Abstract
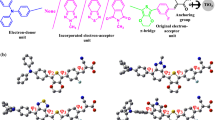
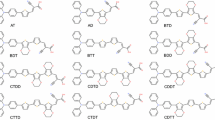
This research focuses on a theoretical study of diphenylsulfone-based emitters for thermally activated delayed fluorescence (TADF). The acceptor moiety employed in this study is sulfonyldibenzene, while the donor moiety is 9H-carbazole. The effects of methoxy, thiophene, pyridine, and pyrazine adding on the donor moiety were investigated, resulting in the design of six distinct molecules. Density functional theory (DFT) and time-dependent density functional theory (TDDFT) methods, utilizing the B3LYP functional with 6-311G(d,p) basis sets, were used to assess the properties of these compounds in their singlet, doublet, and triplet states. The calculations encompassed various aspects, including ground state molecular geometry, electronic properties, dipole moments, polarizabilities, frontier orbital energies, density of states, UV-Vis spectra, and IR spectra. The investigation revealed that adding the donor moiety with rings, particularly thiophene and pyridine, resulted in improved properties, including molecular geometry. Furthermore, increasing the D-A dihedral angle was found to increase the energy gap, optical gap, dipole moments, and polarizabilities, while reducing the ∆EST and the overlap between the HOMO and LUMO. Hole-electron analysis demonstrated that all the compounds exhibited localized excitation characteristics, with the electrons being photoinduced locally. These compounds exhibited strong blue emissions, with maximum wavelengths ranging from 387.462 to 458.286 nm in the singlet-triplet states. Moreover, the designed compounds exhibited coverage of the entire visible region in their UV-Vis spectra when in the doublet state. Based on these findings, these compounds show promise as TADF emitters in organic light-emitting diodes (OLEDs), providing improvements in efficiency and properties.











Similar content being viewed by others
Availability of data and material
Not applicable
Code availability
The authors confirm that the data supporting this study’s findings are available within the article.
References
Pope M, Kallmann HP, Magnante P (1963) Electroluminescence in organic crystals. The J Chem Phys 38(8):2042–2043
Im Y et al (2017) Molecular design strategy of organic thermally activated delayed fluorescence emitters. Chem Mater 29(5):1946–1963
Uoyama H et al (2012) Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 492(7428):234–238
Forrest SR (2004) The path to ubiquitous and low-cost organic electronic appliances on plastic. Nature 428(6986):911-918
Hong G et al (2021) A brief history of OLEDs—emitter development and industry milestones. Adv Mater 33(9):2005630
Li Y et al (2017) Tuning the twist angle of thermally activated delayed fluorescence molecules via a dendronization strategy: high-efficiency solution-processed non-doped OLEDs. J Mater Chem C 5(14):3480–3487
Zhang Q et al (2019) Highly efficient TADF OLEDs with low efficiency roll-off based on novel acridine–carbazole hybrid donor-substituted pyrimidine derivatives. J Mater Chem C 7(39):12248–12255
Dos Santos PL, Dias FB, Monkman AP (2016) Investigation of the mechanisms giving rise to TADF in exciplex states. The J Phys Chem C 120(32):18259–18267
Geffroy B, Le Roy P, Prat C (2006) Organic light-emitting diode (OLED) technology: materials, devices and display technologies. Polymer Int 55(6):572–582
Huang J et al (2017) Highly efficient nondoped OLEDs with negligible efficiency roll-off fabricated from aggregation-induced delayed fluorescence luminogens. Angew Chem Int Ed 56(42):12971–12976
Guo R et al (2021) Donor engineering for diphenylsulfone derivatives with both thermally activated delayed fluorescence and aggregation-induced emission properties. Dyes Pigm 184:108781
Zhang C et al (2022) Thermally activated delayed fluorescence dendrimers achieving 20% external quantum efficiency for solution-processed OLEDs. Mater Chem Front 6(22):3442–3449
Lee GH, Kim YS (2016) High-efficiency diphenylsulfone derivative-based organic light-emitting diode exhibiting thermally-activated delayed fluorescence. J Korean Phys Soc 69:398–401
JM DRKTM (2008) GaussView 5.0. 8. Gaussian Inc
Frisch M et al (1988) Gaussian 09, revision A. 02, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed;(b) C. Lee, W. Yang and RG Parr. Phys Rev B: Condens Matter Mater Phy 37:785
Al-Temimei FA, Alkhayatt AHO (2020)A DFT/TD-DFT Investigation on the efficiency of new dyes based on ethyl red dye as a dye-sensitized solar cell light-absorbing material. Optic 208:63920, 0030-4026
Hameka HF (2004) Quantum mechanics: a conceptual approach. John Wiley & Sons
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580–592
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graphics 14(1):33–38
Gangala S, Mahalingavelar P et al (2019) Energy level tuning of ‘Z’-shaped small molecular non-fullerene electron acceptors based on a dipyrrolo[2,3-b:2′,3′-e]pyrazine-2,6(1H,5H)-dione acceptor unit for organic photovoltaic applications: a joint experimental and DFT investigation on the effect of fluorination. J Chem 43:5173–5186
Ku J, Lansac Y, Jang YH (2011) Time-dependent density functional theory study on benzothiadiazole-based low-band-gap fused-ring copolymers for organic solar cell applications. The J Phys Chem C 115(43):21508–21516
Janssen RAJ, Nelson J (2013) Factors limiting device efficiency in organic photovoltaics. Adv Mater 25(13):1847–1858
Miyasaka H (2013) Control of charge transfer in donor/acceptor metal–organic frameworks. Acc Chem Res 46(2):248–257
Hehre WJ (1976) Ab initio molecular orbital theory. Acc Chem Res 9(11):399–406
Kalb WL et al (2010) Trap density of states in small-molecule organic semiconductors: a quantitative comparison of thin-film transistors with single crystals. Phys Rev B 81(15):155315
Abby C et al (2015) Cyano substituted benzothiadiazole: a novel acceptor inducing n-type behaviour in conjugated polymers. J Phys Chem C 119(30):17053
AL-Temimei FA (2022) Design high—efficiency organic dyes based on fluorescein toward dye—sensitized solar cells: a DFT/TD-DFT study. Opt Quant Elect 54, 600
Hickey AL, Rowley CN (2014) Benchmarking quantum chemical methods for the calculation of molecular dipole moments and polarizabilities. The J Phys Chem A 118(20):3678–3687
Mahmood A, Irfan A, Wang JL (2022) Molecular level understanding of the chalcogen atom effect on chalcogen-based polymers through electrostatic potential, non-covalent interactions, excited state behaviour, and radial distribution function. Polym Chem 13:5993–6001
Al-Temimei FA, Alasadi LA, Alaboodi AS (2020) DFT study of new donor-π-acceptor materials based on thieno [2, 3-b] indole candidate for organic solar cells application. Mater Sci Forum 1002, 1662-9752
Murray JS, Politzer P (2011) The electrostatic potential: an overview. WIREs Comput Mol Sci 1(2):153–163
Jang JS et al (2019) Electrostatic potential dispersing pyrimidine-5-carbonitrile acceptor for high efficiency and long lifetime thermally activated delayed fluorescence organic light-emitting diodes. J Mater Chem C 7(40):12695–12703
Hirshfeld FL (1977) Bonded-atom fragments for describing molecular charge densities. Theoretica Chimica Acta 44(2):129–138
Spackman MA, Jayatilaka D (2009) Hirshfeld surface analysis. Cryst Eng Comm 11(1):19–32
Spackman MA, McKinnon JJ, Jayatilaka D (2008) Electrostatic potentials mapped on Hirshfeld surfaces provide direct insight into intermolecular interactions in crystals. Cryst Eng Comm 10(4):377–388
Cioslowski J (1991) A theory of molecules: atoms in molecules. A quantum theory. Richard F. W. Bader. Clarendon (Oxford University Press), New York, 1990. xviii, 438 pp., illus. $120. International Series of Monographs on Chemistry 22. Sci 252(5012):1566-1567
Varadwaj PR, Varadwaj A, Marques HM (2020) Does chlorine in CH3Cl behave as a genuine halogen bond donor? Crystals 10(3):146
Parr RG (1980) Density functional theory of atoms and molecules. In Horiz. Quantum Chem. Dordrecht: Springer Netherlands
AL-Temimei FA, Mhaimeed MS (2020) Electronic structure, photovoltaic and absorption properties of designed photo-efficient new organic dyes with D-π-A framework. Int J Nuclear Energy Sci Technol 14(1):61-70
Lemaur V et al (2005) Photoinduced charge generation and recombination dynamics in model donor/acceptor pairs for organic solar cell applications: a full quantum-chemical treatment. J Am Chem Soc 127(16):6077–6086
Clarke TM, Durrant JR (2010) Charge photogeneration in organic solar cells. Chem Rev 110(11):6736–6767
AL-Temimei FA, Mraity HAA (2022) DFT/TD-DFT investigation of novel D–π–A configuration dyes for improving solar cell efficiency. Struct Chem 33, 859–869
Dias FB, Penfold TJ, Monkman, AP (2017) Photophysics of thermally activated delayed fluorescence molecules. Methods Appl Fluoresc 5(1):012001
Lee J et al (2013) Oxadiazole- and triazole-based highly-efficient thermally activated delayed fluorescence emitters for organic light-emitting diodes. J Mater Chem C 1(30):4599–4604
Lee SY et al (2015) X-shaped benzoylbenzophenone derivatives with crossed donors and acceptors for highly efficient thermally activated delayed fluorescence. Dalton Transact 44(18):8356–8359
Mahmood A, Irfan A, Wang JL (2022) Machine learning for organic photovoltaic polymers: a minireview. Chin J Polym Sci 40:870–876
Mahmood A, Yahya S, Jin-Liang W (2023) Easy and fast prediction of green solvents for small molecule donor-based organic solar cells through machine learning. Phys Chem Chem Phys 25:10417
Acknowledgements
The authors thank the Department of Physics, College of Science, University of Kufa, for cooperation in completing the research.
Author information
Authors and Affiliations
Contributions
The presented idea was conceived by Faeq and Bahjat. Faeq was responsible for calculations, writing, and data analysis. Bahjat also contributed to conceiving the problem and analyzing the results, developed the structures and performed the computations. Faeq and Bahjat wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
AL-Temimei, F.A., Hameed, B.S. Theoretical investigation of new series diphenylsulfone derivatives suitable candidates for organic light-emitting diodes (OLEDS) applications. Struct Chem 35, 161–179 (2024). https://doi.org/10.1007/s11224-023-02202-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-023-02202-3