Abstract
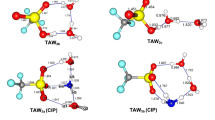
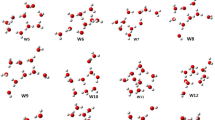
Chemical clusters with composition (H2nOn), (H2n+1On)+, and (H2n−1On)− with n = 4 are crystallographically known. Moreover, for each of those classes, there are documented examples in more than one stereochemical conformation. In this study, we consider the relation between their stereochemical characteristics in the solid and in their free form as fluids, by calculating their energies as crystallographically reported, as well as by energy optimization. Also, since accurate crystallographic studies provided these species in more than one geometrical isomer, quantum mechanical comparisons of their energies in crystalline and free molecular form are herein reported. Finally, for those in which energy minimization(s) resulted in stereochemical rearrangements, the new stereoisomer resulting is herein described. Quantum calculations show the molecular energy increases from deprotonated, to neutral, to protonated fragments. Energy optimization was carried out for each of the crystal structures studied, which allowed us to determine the structural relationship between the crystalline and free form of these fragments. Among these aquo-fragments, the most frequent structures were quadrangular and triangular motifs with either four or three hydrogen bonds. The quantum chemical calculated energy of all crystal and optimized structures are reported.








Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
CSD = The Cambridge Structural Database, C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta Cryst. (2016). B72, 171–179.
Bernal I (2006) C R Chimie 9:1454
Bernal I (2007) C R Chimie 10:1209
Bernal I, Watkins SF (2014) Acta Cryst C70:566
Gaussian 09: Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G. A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H. P., Izmaylov, A. F., Bloino, J., Zheng, G., Sonnenberg, J. L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery Jr, J. A., Peralta, J. E., Ogliaro, F., Bearpark, M., Heyd, J. J., Brothers, E., Kudin, K. N., Staroverov, V. N., Keith, T., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J. C., Iyengar, S. S., Tomasi, J., Cossi, M., Rega, N., Millam, J. M., Klene, M., Knox, J. E., Cross, J. B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R. E., Yazyev, O., Austin, A. J. , Cammi, R., Pomelli, C., Ochterski, J. W., Martin, R. L., Morokuma, K., Zakrzewski, V. G., Voth, G. A., Salvador, P., Dannenberg, J. J., Dapprich, S., Daniels, A. D., Farkas, O., Foresman, J. B., Ortiz, J. V., Cioslowski, J., and Fox, D. J.; Gaussian Inc.: Wallingford CT, 2010.
Density functional theory (1989) Parr RG. Yang W. Density-functional theory of atoms and molecules. Oxford University Press, Oxford
Koch W, Holthausen MC (2001) A Chemist’s Guide to Density Functional Theory, 2nd edn. Wiley-VCH, New York
GaussView: Dennington R, Keith TA, Millam JM (2009) Gauss View, Version 5. Semi chem Inc., Shawnee Mission, Kansas
MATLEY = Wiebcke M, Felsche J (2000) Acta Crystallogr., Sect. C: Cryst Struct Commun 56, 901. Sp. Gr. = Pnma, z = 4.0, R = 4.73, T = 153 K
VORYEG = Burshtein IF, Gerbeleu NV, Bologa OA, Lozan VI, Malinovskii TI, Dokl. Akad. Nauk SSSR (Russ.) Proc Nat Acad Sci USSR, 316, 368 (1991). Sp. Gr. = R(-3), z = 3.0, R = 6.30, T = 283–303 K
AQUNAB = Michels JJ, O'Connell MJ, Taylor PN, Wilson JS, Cacialli F, Anderson HL (2003) Chem -Eur J 9, 6167. Sp. Gr. = P(-1), z = 2.0, R = 1.82, T = 150 K
FMPECW = Greenhough TJ, Ladd MFC (1978) Acta Crystallogr., Sect. B: Struct Crystallogr Cryst Chem 34, 2619 Sp. Gr. = P21/c, z = 4.0, R = 3.40, T = 296 K
IMCDCP01 = Jian F, Zhao P, Wang S, Zhang S (2002) J Chem Cryst 32, 395 . Sp. Gr. = P63/m, z = 2.0, R = 2.85, T = 293 K
JEDYAT = Matczak-Jon E, Slepokura K, Kafarski P (2006) J Mol Struct 782, 81–93. Sp. Gr. = P21, z = 2.0, R = 4.02, T = 100 K
NUDCEV = Constable EC, Zhang G, Housecroft CE, Neuburger M, Schaffner S (2009) Cryst Eng Comm 11, 1014 Sp. Gr. = P21/n, z = 4.0, R = 2.89, T = 223 K
ASUHIF = Rafizadeh M, Ranjbar M, Amani V (2004) Acta Crystallogr., Sect. E: Struct Rep Online 60, m479
Allen FH (2002) MERCURY is the computational and graphics routine accompanying CSD (see above, reference [1]). Acta Cryst B58:380–388
Wallace S, Huang L, Massa L, Mukhopadhyay U, Bernal I, Karle J (2007) The structures of cyclic dihydronium cations. Proc Natl Acad Sci 104(43):16798–16803. https://doi.org/10.1073/pnas.0708249104
Huang L, Matta CF, Wallace S, Massa L, Bernal I (2015) A unique trapping by crystal forces of a hydronium cation displaying a transition state structure. C R Chim 18:511–515
Quantifying hydrogen bond cooperativity in water: VRT spectroscopy of the water tetramer. Science. 271, 59–61
Cruzan JD, Brown MG, Liu K, Braly LB, Saykally RJ (1996) The far-infrared vibration–rotation–tunneling spectrum of the water tetramer-d8. J Chem Phys 105:6634–6644
Keutsch FN, Saykally RJ (2001) Water clusters: untangling the mysteries of the liquid, one molecule at a time. Proc Natl Acad Sci USA 98 10533–10540
Liu K, Cruzan J, Saykally R (1996) Water Clusters. Science 271:929–933
Xantheas SS, Dunning TH Jr (1993) Ab initio studies of cyclic water clusters (H2O)n , n=1–6. I. Optimal structures and vibrational spectra. J Chem Phys 99, 8774
Miliordos E, Aprà E, Xantheas SS (2013) Optimal geometries and harmonic vibrational frequencies of the global minima of water clusters (H2O)n, n = 2–6, and several hexamer local minima at the CCSD(T) level of theory. J Chem Phys 139(114302–1):114302–114313
Xantheas SS, Burnham CJ, Harrison RJ (2002) Harrison, Development of transferable interaction models for water. II. Accurate energetics of the first few water clusters from first principles. J Chem Phys 116, 1493–1499
Howard JC, Tschumper GS (2014) Wavefunction methods for the accurate characterization of water clusters. Comput Mol Sci https://doi.org/10.1002/wcms.1168
Bates DM, Smith JR, Tschumper GS (2011) Efficient and accurate methods for the geometry optimization of water clusters: application of analytic gradients for the two-body:many-body QM:QM fragmentation method to (H2O)n, n = 3–10. J Chem Theory Comput 7:2753–2760
Howard JC, Tschumper GS (2015) Benchmark structures and harmonic vibrational frequencies near the CCSD(T) complete basis set limit for small water clusters: (H2O)n = 2, 3, 4, 5, 6, Chem Theory Comput 11, 2126−2136
Author information
Authors and Affiliations
Contributions
All authors, HA, IB, LM, contributed equally to all sections of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ajiki, H., Bernal, I. & Massa, L. Acid-based analogs of certain water tetramers: an examination of some crystal structures in the literature. Struct Chem 33, 1177–1188 (2022). https://doi.org/10.1007/s11224-022-01924-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-022-01924-0