Abstract
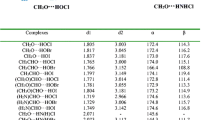
Noncovalent interactions involving halogen bonding interactions, one of the emerging interactions due to its directionality, have been a subject of interest for various researchers, owing to its role played in construction of supramolecular structures and crystal engineering. In this article, the RCHO⋯X–Y {X = Cl, Br, I; Y = –CF3, –CF2H, –CFH2, –CN, –CCH, –CCCN; R = –OH, –OCH3, –NH2, –O−} halogen-bonded complexes have been investigated with aid of quantum chemical calculations at MP2/aug-cc-pVDZ(-PP) level. The geometrical, spectroscopic and energetic properties have been analyzed for these complexes with QTAIM, MEP and NBO analyses. For same RCHO molecule, the interaction energies increase in the order of O⋯Cl < O⋯Br < O⋯I and for the same X–Y; the trend is (HO)CHO < (CH3O)CHO < (H2N)CHO. The present article also compares the neutral complexes involving (HO)CHO, (CH3O)CHO and (H2N)CHO carbonyl molecules with those of charged carbonyl species HCOO−. It has been found that strength of the latter complexes is highest among all of the four. The role of substituents in the complex formation has been analyzed on the basis of results obtained from MEP, QTAIM and NBO studies.









Similar content being viewed by others
References
Scheiner S (1997) Hydrogen bonding: a theoretical perspective. Oxford University Press, New York
Feng Y, Rainteau D, Chachaty C, Yu ZW, Wolf C, Quinn PJ (2004) Biophys J 86:2208–2217
Muller-Dethlefs K, Hobza P (2000) Chem Rev 100:143–168
Wu FG, Wang NN, Yu ZW (2009) Langmuir 25:13394–13401
Metrangolo P, Pilati R, Resnati G (2006) CrystEngComm 8:946–947
Metrangolo P, Resnati G (2008) Halogen bonding: fundamentals and applications, structure and bonding. Springer, Berlin
Legon AC (2010) Phys Chem Chem Phys 12:7736–7747
Metrangolo P, Resnati G (2012) Cryst Growth Des 12:5835–5838
Lu Y, Wang Y, Zhu W (2010) Phys Chem Chem Phys 12:4543–4551
Lu Y, Shi T, Wang Y, Yang H, Yan X, Luo X, Jiang H, Zhu W (2009) J Med Chem 52:2854–2862
Parisini E, Metrangolo P, Pilati T, Resnati G, Terraneo G (2011) Chem Soc Rev 40:2267–2278
Jentzsch AV, Matile S (2013) J Am Chem Soc 135:5302–5303
Metrangolo P, Resnati G (2001) Chem Eur J 7:2511–2519
Metrangolo P, Meyer F, Pilati T, Resnati G, Terraneo G (2008) Angew Chem Int Ed 47:6114–6127
Voth AR, Hays FA, Ho PS (2007) Proc Natl Acad Sci USA 104:6188–6193
Minguez Espallargas G, Zordan F, Arroyo Marin L, Adams H, Shankland K, van de Streek J, Brammer L (2009) Chem Eur J 15:7554–7568
Vidal F, Dávila MA, Torcuato AS, Gómez-Sal P, Mosquera ME (2013) Dalton Trans 42:7074–7084
Brinck T, Murray JS, Politzer P (1992) Int J Quantum Chem 44:57–64
Clark T, Hennemann M, Murray JS, Politzer P (2007) J Mol Model 13:291–296
Politzer P, Lane P, Concha MC, Ma Y, Murray JS (2007) J Mol Model 13:305–311
Murray JS, Lane P, Clark T, Politzer P (2007) J Mol Model 13:1033–1038
Politzer P, Murray JS, Concha MC (2008) J Mol Model 14:659–665
Murray JS, Concha MC, Lane P, Hobza P, Politzer P (2008) J Mol Model 14:699–704
Murray JS, Lane P, Politzer P (2009) J Mol Model 15:723–729
Riley KE, Murray JS, Fanfrlík J, Řezáč J, Solá RJ, Concha MC, Ramos FM, Politzer P (2011) J Mol Model 17:3309–3318
Riley KE, Murray JS, Fanfrlík J, Řezáč J, Solá RJ, Concha MC, Ramos FM, Politzer P (2013) J Mol Model 19:4651–4659
Han N, Zeng Y, Sun C, Li X, Sun Z, Meng L (2014) J Phys Chem A 118:7058–7065
Han N, Zeng Y, Li X, Zheng S, Meng L (2013) J Phys Chem A 117:12959–12968
Riley KE, Merz KM (2007) J Phys Chem A 111:1688–1694
Lu Y-X, Zou J-W, Wang Y-H, Jiang Y-J, Yu Q-S (2007) J Phys Chem A 111:10781–10788
Riley KE, Hobza P (2008) J Chem Theory Comput 4:232–242
Riley KE, Murray JS, Politzer P, Concha MC, Hobza P (2009) J Chem Theory Comput 5:155–163
Bauzá A, Quińonero D, Frontera A, Deyá PM (2011) Phys Chem Chem Phys 13:20371–20379
Palusiak M (2010) J Mol Struct (Theochem) 945:89–92
Politzer P, Murray JS, Clark T (2010) Phys Chem Chem Phys 12:7748–7757
Zierkiewicz W, Wieczorek R, Hobza P, Michalska D (2011) Phys Chem Chem Phys 13:5105–5113
Li Q, Xu X, Liu T, Jing B, Li W, Cheng J, Gong B, Sun J (2010) Phys Chem Chem Phys 12:6837–6843
Grabowski SJ, Bilewicz E (2006) Chem Phys Lett 427:51–55
Chen Y (2013) J Phys Chem A 117(33):8081–8090
Solimannejad M, Malekani M (2013) J Phys Chem A 117:5551–5557
Syzgantseva OA, Tognetti V, Joubert L (2013) J Phys Chem A 117:8969–8980
Møller C, Plesset MS (1934) Phys Rev 46:618–622
Dunning TH (1989) J Chem Phys 90:1007–1023
Wood DE, Dunning TH (1995) J Chem Phys 103:4572–4585
Peterson KA, Figgen D, Goll E, Stoll H, Dolg M (2003) J Chem Phys 119:11113–11123
Boys SF, Bernadi F (1970) Mol Phys 19:553–566
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, KItao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendel A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo C, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian, Inc., Wallingford CT
Bulat FA, Toro-Labbe A, Brinck T, Murray JS, Politzer P (2010) J Mol Model 16:1679–1691
Politzer P, Truhlar DG (1981) Chemical applications of atomic and molecular electrostatic potentials. Plenum, New York
Keith TA (2012) AIMALL, Version 12.06.03. AIMALL, Overland Park
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899–926
Naray-Szabo G, Ferenczy GG (1995) Chem Rev 95(4):829–847
Murray JS, Politzer P (1998) J Mol Struct (Theochem) 425:107–114
Politzer P, Murray JS, Concha MC (2002) Int J Quantum Chem 88:19–27
Hathwar VR, Gonnade RG, Munshi P, Bhadbhade MM, Row TNG (2011) Cryst Growth Des 11:1855–1862
Glaser R, Chen N, Wu H, Knotts N, Kaupp M (2004) J Am Chem Soc 126:4412–4419
Hauchecorne D, Herrebout WA (2013) J Phys Chem A 117:11548–11557
Kaur D, Kaur R (2014) J Chem Sci 126(6):1763–1779
Politzer P, Murray JS, Lane P (2007) Int J Quantum Chem 107:3046–3052
Politzer P, Murray JS, Concha MC (2007) J Mol Model 13:643–650
Voth AR, Khuu P, Oishi K, Ho PS (2009) Nat Chem 1:74–79
Aakeröy CB, Schultheiss NC, Rajbanshi A, Desper J, Moore C (2009) Cryst Growth Des 9(1):432–441
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, Oxford
Popelier P (2000) Atoms in molecules: an introduction. UMIST, Manchester
Amezaga NJ, Pamies SC, Peruchena NM, Sosa GL (2010) J Phys Chem A 114:552–562
Duarte DJ, Sosa GL, Peruchena NM (2013) J Mol Model 19:2035–2041
Bone RGA, Bader RFW (1996) J Phys Chem 100:10892–10911
Cremar D, Kraka E (1984) Angew Chem Int Ed 23:627–628
Bent HA (1961) Chem Rev 61:275–311
Acknowledgments
We are thankful to DST (INSPIRE Fellowship Programme) for their financial assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaur, D., Kaur, R. & Shiekh, B.A. Effects of substituents and charge on the RCHO⋯X–Y {X = Cl, Br, I; Y = –CF3, –CF2H, –CFH2, –CN, –CCH, –CCCN; R = –OH, –OCH3, –NH2, –O−} halogen-bonded complexes. Struct Chem 27, 961–971 (2016). https://doi.org/10.1007/s11224-015-0680-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-015-0680-y