repressor complex OFP1/4–MYB75–KNAT7–BLH6. Front Plant Sci 12:1–13. https://doi.org/10.3389/fpls.2021.825810
Hirakawa Y, Shinohara H, Kondo Y et al (2008) Non-cell-autonomous control of vascular stem cell fate by a CLE peptide/receptor system. PNAS 105:15208–15213
Hirakawa Y, Kondo Y, Fukuda H (2010) TDIF peptide signaling regulates vascular stem cell proliferation via the WOX4 homeobox gene in Arabidopsis. Plant Cell 22:2618–2629. https://doi.org/10.1105/tpc.110.076083
Houghton RA, Hole W (2008) Quantities of biomass importance.Encycl Ecol448–453
Hua Su Y, Ping Tang L, Yu Zhao X et al (2020) Plant cell totipotency: insights into cellular reprogramming. J Integr Plant Biol. https://doi.org/10.1111/jipb.12972
Iakimova ET, Woltering EJ (2017) Xylogenesis in zinnia (Zinnia elegans) cell cultures: unravelling the regulatory steps in a complex developmental programmed cell death event. Planta 245:681–705. https://doi.org/10.1007/s00425-017-2656-1
Ikeuchi M, Sugimoto K, Iwase A (2013) Plant callus: mechanisms of induction and repression. Plant Cell 25:3159–3173. https://doi.org/10.1105/tpc.113.116053
Kákošová A, Digonnet C, Goffner D, Lišková D (2013) Galactoglucomannan oligosaccharides are assumed to affect tracheary element formation via interaction with auxin in Zinnia xylogenic cell culture. Plant Cell Rep 32:479–487. https://doi.org/10.1007/s00299-012-1379-9
Kamon E, Ohtani M (2021) Xylem vessel cell differentiation: a best model for new integrative cell biology? Curr Opin Plant Biol 64:102135. https://doi.org/10.1016/j.pbi.2021.102135
Kamon E, Noda C, Higaki T et al (2021) Calcium signaling contributes to xylem vessel cell differentiation via post-transcriptional regulation of VND7 downstream events. Plant Biotechnol 38:331–337. https://doi.org/10.5511/plantbiotechnology.21.0519a
Kärkönen A, Santanen A, Iwamoto K, Fukuda H (2011) Plant tissue cultures. In: Popper ZA (ed) Methods in Molecular Biology. Springer, Clifton NJ, pp 1–20
Kawabe H, Ohtani M, Kurata T et al (2018) Protein S-nitrosylation regulates xylem vessel cell differentiation in Arabidopsis. Plant Cell Physiol 59:17–29. https://doi.org/10.1093/pcp/pcx151
Keret R, Nakhooda M, Jones NB, Hills PN (2021) Optimisation of micropropagation protocols for temperate eucalypt hybrids in South Africa, with a focus on auxin transport proteins. South For 83:254–263. https://doi.org/10.2989/20702620.2021.1987177
Kim MH, Bae EK, Lee H, Ko JH (2022) Current understanding of the genetics and molecular mechanisms regulating wood formation in plants. Genes (Basel) 13:1181. https://doi.org/10.3390/genes13071181
Konar R (1974) in vitro studies on Pinus I. Establishment and growth of callus. Physiol Plant 32: 193–197. https://doi.org/10.1111/j.1399-3054.1974.tb03121.x
Kondo Y, Ito T, Nakagami H et al (2014) Plant GSK3 proteins regulate xylem cell differentiation downstream of TDIF-TDR signalling. Nat Commun 5:1–11. https://doi.org/10.1038/ncomms4504
Kondo Y, Fujita T, Sugiyama M, Fukuda H (2015) A novel system for xylem cell differentiation in Arabidopsis thaliana. Mol Plant 8:612–621. https://doi.org/10.1016/j.molp.2014.10.008
Kondo Y, Nurani AM, Saito C et al (2016) Vascular cell induction culture system using Arabidopsis leaves (VISUAL) reveals the sequential differentiation of sieve element-like cells. Plant Cell 28:1250–1262. https://doi.org/10.1105/tpc.16.00027
Kondo Y (2018) Reconstitutive approach for investigating plant vascular development. J Plant Res 131:23–29. https://doi.org/10.1007/s10265-017-0998-1
Kubo M, Udagawa M, Nishikubo N et al (2005) Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev 1855–1860. https://doi.org/10.1101/gad.1331305.GENES
Li Z, Fernie A, Persson S (2016) Transition of primary to secondary cell wall synthesis. Sci Bull 61:838–846. https://doi.org/10.1007/s11434-016-1061-7
López-Serrano M, Fernández MD, Pomar F et al (2004) Zinnia elegans uses the same peroxidase isoenzyme complement for cell wall lignification in both single-cell tracheary elements and xylem vessels. J Exp Bot 55:423–431. https://doi.org/10.1093/jxb/erh036
Lucas WJ, Groover A, Lichtenberger R et al (2013) The plant vascular system: evolution, development and functions. J Integr Plant Biol 55:294–388. https://doi.org/10.1111/jipb.12041
Mathur J, Szabados L, Schaefer S et al (1998) Gene identification with sequenced T-DNA tags generated by transformation of Arabidopsis cell suspension. Plant J 13:707–716. https://doi.org/10.1046/j.1365-313X.1998.00059.x
Mehra PN, Anand M (1979) Cytology of callus of Cryptomeria japonica. Physiol Plant 45:127–131. https://doi.org/10.1111/j.1399-3054.1979.tb01676.x
Mellerowicz EJ, Baucher M, Sundberg B, Boerjan W (2001) Unravelling cell wall formation in the woody dicot stem. Plant Mol Biol 47:239–274. https://doi.org/10.1023/A:1010699919325
Milioni D, Sado PE, Stacey NJ et al (2002) Early gene expression associated with the commitment and differentiation of a plant tracheary element is revealed by cDNA-amplified fragment length polymorphism analysis. Plant Cell 14:2813–2824. https://doi.org/10.1105/tpc.005231
Mira MM, Ciacka K, Hill RD, Stasolla C (2019) in vitro differentiation of tracheary elements is induced by suppression of Arabidopsis phytoglobins. Plant Physiol Biochem 135:141–148. https://doi.org/10.1016/j.plaphy.2018.11.036
Möller R, McDonald AG, Walter C, Harris PJ (2003) Cell differentiation, secondary cell-wall formation and transformation of callus tissue of Pinus radiata D. Don. Planta 217:736–747. https://doi.org/10.1007/s00425-003-1053-0
Möller R (2006) Tracheary element differentiation and secondary cell-wall formation in cell cultures of coniferous gymnosperms. New Zeal J For Sci 36:156–171
Möller R, Koch G, Nanayakkara B, Schmitt U (2006) Lignification in cell cultures of Pinus radiata: activities of enzymes and lignin topochemistry. Tree Physiol 26:201–210. https://doi.org/10.1093/treephys/26.2.201
Myburg AA, Grattapaglia D, Tuskan GA et al (2014) The genome of Eucalyptus grandis. Nature 510:356–362. https://doi.org/10.1038/nature13308
Noguchi M, Fujiwara M, Sano R et al (2018) Proteomic analysis of xylem vessel cell differentiation in VND7-inducible tobacco BY-2 cells by two-dimensional gel electrophoresis. Plant Biotechnol 35:31–37. https://doi.org/10.5511/plantbiotechnology.18.0129a
Oda Y, Mimura T, Hasezawa S (2005) Regulation of secondary cell wall development by cortical microtubules during tracheary element differentiation in Arabidopsis cell suspensions. Plant Physiol 137:1027–1036. https://doi.org/10.1104/pp.104.052613
Oda Y, Iida Y, Kondo Y, Fukuda H (2010) Wood cell-wall structure requires local 2D-microtubule disassembly by a novel plasma membrane-anchored protein. Curr Biol 20:1197–1202. https://doi.org/10.1016/j.cub.2010.05.038
Oda Y, Fukuda H (2012) Secondary cell wall patterning during xylem differentiation. Curr Opin Plant Biol 15:38–44. https://doi.org/10.1016/j.pbi.2011.10.005
Oda Y (2017) VND6-induced xylem cell differentiation in Arabidopsis cell cultures. In: de Lucas M, Etchells JP (eds) Xylem: methods and protocols, methods in molecular biology. Humana Press, New York, pp 67–73
Ohashi-Ito K, Iwamoto K, Fukuda H (2018) LOB DOMAIN-CONTAINING PROTEIN 15 positively regulates expression of VND7, a master regulator of tracheary elements. Plant Cell Physiol 59:989–996. https://doi.org/10.1093/pcp/pcy036
Ohlsson AB, Djerbi S, Winzell A et al (2006) Cell suspension cultures of Populus tremula x P. tremuloides exhibit a high level of cellulose synthase gene expression that coincides with increased in vitro cellulose synthase activity. Protoplasma 228:221–229. https://doi.org/10.1007/s00709-006-0156-4
Ohtani M, Kawabe H, Demura T (2018) Evidence that thiol-based redox state is critical for xylem vessel cell differentiation. Plant Signal Behav 13:1–4. https://doi.org/10.1080/15592324.2018.1428512
Pesquet E, Ranocha P, Legay S et al (2005) Novel markers of xylogenesis in zinnia are differentially regulated by auxin and cytokinin. Plant Physiol 139:1821–1839. https://doi.org/10.1104/pp.105.064337
Pesquet E, Korolev AV, Calder G, Lloyd CW (2010) The microtubule-associated protein AtMAP70-5 regulates secondary Wall patterning in Arabidopsis wood cells. Curr Biol 20:744–749. https://doi.org/10.1016/j.cub.2010.02.057
Pesquet E, Tuominen H (2011) Ethylene stimulates tracheary element differentiation in Zinnia elegans cell cultures. New Phytol 190:138–149. https://doi.org/10.1111/j.1469-8137.2010.03600.x
Pesquet E, Zhang B, Gorzsás A et al (2013) Non-cell-autonomous postmortem lignification of tracheary elements in Zinnia elegans. Plant Cell 25:1314–1328. https://doi.org/10.1105/tpc.113.110593
Pesquet E, Wagner A, Grabber JH (2019) Cell culture systems: invaluable tools to investigate lignin formation and cell wall properties. Curr Opin Biotechnol 56:215–222. https://doi.org/10.1016/j.copbio.2019.02.001
Phillips GC, Garda M (2019) Plant tissue culture media and practices: an overview. Vitr Cell Dev Biol - Plant 55:242–257. https://doi.org/10.1007/s11627-019-09983-5
Pillai KV, McDonald AG, Wagner FG (2011) Developing a model system in vitro to understand tracheary element development in douglas-fir (Pseudostuga mensziesii). Maderas Cienc y Tecnol 13:3–18. https://doi.org/10.4067/S0718-221X2011000100001
Pleguezuelo CRR, Zuazo VHD, Bielders C et al (2015) Bioenergy farming using woody crops. A review. Agron Sustain Dev 35:95–119. https://doi.org/10.1007/s13593-014-0262-1
Ramsden L, Northcote D (1987) Tracheid formation in cultures of pine (Pinus sylvestris). J Cell Sci 88:467–474. https://doi.org/10.1242/jcs.88.4.467
Roberts AW, Koonce LT, Haigler CH (1992) A simplified medium for in vitro tracheary element differentiation in mesophyll suspension cultures from Zinnia elegans L. Plant Cell Tissue Organ Cult 28:27–35. https://doi.org/10.1007/BF00039912
Roberts K, McCann MC (2000) Xylogenesis: the birth of a corpse. Curr Opin Plant Biol 3:517–522. https://doi.org/10.1016/S1369-5266(00)00122-9
Ruonala R, Ko D, Helariutta Y (2017) Genetic networks in plant vascular development. Annu Rev Genet 51:335–359. https://doi.org/10.1146/annurev-genet-120116-024525
Růžička K, Ursache R, Hejátko J, Helariutta Y (2015) Xylem development - from the cradle to the grave. New Phytol 207:519–535. https://doi.org/10.1111/nph.13383
Savidge RA (1983) The role of plant hormones in higher plant cellular differentiation. II. Experiments with the vascular cambium, and sclereid and tracheid differentiation in the pine, Pinus contorta. Histochem J 15:447–466
Serk H, Gorzsás A, Tuominen H, Pesquet E (2015) Cooperative lignification of xylem tracheary elements. Plant Signal Behav 10:1–5. https://doi.org/10.1080/15592324.2014.1003753
Shi D, Lebovka I, Loṕez-Salmeroń V et al (2019) Bifacial cambium stem cells generate xylem and phloem during radial plant growth. Dev 146:1–8. https://doi.org/10.1242/dev.171355
Shwe SS, Alizadeh H, Tayagui A, Leung DWM (2022) Diverse forms of xylem-like cells and strand formation in xylogenic Eucalyptus bosistoana callus culture. Plant Cell Tissue Organ Cult 152:129–138. https://doi.org/10.1007/s11240-022-02393-3
Sorce C, Giovannelli A, Sebastiani L, Anfodillo T (2013) Hormonal signals involved in the regulation of cambial activity, xylogenesis and vessel patterning in trees. Plant Cell Rep 32:885–898. https://doi.org/10.1007/s00299-013-1431-4
Spicer R, Groover A (2010) Evolution of development of vascular cambia and secondary growth. New Phytol 186:577–592. https://doi.org/10.1111/j.1469-8137.2010.03236.x
Szechynska-Hebda M, Skrzypek E, Grazyna D et al (2012) The effect of endogenous hydrogen peroxide induced by cold treatment in the improvement of tissue regeneration efficiency. Acta Physiol Plant 34:547–560. https://doi.org/10.1007/s11738-011-0852-3
Takenaka Y, Watanabe Y, Schuetz M et al (2018) Patterned deposition of xylan and lignin is independent from that of the secondary wall cellulose of Arabidopsis xylem vessels. Plant Cell 30:2663–2676. https://doi.org/10.1105/tpc.18.00292
Takeuchi C, Nagatani K, Sato Y (2013) Chitosan and a fungal elicitor inhibit tracheary element differentiation and promote accumulation of stress lignin-like substance in Zinnia elegans xylogenic culture. J Plant Res 126:811–821. https://doi.org/10.1007/s10265-013-0568-0
Tan TT, Endo H, Sano R et al (2018) Transcription factors VND1-VND3 contribute to cotyledon xylem vessel formation. Plant Physiol 176:773–789. https://doi.org/10.1104/pp.17.00461
Tan TT, Demura T, Ohtani M (2019) Creating vessel elements in vitro: towards a comprehensive understanding of the molecular basis of xylem vessel element differentiation. Plant Biotechnol 36:1–6. https://doi.org/10.5511/plantbiotechnology.18.1119b
Tong H, Chen H, Williams CM (2021) Gene regulatory network of secondary cell wall biosynthesis during VND7 induced de novo xylem formation. Int J Biosci Biochem Bioinforma 11:74–81. https://doi.org/10.17706/ijbbb.2021.11.4.74-81
Turley EK, Etchells JP (2022) Laying it on thick: a study in secondary growth. J Exp Bot 73:665–679. https://doi.org/10.1093/jxb/erab455
Turner S, Gallois P, Brown D (2007) Tracheary element differentiation. Annu Rev Plant Biol 58:407–433. https://doi.org/10.1146/annurev.arplant.57.032905.105236
Tuskan G, Di Fazio S, Jansson S et al (2006) The genome of black cottonwood, Populus trichocarpa. Science 313:1596–1605
Twumasi P, Schel JHN, van Ieperen W et al (2009) Establishing in vitro Zinnia elegans cell suspension culture with high tracheary element differentiation. Cell Biol Int 33:524–533. https://doi.org/10.1016/j.cellbi.2009.01.019
Twumasi P, Iakimova ET, Qian T et al (2010) Caspase inhibitors affect the kinetics and dimensions of tracheary elements in xylogenic zinnia (Zinnia elegans) cell cultures. BMC Plant Biol 10:1–15. https://doi.org/10.1186/1471-2229-10-162
Von Arx G, Crivellaro A, Prendin AL et al (2016) Quantitative wood anatomy—practical guidelines. Front Plant Sci 7:1–13. https://doi.org/10.3389/fpls.2016.00781
Washer J, Reilly KJ, Barnett JR (1977) Differentiation in Pinus radiata callus culture: the effect of nutrients. New Zeal J For Sci 7:321–328
Watanabe Y, Schneider R, Barkwill S et al (2018) Cellulose synthase complexes display distinct dynamic behaviors during xylem transdifferentiation. Proc Natl Acad Sci U S A 115:E6366–E6374. https://doi.org/10.1073/pnas.1802113115
Wedzony M, Szechynska-Hebda M, Zur I et al (2014) Tissue culture and regeneration: a prerequisite for alien gene transfer. In: Pratap A, Kumar J (eds) Alien gene transfer in crop plants, vol 1. Springer, New York, NY, pp 43–75. https://doi.org/10.1007/978-1-4614-8585-8_3
Woodward AW, Bartel B (2018) Biology in bloom: a primer on the Arabidopsis thaliana model system. Genetics 208:1337–1349. https://doi.org/10.1534/genetics.118.300755
Xu H, Giannetti A, Sugiyama Y et al (2022) Secondary cell wall patterning-connecting the dots, pits and helices. Open Biol 12. https://doi.org/10.1098/rsob.210208
Yamagishi Y, Sato T, Uchiyama H et al (2012) Tracheary elements that resemble secondary xylem in calli derived from the conifers, Torreya nucifera and Cryptomeria japonica. J Wood Sci 58:557–562. https://doi.org/10.1007/s10086-012-1288-0
Yamagishi Y, Yoshimoto J, Uchiyama H et al (2013) in vitro induction of secondary xylem-like tracheary elements in calli of hybrid poplar (Populus sieboldii × P. grandidentata). Planta 237:1179–1185. https://doi.org/10.1007/s00425-013-1839-7
Yamagishi Y, Uchiyama H, Sato T et al (2015) in vitro induction of the formation of tracheary elements from suspension-cultured cells of the conifer Cryptomeria japonica. Trees - Struct Funct 29:1283–1289. https://doi.org/10.1007/s00468-014-1139-2
Yamagishi Y, Yoshimoto J, Ide S et al (2017) Partial desiccation enhances induction of secondary xylem-like tracheary elements from calli of hybrid poplar (Populus sieboldii x P. grandidentata). Trees - Struct Funct 31:1083–1089. https://doi.org/10.1007/s00468-016-1411-8
Yamagishi Y, Kudo K, Yoshimoto J et al (2021) Tracheary elements from calli of japanese horse chestnut (Aesculus turbinata) form perforation-like structures. Planta 253:1–9. https://doi.org/10.1007/s00425-021-03621-4
Yamaguchi M, Kubo M, Fukuda H, Demura T (2008) Vascular-related NAC-DOMAIN7 is involved in the differentiation of all types of xylem vessels in Arabidopsis roots and shoots. Plant J 55:652–664. https://doi.org/10.1111/j.1365-313X.2008.03533.x
Yamaguchi M, Goué N, Igarashi H et al (2010) VASCULAR-RELATED NAC-DOMAIN6 and VASCULAR-RELATED NAC-DOMAIN7 effectively induce transdifferentiation into xylem vessel elements under control of an induction system. Plant Physiol 153:906–914. https://doi.org/10.1104/pp.110.154013
Yamamoto R, Fujioka S, Demura T et al (2001) Brassinosteroid levels increase drastically prior to morphogenesis of tracheary elements. Plant Physiol 125:556–563. https://doi.org/10.1104/pp.125.2.556
Yang Y, Yoo CG, Guo H-B et al (2017) Overexpression of a domain of unknown function 266-containing protein results in high cellulose content, reduced recalcitrance, and enhanced plant growth in the bioenergy crop Populus. Biotechnol Biofuels 10:74. https://doi.org/10.1186/s13068-017-0760-x
Yaseen M, Ahmad T, Sablok G et al (2013) Review: role of carbon sources for in vitro plant growth and development. Mol Biol Rep 40:2837–2849. https://doi.org/10.1007/s11033-012-2299-z
Ye ZH, Varner JE (1996) Induction of cysteine and serine proteases during xylogenesis in Zinnia elegans. Plant Mol Biol 30:1233–1246. https://doi.org/10.1007/BF00019555
Zimin A, Stevens KA, Crepeau MW et al (2014) Sequencing and assembly of the 22-Gb loblolly pine genome. Genetics 196:875–890. https://doi.org/10.1534/genetics.113.159715
Zhou J, Zhong R, Ye ZH (2014) Arabidopsis NAC domain proteins, VND1 to VND5, are transcriptional regulators of secondary wall biosynthesis in vessels. PLoS ONE 9. https://doi.org/10.1371/journal.pone.0105726
Acknowledgements
The authors thank the Hans Merensky Foundation for the funding of RK and DD during the period in which this review was written.
Funding
RK and DD were supported by funding from the Hans Merensky Foundation through the Hans Merensky Chair in Advanced Modelling of Eucalypt Wood Formation (EucXylo). PH received no support, grants, or funding during the preparation of this manuscript.
Open access funding provided by Stellenbosch University.
Author information
Authors and Affiliations
Contributions
All of the named authors contributed to the conception of this review paper. RK was responsible for literature search and initial drafting of the manuscript, and all authors have provided input to ensure scientific and grammatical soundness thereof. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Compliance with ethical standards
The authors have no conflicts of interest to declare, nor have any experiments been conducted on human or animal participants during the development of this Review paper. The authors hereby provide consent should any data or figures be required by the journal or reviewers.
Additional information
Communicated by Maria Antonietta Germanà.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Keret, R., Hills, P. & Drew, D. The evolution of in vitro tracheary element systems from annual to perennial plant species. Plant Cell Tiss Organ Cult 153, 257–271 (2023). https://doi.org/10.1007/s11240-023-02478-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-023-02478-7