Abstract
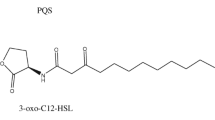
Pseudomonas aeruginosa is an opportunistic pathogen, having complicated quorum sensing (QS) system utilizing multiple signals and receptors to coordinate virulence and pathogenicity. N-acyl-homoserine-lactones (AHLs) are the most common autoinducers responsible for regulation of QS-mediated virulence gene expression. There are four QS systems in P. aeruginosa among which the LasI/R and RhlI/R systems are regulated by 3-oxo-C12-HSL and C4-HSL respectively. They play a major role in host-associated pathogenesis. The LasR and RhlR binding specificity to cognate or non-cognate HSLs influences the QS-mediated responses. Here, we used computational approaches to consolidate the interaction of different types of HSLs produced by P. aeruginosa with LasR and RhlR receptors. To explore the binding affinity, fourteen different AHLs were subjected for molecular docking analysis with LasR and RhlR receptors. The RhlR was modelled using MMseqs2 in ColabFold: Alpha fold 2. Further, to validate the stability and interaction mechanism, molecular dynamic simulations was performed with the top docked six HSLs for 100 ns. In docking results, apart from 3-oxo-C12-HSL and C4-HSL, other HSLs such as C16-HSL and C6-HSL showed better binding affinity towards LasR and RhlR proteins, respectively. Further validation by molecular dynamic simulations showed that 3-oxo-C10-HSL and 3-oxo-C6-HSL formed stable complex with LasR and RhlR, respectively. Our comprehensive in silico study results may provide promising targets for development of anti-QS drugs against Las/Rhl QS systems.






Similar content being viewed by others
Availability of data and material
All data generated or analysed during this study are included in this published article and its supplementary information files.
Code availability
Not applicable.
References
Gellatly SL, Hancock RE (2013) Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis 67:159–173. https://doi.org/10.1111/2049-632X.12033
Govindan Nadar R, Chackaravarthy G, Ramachandran G et al (2021) Isolation and molecular identification of biofilm producing P. aeruginosa and K. pneumoniae from urinary tract infections patient urine sample. J Infect Public Health 14:1875–1880. https://doi.org/10.1016/j.jiph.2021.11.004
Horner C, Mushtaq S, Livermore DM, Committee BRSS (2019) Potentiation of imipenem by relebactam for Pseudomonas aeruginosa from bacteraemia and respiratory infections. J Antimicrob Chemother 74:1940–1944. https://doi.org/10.1093/jac/dkz133
Fazeli H, Akbari R, Moghim S et al (2012) Pseudomonas aeruginosa infections in patients, hospital means, and personnel’s specimens. J Res Med Sci 17:332–337
Cobb LM, Mychaleckyj JC, Wozniak DJ, Lopez-Boado YS (2004) Pseudomonas aeruginosa flagellin and alginate elicit very distinct gene expression patterns in airway epithelial cells: implications for cystic fibrosis disease. J Immunol 173:5659–5670. https://doi.org/10.4049/jimmunol.173.9.5659
Werthen M, Davoudi M, Sonesson A et al (2004) Pseudomonas aeruginosa-induced infection and degradation of human wound fluid and skin proteins ex vivo are eradicated by a synthetic cationic polymer. J Antimicrob Chemother 54:772–779. https://doi.org/10.1093/jac/dkh407
Rutherford ST, Bassler BL (2012) Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med 2:a012427. https://doi.org/10.1101/cshperspect.a012427
Miller MB, Bassler BL (2001) Quorum sensing in bacteria. Annu Rev Microbiol 55:165–199. https://doi.org/10.1146/annurev.micro.55.1.165
Li H, Li X, Song C et al (2017) Autoinducer-2 facilitates Pseudomonas aeruginosa PAO1 pathogenicity invitro and invivo. Front Microbiol 8:1944. https://doi.org/10.3389/fmicb.2017.01944
Lee J, Zhang L (2015) The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 6:26–41. https://doi.org/10.1007/s13238-014-0100-x
LaSarre B, Federle MJ (2013) Exploiting quorum sensing to confuse bacterial pathogens. Microbiol Mol Biol Rev 77:73–111. https://doi.org/10.1128/MMBR.00046-12
Smith RS, Harris SG, Phipps R, Iglewski B (2002) The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl) homoserine lactone contributes to virulence and induces inflammation in vivo. J Bacteriol 184:4. https://doi.org/10.1128/jb.184.4.1132-1139.2002
Papenfort K, Bassler BL (2016) Quorum sensing signal-response systems in Gram-negative bacteria. Nat Rev Microbiol 14:576–588. https://doi.org/10.1038/nrmicro.2016.89
Williams P, Camara M (2009) Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: a tale of regulatory networks and multifunctional signal molecules. Curr Opin Microbiol 12:182–191. https://doi.org/10.1016/j.mib.2009.01.005
Churchill ME, Sibhatu HM, Uhlson CL (2011) Defining the structure and function of acyl-homoserine lactone autoinducers. Methods Mol Biol 692:159–171. https://doi.org/10.1007/978-1-60761-971-0_12
Charlton TS, de Nys R, Netting A et al (2000) A novel and sensitive method for the quantification of N-3-oxoacyl homoserine lactones using gas chromatography-mass spectrometry: application to a model bacterial biofilm. Environ Microbiol 2:530–541. https://doi.org/10.1046/j.1462-2920.2000.00136.x
Moradali MF, Ghods S, Rehm BH (2017) Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol 7:39. https://doi.org/10.3389/fcimb.2017.00039
Passador L, Cook JM, Gambello MJ, Rust L, Iglewski BH (1993) Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 260:1127–1130. https://doi.org/10.1126/science.8493556
Steindler L, Bertani I, De Sordi L et al (2009) LasI/R and RhlI/R quorum sensing in a strain of Pseudomonas aeruginosa beneficial to plants. Appl Environ Microbiol 75:5131–5140. https://doi.org/10.1128/AEM.02914-08
Pearson JP, Pesci EC, Iglewski BH (1997) Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol 179:5756–5767. https://doi.org/10.1128/jb.179.18.5756-5767.1997
Cooley MA, Whittall C, Rolph MS (2010) Pseudomonas signal molecule 3-oxo-C12-homoserine lactone interferes with binding of rosiglitazone to human PPARgamma. Microbes Infect 12:231–237. https://doi.org/10.1016/j.micinf.2009.12.009
Davis BM, Jensen R, Williams P, O’Shea P (2010) The interaction of N-acylhomoserine lactone quorum sensing signaling molecules with biological membranes: implications for inter-kingdom signaling. PLoS ONE 5:e13522. https://doi.org/10.1371/journal.pone.0013522
Ortori CA, Dubern JF, Chhabra SR et al (2011) Simultaneous quantitative profiling of N-acyl-L-homoserine lactone and 2-alkyl-4(1H)-quinolone families of quorum-sensing signaling molecules using LC-MS/MS. Anal Bioanal Chem 399:839–850. https://doi.org/10.1007/s00216-010-4341-0
Kumari A, Pasini P, Daunert S (2008) Detection of bacterial quorum sensing N-acyl homoserine lactones in clinical samples. Anal Bioanal Chem 391:1619–1627. https://doi.org/10.1007/s00216-008-2002-3
Middleton B, Rodgers HC, Camara M et al (2002) Direct detection of N-acylhomoserine lactones in cystic fibrosis sputum. FEMS Microbiol Lett 207:1–7. https://doi.org/10.1111/j.1574-6968.2002.tb11019.x
Shukla A, Shukla G, Parmar P et al (2021) Exemplifying the next generation of antibiotic susceptibility intensifiers of phytochemicals by LasR-mediated quorum sensing inhibition. Sci Rep 11:22421. https://doi.org/10.1038/s41598-021-01845-8
Mohanvel SK, Ravichandran V, Kamalanathan C et al (2019) Molecular docking and biological evaluation of novel urea-tailed mannich base against Pseudomonas aeruginosa. Microb Pathog 130:104–111. https://doi.org/10.1016/j.micpath.2019.02.037
Bottomley MJ, Muraglia E, Bazzo R, Carfì A (2007) Molecular insights into quorum sensing in the human pathogen Pseudomonas aeruginosa from the structure of the virulence regulator LasR bound to its autoinducer. J Biol Chem 282:13592–13600. https://doi.org/10.1074/jbc.M700556200
Zou Y, Nair SK (2009) Molecular basis for the recognition of structurally distinct autoinducer mimics by the Pseudomonas aeruginosa LasR quorum-sensing signaling receptor. Chem Biol 16:961–970. https://doi.org/10.1016/j.chembiol.2009.09.001
Jumper J, Evans R, Pritzel A et al (2021) Highly accurate protein structure prediction with AlphaFold. Nature 596:583–589. https://doi.org/10.1038/s41586-021-03819-2
Sadikot RT, Blackwell TS, Christman JW, Prince AS (2005) Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med 171:1209–1223. https://doi.org/10.1164/rccm.200408-1044SO
Abd El-Aziz NK, Abd El-Hamid MI, El-Naenaeey EY (2018) A complex hierarchical quorum-sensing circuitry modulates phenazine gene expression in Pseudomonas aeruginosa. J Infect Dev Ctries 11:919–925. https://doi.org/10.3855/jidc.8775
Luo J, Dong B, Wang K et al (2017) Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PLoS ONE 12:e0176883. https://doi.org/10.1371/journal.pone.0176883
Feltner JB, Wolter DJ, Pope CE et al (2016) LasR variant cystic fibrosis isolates reveal an adaptable quorum-sensing hierarchy in Pseudomonas aeruginosa. mBio 7:5. https://doi.org/10.1128/mBio.01513-16
McCready AR, Paczkowski JE, Cong JP, Bassler BL (2019) An autoinducer-independent RhlR quorum-sensing receptor enables analysis of RhlR regulation. PLoS Pathog 15:e1007820. https://doi.org/10.1371/journal.ppat.1007820
Parsek MR, Greenberg EP (2000) Acyl-homoserine lactone quorum sensing in gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc Natl Acad Sci U S A 97:8789–8793. https://doi.org/10.1073/pnas.97.16.8789
Hartmann A, Klink S, Rothballer M (2021) Importance of N-acyl-homoserine lactone-based quorum sensing and quorum quenching in pathogen control and plant growth promotion. Pathogens 10:1561. https://doi.org/10.3390/pathogens10121561
Acknowledgements
We thank PSGCP for Schrodinger support.
Author information
Authors and Affiliations
Contributions
Punchappady Devasya Rekha, Jaikanth Chandrasekaran and Devasahayam Arokia Balaya Rex have equally contributed in designing and executing the study. Jaikanth Chandrasekaran, Devasahayam Arokia Balaya Rex and Kanekar Saptami analysed the results and prepared the manuscript. Jaikanth Chandrasekaran and Devasahayam Arokia Balaya Rex performed the in silico studies, homology modelling and data analysis and interpretation. Jaikanth Chandrasekaran and Punchappady Devasya Rekha framed the study, prepared and evaluated the manuscript. All the authors have read and approved the manuscript for submission.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rex, D.A.B., Saptami, K., Chandrasekaran, J. et al. Pleotropic potential of quorum sensing mediated N-acyl homoserine lactones (AHLs) at the LasR and RhlR receptors of Pseudomonas aeruginosa. Struct Chem 34, 1327–1339 (2023). https://doi.org/10.1007/s11224-022-02115-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-022-02115-7