Abstract
Energy and environmental issues related to conventional fossil-derived products and fuels have led researchers to focus on alternative, more environmentally-friendly processes, such as the production of microbial oils from renewable feedstocks or even pollutants as sustainable sources of biofuels, allowing to progressively move away from the use of fossil fuels. Among the oleaginous yeasts, Yarrowia lipolytica is a highly promising cell factory and microbial oil producer because of its high capacity to accumulate lipids for subsequent biofuel production. Y. lipolytica also stands out for its ability to assimilate various carbon sources, even at low cost, reaching lipid concentrations of at least 30% by weight with non-genetically modified strains, and even much higher values with engineered organisms. Among others, fatty acids have attracted recent interest as substrates for their lower cost and possible production from pollutants compared to sugars. This review pays special attention to some of those emerging carbon sources, i.e., carboxylic acids and even greenhouse gases. Besides, another focus is to provide detailed up to date information on the main characteristics and factors that most influence the fermentation process of this yeast, with the ultimate aim of optimising the bioconversion process and the synthesis of useful metabolites. Besides, the reader will find comprehensive information on the industrial applicability of the synthesised lipids, in addition to the production of biofuels. Apart from lipids, other metabolites of interest that can be synthesised by Y. lipolytica are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The relationship between environmental quality, human health and global warming is currently a highly topical issue. Numerous studies have focused on this issue, with the combustion of fossil fuels being a major contributor to environmental pollution. It is, in anthropological terms, the main source of CO2 emissions to the atmosphere, involved in climate change. Besides, global energy consumption has been increasing considerably over the years, and the availability of fossil fuels may come to an end soon (Höök and Tang 2013). These are some of the reasons why much attention is being paid to the production of more environmentally-friendly alternative fuels, in a sustainable way and from renewable or even polluting sources (Fernández-Naveira et al. 2017). In that respect, there is an increasing interest in developing fermentation processes to obtain more sustainable products, based on a circular economy approach and on the valorisation of pollutants and renewable sources as raw materials, gradually leaving behind the use of fossil, non-renewable and non-sustainable, raw materials.
Biofuels have some excellent characteristics that make them good candidates to replace conventional fuels. Some of those can be produced through bioconversion processes catalysed by yeasts, e.g., biodiesel being the most common ones. Biodiesel, which is considered a sustainable biofuel, can be classified into three different categories, depending, generally, on the oil feedstock, namely first, second and third-generation biofuels or biodiesel, although sometimes other parameters than the feedstock are also used (e.g., the process or technology itself), though this is less common. Figure 1 shows a comparison between fossil fuels and the different types of biofuels.
Due to certain limitations of first generation biofuels which result in food/fuel competition and limitations for biodiesel derived from vegetable oils (e.g., palm oil), the use of microorganisms such as fungi, bacteria or yeasts grown on second generation feedstocks or on solid and liquid pollutants, as well as gas emissions, may become one of the best options (Robles-Iglesias et al. 2021; Rene et al. 2022; Kennes 2023). If the second-generation feedstock is lignocellulosic biomass, there may still be some indirect competition for land that could also be needed for food/feed production. Such competition disappears if the feedstock is a pollutant, e.g., waste, wastewater or even greenhouse gases such as CO2, which are seen as innovative, emerging, alternatives.
Related to the above comments, it should be noted that microorganisms capable of accumulating lipids at concentrations exceeding 20% of their DCW are known as oleaginous species, and those lipids are suitable for the production of biofuels and other bioproducts. Among the lipid-accumulating oleaginous strains, yeasts stand out as having several advantages over other organisms. For example, they have the ability to synthesise lipids from a variety of carbon sources and they are not limited by physicochemical factors such as light or temperature as microalgae are. They are more tolerant to metal ions than moulds and lipid extraction is generally easier than with bacteria, in which lipids are tightly bound to the bacterial membrane. These and other advantages have led to an increased interest in the study of oleaginous yeasts for optimized lipid synthesis (Bao et al. 2021).
This manuscript will focus on the yeast Yarrowia lipolytica, which, in recent years, has been considered a highly suitable microorganism for biofuels (e.g., biodiesel) production due to its ability to accumulate lipids efficiently (Yook et al. 2019). It should be noted that the non-conventional yeast Y. lipolytica is considered a model organism for lipid production, with a lipid accumulation capacity commonly exceeding 30% of its dry weight (Bao et al. 2021). On the other hand, with regard to engineered Y. lipolytica strains, lipid accumulation over 90% and lipid concentrations over 25 g/L have been obtained (Blazeck et al. 2014). These and other promising characteristics of this yeast, which will be discussed in later sections, highlight its remarkable efficiency in the production of oleochemical products. The next sections provide a comprehensive overview of the main characteristics of the species Y. lipolytica, the possibility of obtaining products of great industrial interest, such as lipids, among others, besides other products, as well as key factors affecting the fermentation process.
2 History, taxonomy and most relevant morphological characteristics
The dimorphic oleaginous yeast Y. lipolytica belongs to the phylum Ascomycota, subdivision Saccharomycotina, and has both good lipolytic and proteolytic activity. The common name Yarrowia was introduced in 1980 by Van der Walt and Von Arx in gratitude to David Yarrow for the recognition of a new genus. On the other hand, the name "lipolytica" was established because of its ability to hydrolyse lipids (Nicaud 2012; Madzak 2015).
Y. lipolytica can be found naturally in soils, seawater, and foodstuffs (mostly in dairy products such as cheese or meat), among others (Madzak 2015). As mentioned above, this yeast has gained significant interest in the industry for the production of certain metabolites and also for the development of heterologous proteins. These and other aspects, e.g., bioremediation of contaminated soils and water (Bankar et al. 2009), together with the peculiar characteristics of this type of yeast, make it a mainstay of research.
Specifically, in 1969, interest in Y. lipolytica emerged for its cultivation in fermenters due to its ability to use hydrophobic substrates (e.g., alkanes) to obtain single-cell proteins and some other valuable intermediate metabolites, e.g., citric acid. Later, in the 1990s, concomitant with the development of genetic tools, the use of this yeast to produce heterologous proteins also attracted some interest, due to the dimorphism of Y. lipolytica (ability to grow as a yeast or as a hyphae); until the 2000s, when its genome was sequenced (Fickers et al. 2005).
Regarding the dual morphology of Y. lipolytica, it can be stated that wild-type strains show different colony morphologies depending on growth conditions and genetics. Besides, one can find very wiry and dull morphology types or, otherwise, smooth and shiny ones (Nicaud 2012). This peculiarity was reported in 2002 (Ruiz-Herrera and Sentandreu 2002), obtaining results showing that this dimorphism was specifically affected by stimuli such as starvation and heat shock; in addition to the pH of the medium, the nature of the nitrogen and carbon sources, anaerobic stress, as well as non-metabolised substances. Furthermore, the importance of taking into account the type of medium, whether solid or liquid, as well as the type of strain was also highlighted, as it also greatly affects the dimorphism of Y. lipolytica. An example is given in Fig. 2, illustrating the morphology of Y. lipolytica strain W29 cells grown on 20 g/L glucose and 5 g/L yeast extract, with agitation (150 rpm) at a temperature of 33 °C, at two different pH values, showing oval forms at pH 3 and additionally some more elongated mycelial-like forms at pH 7.
Thus, Y. lipolytica is considered a convenient template for dimorphism studies, as it has the ability to grow in a more oval or pseudo-hyphal or mycelial forms. Furthermore, among the factors discussed above, the pH of the medium seems to have a strong influence on morphology. Besides, the transition from oval cells to filamentous morphotype is a necessary requirement for yeast growth on hydrophobic substrates (Lopes et al. 2022).
3 Substrates
Y. lipolytica is characterised by its ability to grow in simpler and less nutrient-dense cultures, to be genetically manipulated in a very simple way, and has a high stability compared to e.g. moulds (Cavallo et al. 2020). Y. lipolytica is able to consume and ferment different carbon substrates, either hydrophobic or hydrophilic, e.g., glucose, glycerol, alkanes, fatty acids, triglycerides (Fickers et al. 2005; Cavallo et al. 2017, 2020).
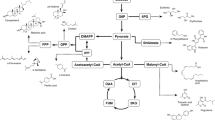
In the process of lipid accumulation in Y. lipolytica, the use of hydrophilic substrates is carried out through de novo lipid synthesis, and the use of hydrophobic substrates through ex novo synthesis. Y. lipolytica stands out for its ability to survive in hydrophobic media, accumulating lipids through this latter pathway (Patel and Matsakas 2019). The main reasons for this variety of uptake options are the existence of different multigenic families and different assimilation pathways (Bao et al. 2021; Lopes et al. 2022). Figure 3 summarises the metabolic processes involved in lipid production by Y. lipolytica using different carbon sources.
Metabolic processes involved in lipid production by Y. lipolytica. ACC acyl-CoA carboxylase, ACL ATP-citrate lyase, ACS acetyl-Coa synthase, DAG diacylglycerol, DGA1 and DGA2, DAG acyl-transferases I and II; DHAP dihydroxyacetone phosphate, ER endoplasmic reticulum, FAA1 acyl-CoA synthetase, FAS fatty acid synthase, FFA free fatty acids, G3P glycerol 3-phosphate, GPD1, NAD+ dependent G3P dehydrogenase, GUT1, glycerol kinase, GUT2, FAD+ dependent G3P dehydrogenase, LB lipid body, LPA lysophosphatidic acid, MIT mitochondria, NADPH nicotinamide adenine dinucleotide phosphate, PA phosphatidic acid, PAP PA phosphohydrolase, PER peroxisome, SCT1 G3P acyltransferase, SLC1 LPA acyltransferase, TAG triacylglycerol, TCA cycle, tricarboxylic-acid cycle, TGL4 TAG intracellular lipase, TGL3 a positive regulator of TGL4
Regarding de novo production, Y. lipolytica is able to naturally use hydrophilic substrates such as glucose, glycerol and fructose, as well as low amounts of organic acids and alcohols. The growth of this yeast has been reported to be reduced at concentrations above 150 g/L glucose. However, genetically modified strains can achieve a glucose-to-lipid conversion yield of approximately 0.23 g/g and even tolerate glucose concentrations exceeding 300 g/L. On the other hand, with respect to fructose, the growth rate of this yeast is generally lower than with glucose (Carsanba et al. 2018). Glycerol, a by-product of biodiesel manufacture, has also become a substrate of interest due to its low cost and high availability, besides yielding good results (Fontanille et al. 2012; Dobrowolski et al. 2016; Carsanba et al. 2018). Sucrose, although also suitable and valid for numerous oleaginous species for the production of microbial oils, requires the presence of the enzyme invertase for its breakdown into glucose and fructose. However, the latter enzyme is not available in native strains of Y. lipolytica. A further drawback is the high nitrogen concentrations present in molasses, from which sucrose is often obtained. Even so, genetically modified strains showed relatively good results in presence of sucrose. Also, among the hydrophilic substrates, it is worth mentioning the ability of Y. lipolytica to naturally use ethanol, thanks to the action of the enzymes alcohol dehydrogenase and aldehyde dehydrogenase; acetate (Carsanba et al. 2018) and other short-chain volatile fatty acids (VFAs) as more recent, innovative, carbon sources. The latter have attracted recent increased interest, among others, because they are generally cheaper substrates than sugars, estimated at 0.4 $/kg (Bonatsos et al. 2020) (i.e., about 0.37 €/kg) vs 0.1 $/kg (Park et al. 2021a) (i.e., about 0.09 €/kg), for glucose and acetic acid, respectively. They will therefore be discussed more deeply in the next section.
As discussed above, there is also the possibility of using various hydrophobic carbon sources for ex novo lipid accumulation (which is not limited by the depletion of nutrients from the culture medium), such as various vegetable oils, fatty by-products or fish oil residues, soap stocks, fatty esters, pure free fatty acids, n-alkanes, among others (Papanikolaou and Aggelis 2011; Bao et al. 2021). Specifically, Y. lipolytica is usually found in environments rich in hydrophobic substrates (alkanes or lipids), and is therefore able to assimilate fatty acids, TAGs and alkanes in an efficient manner (Beopoulos et al. 2009). Additionally, it is also common to use a combination of hydrophilic substrates, such as glucose, and various fatty materials for ex novo synthesis. For example, the use of vegetable oils to grow Y. lipolytica has been reported and allows the production of single-cell oils (SCOs) (Carsanba et al. 2018). Zhao et al. (2015) achieved very promising results using oleic acid as substrate, reaching lipid and biomass contents of over 30% and 6 g/L, respectively; while with glucose the results obtained were 21% and 7 g/L, respectively. Chai et al. (2019) also achieved better lipid production results by increasing the concentration of rapeseed oil and, in fact, it was found that the action of short-chain compounds on the oil substrate did not improve lipid production.
4 Carboxylic acids: novel sustainable substrates and inhibitors
The synthesis of microbial oils has usually been studied using sugars as substrates, which are expensive and involve high contamination risks (Llamas et al. 2020a). Therefore, the use of less conventional and low-cost carbon sources such as VFAs as substrates is a very promising alternative for lipid accumulation in yeasts (Gao et al. 2020), including Y. lipolytica, but also species such as Cutaneotrichosporon curvatus, Lipomyces lipofer, Rhodotorula toruloides, or Cyberlindnera saturnos (Park et al. 2021b). Interestingly, these VFAs substrates can be obtained from renewable sources and pollutants. They have been produced from feedstocks such as wastes, wastewaters, but also greenhouse gases, such as CO2, and other gases containing one carbon (C1) compounds (Tomás-Pejó et al. 2023). In that sense, the production of VFAs from wastes and wastewaters has been studied for several years, e.g., for their subsequent bioconversion into biopolymers and also a range of other valuable products, by bacteria, mixed cultures, but, more recently, even by yeasts (Lagoa-Costa et al. 2022). Possible waste or wastewater feedstocks include tuna waste (Bermúdez-Penabad et al. 2017), cheese whey (Lagoa-Costa et al. 2020), agro-industrial waste (Iglesias-Iglesias et al. 2021), brewery wastewater (Ben et al. 2016), sewage sludge (Iglesias-Iglesias et al. 2019), among others.
Concerning the use of carboxylic acids, Fontanille et al. (2012) proposed a method of lipid production through the use of VFAs metabolized by Y. lipolytica. In their research, the yeast was first cultured on glucose or glycerol and, in a second step, acetic acid was added sequentially, obtaining a lipid concentration of 12.4 g/L from an initial glucose concentration of 40 g/L. Tests were also carried out to highlight the efficiency of the process with other VFAs, such as butyric acid and propionic acid. Furthermore, Llamas et al. (2020b) also demonstrated that Y. lipolytica CECT 1240 is able to grow on VFAs as sole carbon source, consuming the substrate while efficiently producing microbial oils. Taking this into account, and considering that there are several recent studies on this topic, it can be stated that although sugar-based substrates are the most common carbon sources for lipid production, there is an increasing interest in the use of low-cost substrates such as VFAs.
Studies with Y. lipolytica strains allowed to reach about 26% lipid content when cultivated on fatty acids from food vegetable wastes (Gao et al. 2020). Though anaerobic bacterial conversion of wastes into fatty acids followed by the conversion of those acids into lipids by yeasts is an interesting approach, it should also be compared, in terms of techno-economic sustainability, to the accumulation of lipids by yeasts directly grown on such wastes. Some studies reported lipid contents of up to 61% in Y. lipolytica grown on industrial palm oil mill effluent waste (Cheirsilp and Louhasakul 2013). The suitability of other wastes for growing oleaginous yeasts, such as Y. lipolytica, has been tested and evaluated as well, e.g., sugar beet molasses (El Kantar and Koubaa 2022), among others.
Regarding the potential use of C1-gases as substrates, syngas, which is mostly composed of CO, CO2 and H2, is considered an emerging economical and adaptable substrate that can be used for the production of renewable fuels and chemicals with acetogenic bacteria. These anaerobic microorganisms are able to grow on C1 compounds and produce mainly acetate, through the Wood-Ljungdahl pathway (WLP) (Fernández-Naveira et al. 2017). In this anaerobic microbial fermentation, yielding VFAs as end products, enzymes convert different carbon sources into energy, intermediates of anabolic processes and end metabolites, such as acetic acid, and occasionally, butyric acid and even longer chain fatty acids (e.g., caproic acid) (Baumann and Westermann 2016; Fernández-Naveira et al. 2017). With this in mind, the combination of a first process of anaerobic fermentation of CO2 (together with H2), CO-rich gases or syngas, into VFAs, followed by a second aerobic fermentation using oleaginous yeasts, for the bioconversion of these acids into lipids, is a possible, novel, and appealing approach.
Considering that research focused on the synthesis of biofuels or bioproducts from VFAs derived from pollutant gases containing CO or CO2 is still very limited (Robles-Iglesias et al. 2021), noteworthy are the promising results obtained by Robles-Iglesias et al. (2021) in their research focused on the bioconversion of carbon dioxide into lipids with two reactors inoculated with Acetobacterium woodii and Rhodosporidium toruloides. In the first stage, A. woodii produced concentrations of more than 20 g/L acetic acid from CO2, in the presence of H2. This acetic acid was then used in a second stage for its bioconversion into lipids by R. toruloides. Encouraging results allowed reaching a lipid accumulation close to 20% and where C18:1 represented more than 40% of the total lipids accumulated, which indicates that it is very suitable for the production of biofuels such as biodiesel (Robles-Iglesias et al. 2021). Similarly, in another study with Clostridium aceticum and R. toruloides, better results were observed and reached total lipid accumulation around 40% with 11 g/L acetic acid coming from the fermentation of C1-gases (CO, CO2, syngas) (Robles-Iglesias et al. 2023, submitted manuscript). On the other hand, Naveira-Pazos et al. (2022) also studied lipid accumulation, in this case using the oleaginous yeast Y. lipolytica grown on a mixture of VFAs typically obtained from acetogenic syngas or carbon dioxide fermentation. This research tested the ability of Y. lipolytica to assimilate C2–C6 fatty acids produced from C1-gases, achieving lipid production rates similar as with sugars.
However, when the acid concentration exceeds a certain level, an inhibitory effect occurs, which negatively affects lipid production yields by hindering the activity of these oleaginous microorganisms. Moreover, it is not only the concentration of acids in the medium that affects, but it is also important to consider other factors such as the yeast strain, type of acid, acid chain length (toxicity is proportional to the increase in chain length), and pH, among others. In terms of pH, it is common to use mildly acidic conditions although, even so, working in acidic conditions increases the presence of VFAs in undissociated form, favouring inhibition. Besides, when working with high concentrations of VFAs, more alkaline pH values help mitigate inhibition to some degree. In addition to this substrate inhibition, product inhibition must also be considered, because throughout the yeast's metabolism of VFAs, the pH tends to become more basic, which can also cause inhibition (Gao et al. 2020; Park et al. 2021b).
For example, Naveira-Pazos et al. (2022) and Robles-Iglesias et al. (2021) used either a mixture of even-chain fatty acids typical of anaerobic C1-gas fermentations (acetic, butyric and caproic acids) or acetic acid alone, as carbon sources for the production of lipids by oleaginous yeasts. The results observed by Naveira-Pazos et al. (2022) with a native Y. lipolytica strain confirmed those previously performed by Robles-Iglesias et al. (2021) with Rhodosporidium toruloides. Inhibitory effects were estimated in both studies. Naveira-Pazos et al. (2022) determined the toxicity of acid mixtures on the growth of Y. lipolytica in batch experiments. The lag phases for initial acid concentrations below 16 g/L were short, and these substrates' concentrations started to decrease and be assimilated a few hours after inoculation.
Furthermore, regarding the type of acids, differences in consumption efficiencies between acids could also be observed in this research. Acetic acid showed the highest consumption rates, followed by butyric acid and, finally caproic acid. This demonstrates the preference of Y. lipolytica for acetic acid over potentially more toxic longer-chain acids. These results are in agreement with those obtained by Robles-Iglesias et al. (2021), who concluded, after batch studies, that at initial acetic acid concentrations below 14.2 g/L there is no inhibition, while above 16 g/L, R. toruloides started exhibiting significant inhibition as acetic acid consumption rates decreased and lag phases were extended. Furthermore, it was shown that at concentrations above 20 g/L acetic acid is lethal to the yeast, not being able to assimilate it anymore as a substrate. It is nevertheless possible to feed higher concentration of VFAs substrates (e.g., about 100 g/L) and thus accumulate higher concentrations of metabolites if this is done gradually, in fed-batch mode, thus avoiding reaching inhibitory substrate levels (unplubished data).
The pH value also plays a key role. Indeed, Gao et al. (2020) verified that the use of alkaline conditions decreased the inhibitory effect when using high concentrations of VFAs > 10 g/L. In that study, cultures of Y. lipolytica were compared using concentrations between 30 and 110 g/L acetic acid at different pH values, between 6 and 10, and it was found that the highest production of lipids occurred when using 70 g/L acetic acid and a pH of 8. The authors also concluded that the use of VFAs mixtures was even more favourable for Y. lipolytica CICC 31596 adaptation to return to growth and subsequent lipid production. Also noteworthy are the conclusions of Llamas et al. (2020b), who reported that VFAs with longer chain length provided a higher biomass yield but, on the other side, the predominance of long chains in the VFAs mixture aggravated their inhibitory effect on Y. lipolytica CECT 1240. It was also established that Y. lipolytica constitutes a potential for sustainable lipid production from real digestates of anaerobic fermentations, showing a 2.5-fold increase in biomass in comparison to synthetic mixtures. Furthermore, using real digestates, inhibition was reached at concentrations of 26.5 g/L, while synthetic media resulted in inhibition already at VFAs concentrations slightly above 10.6 g/L. Morales-Palomo et al. (2022), reported that Y. lipolytica ACA DC 50109 was able to grow in anaerobic fermentation media of food waste (real digestate) in the presence of 15 g/L VFAs, obtaining high lipid contents of 43.4% and 37.3% (w/w) when working with a 6:1 acetic:caproic ratio and with a C/N ratio of 200:1 and 150:1, respectively. Furthermore, the lipid yields, i.e., 0.33 and 0.31 g/g, were similar to those reported from sugars. This further supports the use of VFAs from residues for lipid production by oleaginous yeasts.
Not limited to the yeast Y. lipolytica, it should be noted that, although the use of VFAs is a very useful alternative to sugars as a carbon source for lipid production, it is important to analyse all the parameters discussed so far in order to obtain a truly efficient process. In this regard, the research of Rodrigues and Pais (2000) described the estimation of the minimum inhibitory acid concentration (xmin) of individual VFAs (Eq. 1) using the inhibition constant (ki) and taking into account that µx and µ0 are the specific growth rates at “x” concentration of fatty acids and in the absence of fatty acids, respectively.
In addition, it has been shown that when VFAs are initially at concentrations below the inhibition threshold, inhibition is nil and a slight increase in lipid production is observed (Park et al. 2021b). On the other hand, with regard to the influence of the pH of the medium, already mentioned above, it may also be important to be able to calculate the amount of undissociated acid in the medium, as its percentage, and thus its toxicity, increase at extracellular pH values below the pKa of the acid. In fact, in the study of Taherzadeh et al. (1997), where Saccharomyces cerevisiae was used, the undissociated acetic acid concentration in the medium was calculated (Eq. 2) from the pH of the medium and the total acetic acid concentration (Taherzadeh et al. 1997).
To conclude, it is clear that there is a need to further investigate the toxicity caused by these compounds in yeasts. What is already known is that yeast strains generally gain tolerance to fatty acids through adaptive evolutionary assays, overexpression and deletion libraries and comparative multiomics. This makes it possible to identify important genes and modify them (Park and Nicaud 2020).
In addition to the importance of the main substrates used with Y. lipolytica, it is also necessary to consider many other parameters that affect the production of these metabolites of industrial interest and influence biomass growth. Many researchers focused on optimising aspects, such as the composition of the culture medium (which also includes the type of carbon source or substrate), mode of cultivation, genetic engineering, methods of metabolite extraction and some other aspects. In particular, there are also several critical parameters related to the culture medium, apart from the carbon source, such as the nitrogen source, the C/N ratio, pH, but also temperature, aeration and even some micronutrients, which are among the most significant factors affecting the bioconversion process (Bao et al. 2021) (Fig. 4). These parameters are discussed in more detail below. Besides, Table 1 also shows some of the parameters affecting growth and lipid production in the oleaginous yeast Y. lipolytica, according to different recently reported studies.
5 Nitrogen source and C/N ratio
As indicated above, the nitrogen source plays a key role in cell growth and lipid synthesis. Nitrogen is considered to be an important nutrient for microorganisms to synthesise amino acids, proteins, nucleic acids, and different metabolites. In oleaginous microorganisms, the nitrogen source and its depletion in the medium is a key aspect in the process of de novo lipid accumulation. This process is stimulated and generally initiated by the lack of nitrogen, as the organism continues to sequester carbon but cell proliferation stops. This is explained, among others, by the fact that the absence of nitrogen inhibits isocitrate dehydrogenase (Beopoulos et al. 2009; Carsanba et al. 2018; Bao et al. 2021). Conversely, it is also noteworthy that some strains of Y. lipolytica are able to produce lipids in the presence of nitrogen and consume the accumulated lipids when exposed to nitrogen-limiting conditions (Gottardi et al. 2021). In a study carried out by Bellou et al. (2016), the authors cultivated Y. lipolytica not only under nitrogen limitation, but also under magnesium limitation because it had been suggested, in other studies, that this double nutrient limitation favours lipid accumulation. Under conditions of double limitation of nitrogen and magnesium, and using glucose as substrate, the authors obtained 12.2 g/L biomass with almost 50% lipids, i.e., a lipid production of about 6 g/L, which is a very high yield for wild-type strains of Y. lipolytica.
There is some controversy about the most suitable type of nitrogen source to be used, depending on whether cell growth or lipid production is to be promoted. Nitrogen sources used in culture media can be either organic or inorganic. Regarding organic sources, peptone and yeast extract are quite common, while typical inorganic sources include ammonium chloride, ammonium sulphate and ammonium or potassium nitrate. Several researches claim that organic nitrogen sources are suitable for lipid accumulation by oleaginous micro organisms, while inorganic nitrogen sources are more suitable for cell growth (Jianzhong et al. 1998; Bellou et al. 2016; Bao et al. 2021). For example, Li et al. (2007) observed that, in studies with R. toruloides, the latter accumulated more lipids when using organic nitrogen, so they used yeast extract and peptone as nitrogen sources in their batch cultures with R. toruloides Y4 using glucose as a carbon source for microbial lipid production. In the afore cited reference of Bellou et al. (2016), their best results of lipids were obtained using glucose and yeast extract, respectively as carbon and nitrogen sources, for Yarrowia lipolytica. Tsigie et al. (2011), based also on other yeast studies confirmed that organic nitrogen sources promote growth and lipid production. They carried out studies with various sources and demonstrated the same with Yarrowia lipolytica Po1g. However, some other researchers reported that ammonium sulphate was preferred for lipid production (Madani et al. 2017). Fontanille et al. (2012), carried out the bioconversion of VFAs into lipids by Y. lipolytica using ammonium as a nitrogen source, i.e., (NH4)2SO4, under limiting conditions, obtaining a biomass lipid content close to 40%. Recently, Bhutada et al. (2022) also used limiting conditions of (NH4)2SO4, for the manufacture of a human milk fat substitute using genetically modified strains of Y. lipolytica; it was demonstrated that Y. lipolytica could be genetically modified, and grown on different substrates under nitrogen-limited conditions, to synthesise triacylglycerol with a fatty acid composition similar to human milk fat (palmitic, oleic and linoleic acids). Finally, it should also be noted that, other authors, despite not working with Y. lipolytica, concluded in their studies that yeast extract and ammonium sulphate exerted the best effect on lipid synthesis, compared to peptone and ammonium chloride, in other oleaginous yeasts, i.e., Rhodotorula 110 and Candida 14 (Enshaeieh et al. 2012).
Not only are the carbon and the nitrogen sources key factors, but also the C/N ratio plays a relevant role in the lipid content and the amount biomass produced. In fact, it has often been reported that the C/N ratio is even the most important factor affecting lipid accumulation (Madani et al. 2017). Gao et al. (2020) also stated that the C/N ratio is critical in lipogenesis and that, in general, oleaginous yeasts are limited in lipid production when the initial C/N ratio is lower than 20 and that, on the other side, C/N ratios between 40 and 80 are usually the most appropriate. However, although the need for a high C/N ratio is clear, some more recent literature, e.g., Robles-Iglesias et al. (2023), report that optimal conditions for lipid accumulation usually require a C/N ratio ranging between 100 and 200. A low C/N value would imply the absence of nitrogen limitation and thus the suppression of activation of AMP-deaminase, which is responsible for supplying ammonium to nitrogen-deficient cells. In case of nitrogen limitation and when this reaction takes place, the mitochondrial AMP concentration decreases, which blocks the TCA cycle. As a result, excess citrate accumulated in the TCA cycle is excreted and leads to lipid accumulation (Seo et al. 2013). Kuttiraja et al. (2016) studied how the glycerol concentration and C/N ratio affected lipid production in Y. lipolytica strain SKY7 and concluded that a substrate concentration of 112.5 g/L and a C/N ratio of 100 provided the best results for both biomass and lipid production, taking into account that several studies were carried out with different glycerol concentrations (34.43, 52.37, 112.58 and 168.25 g/L) and different C/N ratios (25, 50, 100 and 150).
On the other hand, it is important to keep in mind that increasing the C/N ratio not only improves the lipid yield, but can also have some adverse effects such as a decrease of the biomass concentration and growth rates. Therefore, there is a maximum C/N ratio that a yeast can tolerate, which varies depending on the strain (Robles-Iglesias et al. 2023). For example, in experiments carried out with a C/N ratio = ∞ and using glucose as substrate and the yeast R. toruloides, the amount biomass obtained was half that obtained when the C/N ratio was 20 (Ye et al. 2021). On the other hand, there is also evidence that increasing the C/N ratio can even decrease lipid accumulation when the concentration of substrate used is too high, which can cause substrate inhibition (Robles-Iglesias et al. 2023).
The results found in the literature on the effect of the C/N ratio and, above all, on its optimal value are not always consistent, as different data have been reported. However, this may be due, among others, to the fact that different studies may use different strains, substrates, or fermentation conditions besides the nitrogen source, thus reaching apparently contradictory conclusions while using similar C/N ratios. Despite such apparent contradiction, after carrying out this comprehensive literature review, it can be stated that, although the operating conditions affect the results and the conclusions obtained, a C/N ratio around 100 can generally be considered as an optimal value for lipid overproduction in most oleaginous yeasts.
In a nutshell, the nature of the carbon and nitrogen sources and the C/N ratio are closely related parameters. Their effect is highly dependent on the yeast strain used, and is very important for efficient metabolites production and yields.
6 Temperature
Depending on the yeast strains, the tolerance to temperature is different; some yeasts prefer high temperatures (35–45 °C), while others prefer lower temperatures (25–30 °C) (Bao et al. 2021). Incubation temperature significantly affects lipid production by the yeast Y. lipolytica. According to some studies, it is claimed that temperatures between 24 and 33 °C favour cell growth, while a temperature of 28 °C would greatly favour lipid accumulation (Carsanba et al. 2018). Papanikolaou et al. (2002) obtained promising results of lipid production at pH 6 and temperatures between 28 and 33 °C in their research based on lipid production in an industrial animal fat medium; and they achieved about 10 g/L dry biomass by accumulating high amounts of lipids intracellularly (about 55% (w/w). Furthermore, it has been reported, in the two afore mentioned studies, that the highest lipid accumulation was achieved at a temperature of 28 °C (Papanikolaou et al. 2002; Carsanba et al. 2018), and this could therefore be considered optimal for both biomass growth and production of these metabolites. However, it is necessary to bear in mind that this is approximate, since as with other factors, the optimum temperature may depend on the strain and on other conditions, but values between 28 and 30 °C are most commonly used in the literature (Cavallo et al. 2017). For example, in research carried out by Bruder et al. (2020), in which they focused on the study of different of Y. lipolytica strains in relation to their ability to produce important metabolites such as lipids and carotenoids, they found that the strains did indeed have similar growth rates in the temperature range 28–34 °C, whereas a considerable decline in growth occurred at a temperature of 37 °C.
Besides, it is important to know that temperature also affects the lipid profile and the composition of the fatty acids obtained. It was observed that the degree of saturation is usually reduced when working at lower culture temperatures. On the other hand, despite the increase in unsaturation, lipid storage is not facilitated (Carsanba et al. 2018). This can be confirmed, for example, by the research carried out by Gao et al. (2017). The authors compared the behaviour of Y. lipolytica cultures at 38 and 28 °C, using VFAs as carbon sources. It was concluded, among other aspects, that cultures at 38 ºC provided lipids with less unsaturated fatty acids and more saturated fatty acids; in fact, no C18:3 was detected.
7 pH
Similarly to the parameters discussed in the previous sections, the pH of the medium is another key factor in the production of lipids and other bioproducts. Y. lipolytica has the advantage of being able to grow from pH values as low as 3 to values as high as 10 (Brígida et al. 2014; Sekova et al. 2015; Carsanba et al. 2018; Bao et al. 2021).
Regarding lipid synthesis, most authors recommend a pH between 5 and 6.5 (Timoumi et al. 2018). Gropoșilă-Constantinescu et al. (2015) reported that the optimal pH for lipid biosynthesis in wild-type Y. lipolytica was 6, using glucose and (NH4)2SO4 as carbon and nitrogen sources, respectively. On the other side, Lopes et al. (2018) concluded that the optimal pH was 5.6 in their research aimed at maximising the production of lipids and other metabolites in Y. lipolytica W29 (ATCC 20460) from lard. Also, Papanikolaou et al. (2002) concluded that the optimal pH for lipid production in Y. lipolytica strain ACA-DC 50109 was 6. With the above results, as well as other literature data, it appears that, as with other parameters, it is challenging to establish a single optimal pH value for Y. lipolytica, as it varies between oleaginous yeast strains and depends on other aspects such as the carbon sources (Bao et al. 2021), among others. A value close to 6 or slightly lower is, however, the most common one.
8 Aeration
As mentioned earlier, Y. lipolytica is a strictly aerobic yeast, and oxygenation of the culture medium is a parameter whose effect on its metabolism has then to be taken into account. Indeed, the dissolved oxygen concentration (DOC) in the culture medium greatly influences the growth of this yeast and also the synthesis of lipids, the latter being related to the level of lipid accumulation and their composition (Carsanba et al. 2018; Pereira et al. 2021). On the one hand, DOC significantly affects yeast morphology. In studies carried out with Y. lipolytica at low or zero DOC, the mycelial and/or pseudomycelial forms dominated over the yeast form, regardless of the carbon and nitrogen sources used (Bellou et al. 2014). On the other hand, it has recently been reported that for optimal lipid production, low dissolved oxygen values need to be maintained (Carsanba et al. 2018), as also confirmed by several other studies (Sabra et al. 2017; Rakicka et al. 2015; Unrean et al. 2017). Also, in the case of using fats and oils as substrates for growth and lipid production with Y. lipolytica, it is important to note that lipid synthesis appeared to be more favourable as dissolved oxygen decreased, and indeed, growth with high aeration can result in some cases in lipid-free biomass synthesis (Carsanba et al. 2018). Conversely, it has been reported that under high aeration conditions the production of citric acid (CA) and other non-target compounds may be favoured (Probst et al. 2016; Magdouli et al. 2018). Oxygen at high concentrations causes an increase in the activity of mitochondrial enzymes (citrate synthase, aconitase, malate dehydrogenase and NADP-dependent isocitrate dehydrogenase) involved in CA synthesis (Magdouli et al. 2018). Similarly as Magdouli et al. (2018), Bellou et al. (2014) also stated, in their studies with Y. lipolytica, that under high DOC conditions, the activity of ATP-citrate lyase and malic enzymes increases, up-regulating lipid metabolism. Additionally, at high DOC, NADP-dependent isocitrate dehydrogenase shows low activity, which also promotes lipid accumulation. Although it seems that the influence of dissolved oxygen on Y. lipolytica is more or less clear, it is important to note that during the lipid accumulation phase, however, the oxygen demand is variable depending on the oleaginous yeast species and the culture conditions used (Probst et al. 2016).
It is also worth highlighting the results of some other recent publications, summarized hereafter. Snopek et al. (2021) reported that a significant decrease in the DOC is proportional to an increase in biomass production and normally also to an increase in enzyme activity (Snopek et al. 2021). In their studies on the effect of air flow rate and agitation speed on biomass growth and lipase activity in Y. lipolytica strain KKP 379 with waste fish oil (carbon source), and with an aeration flow rate of 1.75 L/(L x min) (i.e., 1.75 "vvm", volume of air per volume of liquid per minute), they found lipase levels of 14.21 U/cm3, the highest enzyme activity among all cultures they examined. Therefore, the intensity of stirrer rotation and the level of aeration significantly affect the activity of extracellular lipolytic enzymes of the yeast Y. lipolytica. Pereira et al. (2021) studied the accumulation of lipids, from VFAs, in two strains of Y. lipolytica, i.e., strain W29 and strain NCYC 2904, and they concluded that oxygenation of the culture in their stirred tank bioreactor was also a key factor for both uptake of VFAs and production of microbial lipids. The highest VFAs uptake rate and lipid production were achieved in experiments carried out at a kLa of 87 h−1, compared to experiments carried out at a lower kLa of 22 h−1 and at a higher kLa of 125 h−1.
Overall, oxygen plays a key role in aerobic yeast fermentation and dissolved oxygen levels have been shown to affect the synthesis of some enzymes, cell metabolism and product yields, including lipids (Lv et al. 2020).
9 Micronutrients
In lipid production by oleaginous yeasts, it is also essential to consider the presence or absence of certain micronutrients, such as sulphur, phosphorus, magnesium, zinc, among others. In the case of Y. lipolytica, studies have shown that limiting the phosphate concentration favourably affects lipid accumulation while slowing down biomass growth because phosphate interferes with the regulation of chemical oxidative stress that is stimulated by mineral elements (Nambou et al. 2014). For instance, lipid accumulation and the concentration of KH2PO4 in the medium were shown to be directly related (Wierzchowska et al. 2021). It was observed that the amount lipids that accumulated in the yeast Y. lipolytica increased when KH2PO4 concentrations were restricted. Yet, this also created unfavourable conditions for the production of biomass. In the case of sulphate, limiting its availability, even when the nitrogen concentration is high, ensures good lipid production as the excess carbon is directed to lipid synthesis and not to cell growth. It is worth to highlight this fact as it is known that nitrogen limiting conditions favour lipid accumulation when using carbohydrates as carbon source for oleaginous yeasts. Even so, the dual sulphate and nitrogen limitation strategy is significantly more effective in accumulating these microbial oils (Wu et al. 2011). With regard to magnesium, however, it was observed that a higher concentration of magnesium led to larger biomass growth and less limitation of lipid accumulation. This is because magnesium can be involved in vital functions and enzymatic reactions (interaction with proteins, phospholipids and nucleic acids) (Nambou et al. 2014; Bellou et al. 2016; Bao et al. 2021).
From this it can be concluded that depending on the objective pursued, be it cell growth, metabolite production or others, all of the above factors need to be optimized and examined in detail on a case by case approach.
10 Lipids profile
As Y. lipolytica is an oil producer, the quality of its lipid product should be evaluated and compared with that of the final product of interest. The lipid profile observed in some yeasts is related to the experimental conditions, and may show slight variations between different strains, culture media, substrate used (type and concentration), pH and temperature, among others (Bao et al. 2021; Robles-Iglesias et al. 2023). However, despite the existence of these small variations, it should be noted that the lipids predominantly synthesised by Y. lipolytica consist of 16- and 18-carbon fatty acid chains, such as palmitic (C16:0), palmitoleic (C16:1), stearic (C18:0), oleic (C18:1) and linoleic (C18:2) acids. This composition is very similar to that of vegetable oil, which has similar applications, e.g., biodiesel production. Table 2 shows the lipid composition obtained with different strains of Y. lipolytica, using different carbon sources. It can be seen that the lipid profile is quite similar, although with small variations, which is due, in addition to the substrate, to all the factors mentioned above. Different typical lipid profiles are also compared in Fig. 5. Moreover, the degree of saturation of this typical lipid profile can be adjusted by playing with all these parameters. For example, it was reported that lowering the temperature can lead to an increase in unsaturated fatty acids or lowering the pH can lead to a higher percentage of oleic acid (Robles-Iglesias et al. 2023).
Lipid profile obtained from (A) palm oil (Giakoumis 2018); (B) canola oil (Smith et al. 2010); (C) soybean oil (Issariyakul and Dalai 2014); (D) corn oil (Issariyakul and Dalai 2014); (E) rapeseed oil (Sia et al. 2020) (F) Y. lipolytica grown on acetic, butyric and hexanoic acids coming from acetogenic fermentation of syngas by C. carboxidivorans (Unpublished data)
This high capacity to modify the lipid composition depending on the culture conditions, the strain used or even through genetic engineering, makes Y. lipolytica and other oleaginous yeasts even more interesting at an industrial level. In the following section, several applications of the lipids obtained, that does also demonstrate this extraordinary ability to vary the lipid profile, will be discussed.
11 Applications of synthesised lipids
As mentioned above, the remarkable capacity of Y. lipolytica, as a non-conventional yeast to produce lipids, is well known. Having highlighted some of the factors that most influence the production of lipids by this yeast in order to take into account each of the limitations and to be able to improve the process of synthesis of these metabolites, it is essential to study the prospects and applications.
As the production of oils and fats has increased over the last three decades, and is still increasing, this significant demand could be met by lipids produced by oleaginous yeasts. Most research confirms that one of the potential applications of lipids synthesised by oleaginous yeasts is the production of biofuels, followed by their use in the food industry, among others (Abeln and Chuck 2021), as summarized hereafter.
11.1 Biodiesel and sustainable aviation fuel (SAF)
The improvement of the economy and the easing of mobility restrictions after COVID-19 boosted global fuel demand, positively influencing the biofuels market. Global biodiesel consumption reached 55 billion liters in 2021. On the other hand, while demand increased, the cost of feedstocks and production also increased, thus counteracting the previous positive influence on the biofuels market. Regarding biodiesel prices, although they reached very high levels in 2021, they have been decreasing in 2023 due to the drop in raw material prices, although they are expected to remain roughly constant until 2031 due to public policies, the cost of raw materials and crude oil and distribution costs (OECD/FAO 2022).
Biodiesel is a more sustainable alternative compared to fossil-derived diesel. More than 70% of produced biodiesel is first-generation biofuel, derived from vegetable oils (OECD/FAO 2022) (Fig. 1). Although food crops initially seemed to be a promising feedstock for biofuel production, EU policy makers have been debating on the need to limit this type of biofuel and to opt for second-generation biofuels instead, and even to promote other advanced feedstocks, certain microorganisms (e.g., microalgae) and other bio-wastes (Drabik and Venus 2019; Abeln and Chuck 2021), which may also stimulate the production of lipids by oleaginous yeasts.
Although second- and third-generation biofuels, e.g., biodiesel, are currently attracting more attention (Fig. 1), they are still limited in terms of production on an industrial scale due to their relatively high cost and need for subsidies. Their production would be favored if fossil oil and first-generation biodiesel increase in price (Abeln and Chuck 2021). Despite these barriers, the urgent need to reduce direct dependence on fossil fuels and the numerous advantages offered by this type of biodiesel, has boosted research focusing on ways to improve biomass and lipid yields with microorganisms, as well as on economic aspects during the cultivation of oleaginous microorganisms (Leong et al. 2018). In the short term, road transport is expected to be the sector where the use of biofuels would be most widely applied as many vehicles run on diesel, which has a high compatibility with biodiesel.
Besides road traffic, research on aviation and maritime fuels is another recent area of interest. So far, the only fully mature and commercial scale technology recently applied to the production of biojet fuel is based on hydroprocessed esters of fatty acids (HEFA), which are obtained from vegetable oils, waste lipids or animal fats. Feedstock cost, in the production of biojet fuel from vegetable oil, may represent around 30 to up to as much as 80% of the overall production costs, besides the issue of cost fluctuations. Waste-derived feedstocks generally result in the lowest costs (El Kantar and Koubaa 2022). However, many vegetable oils have typical fatty acids carbon chain lengths best suited for biodiesel, e.g., often mainly C16–C18, rather than for aviation fuel. For example, soybean oil is predominantly a C18 oil feedstock, that can be used for biodiesel production, though more jet fuel could still be produced by cracking the diesel down to the jet range; which would however then also lead to increased costs (e.g., higher hydrogen requirements) (Pearlson et al. 2013). This is estimated to result in a biojet fuel price of about 1.07 to 1.24 US-$ per liter (around 1.00 to 1.16 € per liter), which is roughly twice the price of the fossil fuel counterpart (Pearlson et al. 2013). Similar cost comparisons apply to diesel and biodiesel. Another recent study estimated HEFA production costs of 890 € per ton (around 950 US-$ per ton) based on palm oil in this case (Neuling and Kaltschmitt 2018). According to that same study, other technologies, based on feedstocks such as lignocellulosic biomass (e.g., Biomass-to-Liquids (BtL), syngas-based processes) are estimated to result in even higher production costs, i.e., 1054–1325 € per ton (1125–1414 US-$ per ton). Although research on process improvements is going on, biofuels, such as aviation fuels, from vegetable oils are still more expensive than petroleum derived ones. On the other side, as far as maritime transport is concerned, less than 1% uses biofuels at the moment, due to high prices, scarce supplies, and institutional permissions, among others (OECD/FAO 2022; Robles-Iglesias et al. 2023).
A more recent approach has focused on the potential to use microbial oils, besides vegetable oils, as attractive third generation alternative, with Y. lipolytica as a ride horse in that respect. Most yeasts accumulate mainly C16–C18 fatty acids, similar to many vegetable oils, and highly effective for biodiesel production (Robles-Iglesias et al. 2023). However, it has been shown that yeasts, such as Y. lipolytica, can be engineered in order to produce specific target metabolites, i.e., fatty acids in this case, with different properties and different chain lengths, more suitable for the production of aviation fuel rather than biodiesel (Rigouin et al. 2017). In a nutshell, the appealing characteristics of yeasts suggest that biofuels such as biodiesel and SAF from microorganisms, i.e., microbial oils, could be applied soon to a broader extent at larger scale.
Although the production of biodiesel has these numerous advantages and although governments are expected to increasingly promote its production and use, currently it appears that the cost of biodiesel is between 70 and 130% higher than fossil diesel and gasoline in the wholesale market depending on the crop used. By 2022, plant-based biodiesel became almost 100% more expensive than fossil diesel, while used animal-based or cooking oil became almost 130% higher (Vilela 2022). This is mainly because the production of biofuels usually requires a high amount of energy, and their distribution and supply is not simple either. In addition, taking into account that biodiesel on the market is mostly of vegetable origin, additional drawbacks would be added to the previous ones, such as the need for large land areas and land crops, which involves more costs (Priya et al. 2022). This justifies the need to study and optimize the production of biodiesel from waste and pollutants, because the raw material used is the main factor that determines the success and cost-effectiveness of the production of this biofuel, which constitutes around 80% of the operating cost (Skarlis et al. 2012). Since the production of biodiesel using microorganisms and cost-effective substrates,—which is the main subject of this review-, is a quite recent research topic and is still mainly being studied at laboratory scale, it is difficult to compare economically this type of biodiesel with conventional diesel. Even so, it is worth mentioning the study of Sae-ngae et al. (2020), focused on the techno-economic analysis and environmental impact of the biovalorization of agro-industrial wastes for the production of biodiesel using oilseed yeasts. They carried out an economic analysis taking into account the operating costs associated with the conversion of waste into biodiesel by yeasts, not taking into account installation and equipment costs and estimating operating costs on the basis of common operation on an industrial scale. Among the models tested, for different agro-industrial wastes, the one that required the lowest operating cost was 3.0 US$ per kg-biodiesel, using glycerol waste for its bioconversion into biodiesel. Regarding the production costs of biodiesel from microalgae oils, some studies mention values ranging between 2.5 and 5.4 US$/kg (Davis et al. 2011; Richardson et al. 2012). Olkiewicz et al. (2016) carried out a scale-up and economic analysis of biodiesel production from primary sewage sludge. They estimated a biodiesel selling cost of 1.2 US$/Kg. Taking into account that the average price of diesel worldwide is approximately 1.3 $/kg (August 2023) (GlobalPetrolPrices.com 2023), it appears that biodiesel obtained from waste such as municipal sludge has the potential to compete economically with fossil diesel. However, generally, the production of biodiesel is a process that involves large costs, not being really competitive on a large scale today when taking into account that the biodiesel that is marketed is mostly of vegetable origin, which greatly increases costs as mentioned above. Therefore, it is important to continue studying this green technology for optimization and its application in industry.
11.2 Palm oil substitute
Palm oil, extracted from the mesocarp of the oil palm fruit, has a high content of saturated fatty acids, with palmitic acid accounting for about 40% (Spier et al. 2015; Abeln and Chuck 2021). Palm oil is mainly used in food and personal care products (Naylor and Higgins 2017). Its use for biofuels can also be mentioned because palm oil offers high yields cheaply. However, palm oil as a diesel substitute is less suitable than other vegetable oils: it has a high density, high flammability and lower energy density (Fitzherbert et al. 2008). It is one of the cheapest vegetable oils mainly due to its high productivity. Nevertheless, the increase in demand causes significant deforestation, increased carbon dioxide emissions and the formation of smog clouds, mainly in Southeast Asia (Fitzherbert et al. 2008; Whiffin et al. 2016; Naylor and Higgins 2017).
Specific oleaginous yeasts can synthesize an oil similar to conventional palm oil, which is mainly composed of palmitic, stearic, oleic and linoleic acids. Although most oleaginous yeasts produce mostly monounsaturated esters, some are able to produce more saturated oils, e.g., palm oil, such as Rhodotorula glutinis, Lipomyces lipofer or L. starkey (Liu et al. 2015b; Spier et al. 2015; Abeln and Chuck 2021; Robles-Iglesias et al. 2023). Lipid profiles with a high content of saturated esters were also obtained by Carsanba et al. (2020); with Y. lipolytica Po1dL yielding a profile with more than 50% saturated esters, with palmitic acid accounting for 37.2%, thus appearing valid as a substitute for palm oil.
11.3 Cocoa butter equivalent
Cocoa butter, extracted from cocoa beans, contains approximately 60% saturated fatty acids (Abeln and Chuck 2021). The high price of cocoa butter compared to other vegetable fats, the decrease of cocoa crops worldwide, and the fact that the cocoa fruit contains a minimal amount of cocoa butter, has led the food industry to search for alternatives (Jahurul et al. 2013). Oleaginous yeasts, on the other hand, can accumulate lipids rich in unsaturated fatty acids but studies such as those of Papanikolaou et al. (2003) and Papanikolaou et al. (2001), working with Y. lipolytica, demonstrated that it is possible to increase the level of saturated acids in the yeast lipids and obtain a lipid composition similar to that of cocoa butter.
11.4 Polyunsaturated fatty acids (PUFAs)
Polyunsaturated fatty acids, PUFAs, consist of two or more double bonds, usually in the ω-3 or -6 positions, and play vital functions in human health; they have anti-inflammatory and immunomodulatory properties, and they are necessary for the proper operation of the cardiac and immune systems. The predominant sources of PUFAs are vegetable seed oils and fish oils, which are becoming increasingly scarce as demand for these products increases (Cao et al. 2022). However, PUFAS can be obtained from oleaginous yeasts; for example, Y. lipolytica is considered a potential yeast host for PUFAs production, as it has the ability to accumulate lipids in considerable quantities (Palyzová et al. 2022; Robles-Iglesias et al. 2023). In addition, a variety of genetic and metabolic engineering tools are available to enhance the ability of Y. lipolytica to synthesize these products. These strategies include overexpression of synthetic heterologous pathways for de novo fatty acid synthesis, enhancement of acetyl-CoA precursor supply, inhibition of competing pathways, regulation of genes linked to lipid metabolism, among others (Cao et al. 2022).
The benefits of applying PUFAs in animal feed should also be highlighted, which led to the creation of the company Verlasso®, using DuPont's genetically modified Y. lipolytica strain (Zhu and Jackson 2015) to increase the amounts of certain PUFAs in the diet of salmon (Abeln and Chuck 2021).
12 Other products from Y. lipolytica
One of the outstanding features of Y. lipolytica is its ability to metabolise hydrophobic carbon sources such as triglycerides and fatty acids, making it suitable for bioremediation in environments contaminated by oil spills. Furthermore, it is an important bioprocessing carrier for many industrial applications; Y. lipolytica represents an excellent biocatalyst for producing renewable chemicals and enzymes for use in fuels, animal feed, oleochemical and pharmaceutical industries. There is considerable interest in this yeast due to its ability to produce numerous metabolites besides lipids, such as biosurfactants, γ-decalactone, citric acid, and carotenoids, among others (Gonçalves et al. 2014; Zhao et al. 2021). Therefore, besides lipid production reviewed in the previous sections, some other applications of Y. lipolytica are briefly described below.
12.1 Carotenoids
In addition to the great interest in the microbial production of biodiesel, the production of carotenoids is also a relevant topic today, especially for nutraceutical and food industries, among others. Humans do not have the capacity to synthesise vitamin A (Berman et al. 2017), a truly essential nutrient in diets. Carotenoids, present in some fruits and vegetables, are antioxidant compounds that are transformed into vitamin A when ingested. The benefits of this type of nutraceutical product are therefore many.
Carotenoids comprise more than 600 compounds. Moreover, as mentioned above, this group of substances, which have highly relevant industrial applications, have the advantage of being synthesised by different organisms. On the other hand, they are not able to satisfy the current market demand, which is why it is crucial to develop new, efficient, methods to meet the demand for these bioproducts (Liu et al. 2015a).
Even though native Y. lipolytica has the ability to produce high amounts of lipids naturally or even higher concentrations by expressing a gene, or part of a gene, in a host organism through recombinant DNA technology (Bruder et al. 2020), it is important to emphasise that carotenoids are normally not naturally produced by Y. lipolytica (Gottardi et al. 2021). However, there are several genetically modified strains of Y. lipolytica with the ability to synthesise these compounds.
Thus, although Y. lipolytica is unable to synthesise carotenoids naturally, it has the advantage of producing high amounts of acetyl-CoA. With this in mind, Larroude et al. (2018) studied the possibility of transforming Y. lipolytica into a potential yeast for β-carotene production. It was shown that the best engineered strain was able to produce 6.5 g/L and 90 mg/g DCW of β-carotene and 42.6 g/L of lipids. It should also be noted that in this study, different concentrations of carbon as substrate were tested (without varying the amount of nitrogen at any time); both glucose and glycerol were used, with no significant differences in the results obtained. Finally, it was also concluded that the process could be greatly improved by using other low-cost substrates such as lignocellulosic materials or starch. Bruder et al. (2020) conducted a recent study in which they evaluated, among others, the carotenoid production capacity of a total of 12 strains of Y. lipolytica. They observed that the best result obtained was 8 mg/g DCW of β-carotenoids in YNB media. This leads to the conclusion that, as with lipid production, the accumulation of carotenoids is also dependent on the substrate used.
Similarly, as observed for the accumulation of lipids, the efficiency of production of other metabolites, such as carotenoids, may vary depending on the culture conditions discussed above for microbial oils, that is, parameters such as temperature, pH, nutrients, and aeration. In that sense, it was suggested that the pH value might have an impact on β-carotene accumulation as it could play a regulative role at the branching point of acetyl-coA (Bruder et al. 2020). This was also similarly found for extracellular lipase production in Y. lipolytica (Destain et al. 1997).
Other studies have shown that the degree of dissolved oxygen also has a significant influence on the production of these important metabolites. Lv et al. (2020) explored the fermentation with dissolved oxygen feedback control to try to improve the production of β-carotene by a genetically modified strain, Y. lipolytica C11. Using DO-stat fed-batch fermentation, the biomass and β-carotene obtained were almost 1.3 times higher than under fed-batch fermentation; with 94 g/L biomass and 2.01 g/L β-carotene obtained. Kinetic modelling showed that this type of fermentation contributes significantly to the industrial scale production of this metabolite.
Also noteworthy in this section is the production of lycopene, an important central carotenoid that can be synthesised by modifying the metabolic pathway (Liu et al. 2015a). Matthäus et al. (2014) verified, using crude glycerol as a carbon source, the production of lycopene in a genetically modified strain of Y. lipolytica. These authors were able to increase the production of this metabolite, reaching a yield of 16 mg/g DWC in fed-batch fermentation.
12.2 Organic acids
The unconventional yeast Y. lipolytica is also well known for its ability to produce organic acids such as citric or isocitric acids, for example, from glycerol. In addition, advances in genetic engineering have also allowed to greatly favour the production of KGA and succinic acid (Madzak 2018). These four types of organic acids have very important applications (Liu et al. 2015a). Citric acid is a key crucial metabolic intermediate of the tricarboxylic acid (TCA) cycle and, due to its chemical structure, it is of great interest in industrial applications (Liu et al. 2015a); it is widely used in the pharmaceutical, food and chemical industries as it is a safe product with a pleasant acid taste, high water solubility, good chelating and buffering capacities, among other favourable characteristics (Cavallo et al. 2017). In wild-type strains of Y. lipolytica, citric acid is often co-produced together with isocitric acid, a molecule with potential applications in pharmaceuticals, medicine and also in food quality control (Gottardi et al. 2021). KGA and pyruvic keto acids are mainly of great interest in the food, pharmaceutical and animal feed industries; and, as discussed above, Y. lipolytica is also a competitive generator of succinic acid, which is used as a food additive, dietary supplement and as a component of bioplastics (Madzak 2018).
12.3 Flavouring compounds
Consumers are increasingly demanding more and more savoury foods, so in parallel to this, there is a growing requirement to add flavour to products. With this, the production of flavouring compounds is of great importance and has increased considerably as they are used in food and beverage, cosmetics, chemical, pharmaceutical and other industries (Braga and Belo 2016). Lactones, a type of aromatic compounds that can be found in various foods, are of great interest in the food sector because of their peculiar "fruity" aroma. The most interesting lactone is γ-decalactone, which is obtained from ricinoleic acid (Braga and Belo 2016; Gottardi et al. 2021). Although these kinds of compounds are usually obtained from fruits or through chemical synthesis, in recent years the use of enzymes and microorganisms for their synthesis has greatly increased and, in fact, Y. lipolytica is also capable of producing lactones (Braga and Belo 2016). The most commonly used substrates for lactone synthesis by yeasts are castor oil, ricinoleic acid or methyl ricinoleate. There are several microorganisms that can be used for the bioproduction of lactones, in particular for the production of γ-decalactone, but Y. lipolytica is the one that is recurrently most used as it is well adapted to grow on hydrophobic substrates. This advantage is, among others, due to the large number and efficiency of its lipases, cytochrome P450, acyl-CoA oxidases and its ability to produce biosurfactants (Waché et al. 2003; Aguedo et al. 2005; Al Mualad et al. 2022; Nogueira et al. 2022). Furthermore, it should be noted that, for example, during the process of biotransformation of methyl ricinoleate into γ-decalactone by Yarrowia lipolytica, this yeast has the ability to produce also large quantities of other lactones, i.e., 3-hydroxy-γ-decalactone, dec-2-en-4-olide and dec-3-en-4-olide (Waché et al. 2003).
The metabolic pathway of γ-decalactone production from methyl ricinoleate involves peroxisomal β-oxidation (Waché et al. 2002, 2003). However, other lactones, mentioned above, can accumulate in the metabolic pathway from ricinoleyl-CoA to acetyl-CoA, which decreases the yields of the parent compound (Waché et al. 2002). The substrate undergoes β-oxidation and, after a sequence of four reactions catalysed in the peroxisomes, it gives rise to C10 intermediates. Hydrolysis and lactonisation of decanoyl-CoA to γ-decalactone can take place, or its oxidation to 2-decenoyl-CoA and subsequent lactonisation to dec-2-en-4-olide or its hydration to 3-hydroxyacyl-CoA can take place. The 3-hydroxyacyl-CoA, in turn, can either be lactonised to 3-hydroxy-γ-decalactone or dehydrogenated and cleaved and go to the next β-oxidation loop. 3-Hydroxy-γ-decalactone can also be dehydrated to 2- and dec-3-en-4-olide, which can in turn be reduced to γ-decalactone (Waché et al. 2002). On the other hand, Y. lipolytica has a family of six acyl-CoA oxidases—Aox1 to Aox6, encoded by the POX1 to POX6 genes. These genes catalyse the first β-oxidation reaction (Nogueira et al. 2022). Modified strains with reduced Aox activity are known to accumulate more γ-decalactone. Actually, Waché et al. (2002) aimed to optimise γ-decalactone production by modifying the genotype of Y. lipolytica, for which they constructed a strain with multiple copies of POX2 (which codes for the specific long-chain Aox) and which was altered for the main active Aox in the short-chain acyl-CoA. In this way, it should theoretically accumulate the γ-decalactone precursor, thus hindering the accumulation of the other lactones. Indeed, the modified strain reached a higher concentration of γ-decalactone (150 mg/L) than the wild-type strain and, while the concentrations of other lactones were very low.
Finally, it is important to note that Y. lipolytica seems to be the microbial species with the highest productivity of γ-decalactone. There are several microorganisms that have the potential to produce γ-decalactone, but Aguedo et al. (2004), Fickers et al. (2005) and Braga and Belo (2016), claimed that Y. lipolytica should be considered the producer of excellence. Schrader et al. (2004) also report, based on the studies cited below, that the highest concentrations of bioproduced γ-decalactone are obtained with strains of Y. lipolytica. Pagot et al. (1997) used a genetically engineered multiple auxotrophic mutant of Y. lipolytica (POD1) to improve the yields of γ-decalactone and achieved 9.4 g/L of that product. This is a high value compared to those obtained with other microorganisms, such as Rhodotorula glutinis, among others (Schrader et al. 2004). Nevertheless, more recent publications report that, actually, the concentrations of γ-decalactone obtained in patented processes range from a few mg/L to 28 g/L, the latter concentration having been obtained by biotransformation of 12-hydroxystearic acid by Waltomyces lipofer (Nogueira et al. 2022). Thus, this microorganism exceeds the 12 g/L of γ-decalactone observed from castor oil and Y. lipolytica (Rabenhorst and Gatfield 2002; An et al. 2013). Nevertheless, otherwise, generally the highest concentrations have been reached with Y. lipolytica.
12.4 Others
There are still additional high value-added products that can be produced by native or engineered Y. lipolytica strains, including polyols such as erythritol, mannitol and arabitol, which have important industrial applications (Liu et al. 2015a). Erythritol and mannitol are used as flavour enhancers, sweeteners and humectants. However, it should be noted that wild-type strains produce fewer polyols than engineered strains (Gottardi et al. 2021). Y. lipolytica is also considered to be an optimal host for the production of polyhydroxyalkanoates, which are important biodegradable biopolymers that can be produced through genetic engineering. It was also reported that, with Y. lipolytica, a combined strategy with overexpression of some genes and deletion of others, good yields of pentane are obtained, thus affirming that this yeast also has the ability to produce certain hydrocarbons present in petroleum fuels. Finally, it is also important to mention the role of Y. lipolytica in bioremediation, a topic of significant relevance nowadays due to the increase in environmental pollution in recent years. In this regard, this yeast has the ability to produce biosurfactants, substances capable of stimulating the bioavailability of oil-contaminated chemicals, among others (Liu et al. 2015a), which is quite relevant, as oil-contaminated soils and water is a severe environmental problem.
Furthermore, Y. lipolytica can also be considered an important cell factory for other applications because even the unmodified wild-type strain is suitable to produce and secrete endogenous enzymes, e.g., alkaline protease, extracellular protease, lipase, phosphatase, among others and it. It is also an optimal host for the production of heterologous proteins, e.g., laccase and epoxide hydrolase (Liu et al. 2015a; Madzak 2018).
In conclusion, Y. lipolytica has numerous applications, from the production of microbial oils, functional enzymes and the synthesis of numerous metabolites of great industrial interest, to environmental bioremediation.
13 Concluding remarks and perspectives
Climate change, driven largely by CO2 emissions from burning fossil fuels, as well as fossil fuel depletion and current environmental and human health issues, are leading to increased interest in microbial oils produced by oleaginous yeasts. So much so that, over the last decade, annual publications on lipid production using oleaginous yeasts have increased significantly, highlighting the efficient alternative that microbial oils offer compared to conventional fossil fuels. Oleaginous yeasts exhibit lipid profiles that demonstrate the great potential of these microorganisms to play a key role in different fields in the twenty-first century. Y. lipolytica stands out for its great potential for biofuel production, its accumulation of platform chemicals for biorefining, as well as for the wide variety of biotechnological applications it offers. This review highlights the most relevant characteristics of Y. lipolytica, including its ability to metabolise sustainable substrates, the mechanism of lipid synthesis and metabolism, the factors that most influence cell growth and lipid accumulation, as well as the numerous applications of the lipids synthesised and the applications of other metabolites also produced by this oleaginous yeast.
Despite significant advances in lipid accumulation, high production costs are still an obstacle to their cost-effective commercialisation as an alternative to fossil oils or first-generation biofuels. However, the ability of Y. lipolytica to use cheaper substrates, such as VFAs, obtained from organic wastes, wastewaters or even greenhouse gases, would considerably reduce the costs of the process. On the other hand, it is also necessary to take into account the processing costs associated with lipid extraction and cell harvesting. Further processing of the products is costly, which limits a wide application of the lipids. Extraction and purification of lipids represent major obstacles to their industrial commercialisation as the energy and personnel required to perform the operation is high. It is necessary to carry out effective cell wall disruption (Gorte et al. 2020), for example by homogenising the extract well with a solvent and finally using a distillation column to recover the lipids and solvent, making this a simple process on an industrial scale (Robles-Iglesias et al. 2023). There are different methods of cell disruption, such as high-pressure homogenisation (HPH), bead milling, ultrasound, pulsed electric field (PEF), microwave, enzymatic hydrolysis, among others (Dong et al. 2016). For Y. lipolytica, one of the most effective cell disruption techniques for lipid recovery was ultrasound (Gorte et al. 2020). Moreover, although wet extraction with hexane has been modelled on an industrial scale, enzymatic treatment or supercritical fluid extraction with CO2, among others, are cheaper and more environmentally-friendly alternatives, although there is still limited information on these processes applied to oleaginous yeasts (Robles-Iglesias et al. 2023). Still, solvent recovery, as mentioned above, would be a way to save energy at the processing stage (Robles-Iglesias et al. 2023). These savings can be added to those derived from certain fermentation characteristics, which could also facilitate further processing, such as high density cultures, which leads to lower volumes in cell separation, and lipid secretion in the culture broth, which also favours lipid recovery by centrifugation (Abeln and Chuck 2021).
Although much research is still undertaken at laboratory or pilot scale, recent developments are getting closer to achieving cost-effective implementation on an industrial scale, by reducing costs, increasing yields thanks to the optimisation of the process and/or metabolic engineering and thanks to the European Union's commitment to foment the use of advanced sustainable biofuels. Therefore, research in this field needs to go on, as it has been shown that valorization of pollutants with Y. lipolytica to produce biobased products and biofuels is viable and, the latter, can replace fossil fuels to a large extent, while contributing to the circular economy by using renewable resources or pollutants as feedstocks and producing high value-added compounds in a more sustainable way. Furthermore, this detailed literature review highlights the positive impact of biofuel production on the UN Sustainable Development Goals (SDGs), especially if it is carried out using microorganisms and sustainable sources. Despite the obstacles related to the industrialisation of this process, already discussed above, it is necessary to promote its development as it would contribute to several of the SDGs. Indeed, it would, among others, promote poverty reduction mainly related to the oil market (SDG1); the use of waste for the production of biodiesel contributes to the circular economy (SDG 2 and SDG 3); it reduces toxic emissions to our planet through the use of cleaner energy (SDG 7 and SDG 13); it favours land reclamation also leading to the creation of jobs (SDG 8 and SDG 13); and it would also promote an innovative industry (SDG 9) (García‐Franco et al. 2021; United Nations Development Programme 2023). With this in mind, and in order to make progress in this technology, international cooperation would be necessary for the adoption of measures to redirect resources towards the sustainable growth of countries and to increase support for research and the application of this type of innovative and sustainable technology.
Abbreviations
- ACC:
-
Acyl-CoA carboxylase
- ACL:
-
ATP-citrate lyase
- ACS:
-
Acetyl-CoA synthase
- CA:
-
Citric acid
- C/N:
-
Carbon/Nitrogen ratio
- DAG:
-
Diacylglycerol
- DCW:
-
Dry cell weight
- DGA1:
-
DAG acyl-transferase I
- DGA2:
-
DAG acyl-transferase II
- DHAP:
-
Dihydroxyacetone phosphate
- DOC:
-
Dissolved oxygen concentration
- ER:
-
Endoplasmic reticulum
- FAS:
-
Fatty acid synthase
- FFA:
-
Free fatty acids
- FW:
-
Food waste
- G3P:
-
Glycerol 3-phosphate
- GlcNAc:
-
N-Acetylglucosamine
- GPD1:
-
NAD+ dependent G3P dehydrogenase
- GUT1:
-
Glycerol kinase
- GUT2:
-
FAD+ dependent G3P dehydrogenase
- HEFA:
-
Hydroprocessed fatty acid esters
- KGA:
-
α-Ketoglutaric acid
- LB:
-
Lipid body
- LPA:
-
Lysophosphatidic acid
- MIT:
-
Mitochondria
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- OD:
-
Optical density
- PA:
-
Phosphatidic acid
- PAP:
-
PA phosphohydrolase
- PER:
-
Peroxisome
- SCOs:
-
Single cell oils
- SCT1:
-
G3P acyltransferase
- SEM:
-
Scanning electron microscope
- SLC1:
-
LPA acyltransferase
- TAG:
-
Triacylglycerol
- TCA:
-
Tricarboxylic acid
- TGL4:
-
TAG intracellular lipase
- TGL3:
-
A positive regulator of TGL4
- VFAs:
-
Volatile Fatty Acids
- WLP:
-
Wood–Ljungdahl pathway
- w/w:
-
Weight/weight
- YNB:
-
Yeast Nitrogen Base
References
Abeln F, Chuck CJ (2021) The history, state of the art and future prospects for oleaginous yeast research. Microb Cell Factor 20:1–31. https://doi.org/10.1186/S12934-021-01712-1
Aguedo M, Ly MH, Belo I et al (2004) The use of enzymes and microorganisms for the production of aroma compounds from lipids. Food Technol Biotechnol 42:327–336
Aguedo M, Waché Y, Belin J-M, Teixeira JA (2005) Surface properties of Yarrowia lipolytica and their relevance to γ-decalactone formation from methyl ricinoleate. Biotechnol Lett 27:417–422. https://doi.org/10.1007/s10529-005-1776-z
Al Mualad WNA, Bouchedja DN, Selmania A et al (2022) Yeast Yarrowia lipolytica as a biofactory for the production of lactone-type aroma gamma-decalactone using castor oil as substrate. Chem Pap 76:7715–7728. https://doi.org/10.1007/s11696-022-02435-2
An J-U, Joo Y-C, Oh D-K (2013) New biotransformation process for production of the fragrant compound γ-Dodecalactone from 10-Hydroxystearate by permeabilized Waltomyces lipofer Cells. Appl Environ Microbiol 79:2636–2641. https://doi.org/10.1128/AEM.02602-12
Bankar AV, Kumar AR, Zinjarde SS (2009) Environmental and industrial applications of Yarrowia lipolytica. Appl Microbiol Biotechnol 84:847–865. https://doi.org/10.1007/S00253-009-2156-8/FIGURES/6
Bao W, Li Z, Wang X et al (2021) Approaches to improve the lipid synthesis of oleaginous yeast Yarrowia lipolytica: a review. Renew Sustain Energy Rev 149:111386. https://doi.org/10.1016/J.RSER.2021.111386
Baumann I, Westermann P (2016) Microbial production of short chain fatty acids from lignocellulosic biomass: current processes and market. Biomed Res Int 2016:846935. https://doi.org/10.1155/2016/8469357
Bellou S, Makri A, Triantaphyllidou I-E et al (2014) Morphological and metabolic shifts of Yarrowia lipolytica induced by alteration of the dissolved oxygen concentration in the growth environment. Microbiology 160:807–817. https://doi.org/10.1099/mic.0.074302-0
Bellou S, Triantaphyllidou IE, Mizerakis P, Aggelis G (2016) High lipid accumulation in Yarrowia lipolytica cultivated under double limitation of nitrogen and magnesium. J Biotechnol 234:116–126. https://doi.org/10.1016/J.JBIOTEC.2016.08.001
Ben M, Kennes C, Veiga MC (2016) Optimization of polyhydroxyalkanoate storage using mixed cultures and brewery wastewater. J Chem Technol Biotechnol 91:2817–2826. https://doi.org/10.1002/JCTB.4891
Beopoulos A, Chardot T, Nicaud JM (2009) Yarrowia lipolytica: a model and a tool to understand the mechanisms implicated in lipid accumulation. Biochimie 91:692–696. https://doi.org/10.1016/J.BIOCHI.2009.02.004
Berman J, Zorrilla-López U, Sandmann G et al (2017) The silencing of carotenoid β-Hydroxylases by RNA interference in different maize genetic backgrounds increases the β-carotene content of the endosperm. Int J Mol Sci 18:2515. https://doi.org/10.3390/IJMS18122515
Bermúdez-Penabad N, Kennes C, Veiga MC (2017) Anaerobic digestion of tuna waste for the production of volatile fatty acids. Waste Manag (new York, NY) 68:96–102. https://doi.org/10.1016/J.WASMAN.2017.06.010
Bhutada G, Menard G, Bhunia RK et al (2022) Production of human milk fat substitute by engineered strains of Yarrowia lipolytica. Metab Eng Commun 14:e00192. https://doi.org/10.1016/J.MEC.2022.E00192
Blazeck J, Hill A, Liu L et al (2014) Harnessing Yarrowia lipolytica lipogenesis to create a platform for lipid and biofuel production. Nat Commun 5:1–10. https://doi.org/10.1038/ncomms4131
Bonatsos N, Marazioti C, Moutousidi E et al (2020) Techno-economic analysis and life cycle assessment of heterotrophic yeast-derived single cell oil production process. Fuel 264:116839. https://doi.org/10.1016/J.FUEL.2019.116839
Braga A, Belo I (2016) Biotechnological production of γ-decalactone, a peach like aroma, by Yarrowia lipolytica. World J Microbiol Biotechnol 32:1–8. https://doi.org/10.1007/S11274-016-2116-2
Brígida AIS, Amaral PFF, Coelho MAZ, Gonçalves LRB (2014) Lipase from Yarrowia lipolytica: Production, characterization and application as an industrial biocatalyst. J Mol Catal B Enzym 101:148–158. https://doi.org/10.1016/J.MOLCATB.2013.11.016
Bruder S, Melcher FA, Zoll T et al (2020) Evaluation of a Yarrowia lipolytica strain collection for its lipid and carotenoid production capabilities. Eur J Lipid Sci Technol 122:1900172. https://doi.org/10.1002/EJLT.201900172
Cao L, Yin M, Shi TQ et al (2022) Engineering Yarrowia lipolytica to produce nutritional fatty acids: current status and future perspectives. Syn Syst Biotechnol 7:1024–1033. https://doi.org/10.1016/J.SYNBIO.2022.06.002
Carsanba E, Papanikolaou S, Erten H (2018) Production of oils and fats by oleaginous microorganisms with an emphasis given to the potential of the nonconventional yeast Yarrowia lipolytica. Crit Rev Biotechnol 38:1230–1243. https://doi.org/10.1080/07388551.2018.1472065
Carsanba E, Papanikolaou S, Fickers P, Erten H (2020) Lipids by Yarrowia lipolytica strains cultivated on glucose in batch cultures. Microorganisms 8:1–14. https://doi.org/10.3390/MICROORGANISMS8071054
Cavallo E, Charreau H, Cerrutti P, Foresti ML (2017) Yarrowia lipolytica: a model yeast for citric acid production. FEMS Yeast Res 17:fox084. https://doi.org/10.1093/FEMSYR/FOX084
Cavallo E, Nobile M, Cerrutti P, Foresti ML (2020) Exploring the production of citric acid with Yarrowia lipolytica using corn wet milling products as alternative low-cost fermentation media. Biochem Eng J 155:107463. https://doi.org/10.1016/J.BEJ.2019.107463
Chai B, Wang Y, Wang W, Fan P (2019) Effect of carbon source on lipid accumulation and biodiesel production of Yarrowia lipolytica. Environ Sci Pollut Res Int 26:31234–31242. https://doi.org/10.1007/S11356-019-06249-W
Cheirsilp B, Louhasakul Y (2013) Industrial wastes as a promising renewable source for production of microbial lipid and direct transesterification of the lipid into biodiesel. Biores Technol 142:329–337. https://doi.org/10.1016/J.BIORTECH.2013.05.012
Daskalaki A, Vasiliadou IA, Bellou S et al (2018) Data on cellular lipids of Yarrowia lipolytica grown on fatty substrates. Data Brief 21:1037–1044. https://doi.org/10.1016/j.dib.2018.10.116
Davis R, Aden A, Pienkos PT (2011) Techno-economic analysis of autotrophic microalgae for fuel production. Appl Energy 88:3524–3531. https://doi.org/10.1016/j.apenergy.2011.04.018
Destain J, Roblain D, Thonart P (1997) Improvement of lipase production from Yarrowia lipolytica. Biotech Lett 19:105–108. https://doi.org/10.1023/A:1018339709368/METRICS
Dobrowolski A, Mituła P, Rymowicz W, Mirończuk AM (2016) Efficient conversion of crude glycerol from various industrial wastes into single cell oil by yeast Yarrowia lipolytica. Biores Technol 207:237–243. https://doi.org/10.1016/J.BIORTECH.2016.02.039
Dong T, Knoshaug EP, Pienkos PT, Laurens LML (2016) Lipid recovery from wet oleaginous microbial biomass for biofuel production: a critical review. Appl Energy 177:879–895. https://doi.org/10.1016/j.apenergy.2016.06.002
Drabik D, Venus T (2019) EU biofuel policies for road and rail transportation sector. In: EU bioeconomy economics and policies. EU bioeconomy economics and policies: Volume II, pp 257–276
El Kantar S, Koubaa M (2022) Valorization of low-cost substrates for the production of odd chain fatty acids by the Oleaginous Yeast Yarrowia lipolytica. Fermentation 8:284. https://doi.org/10.3390/FERMENTATION8060284
Enshaeieh M, Abdoli A, Nahvi I, Madani M (2012) Selection and optimization of single cell oil production from Rodotorula 110 using environmental waste as substrate. J Cell Mol Res 4:68–75
Fernández-Naveira Á, Veiga MC, Kennes C (2017) H-B-E (hexanol-butanol-ethanol) fermentation for the production of higher alcohols from syngas/waste gas. J Chem Technol Biotechnol 92:712–731. https://doi.org/10.1002/JCTB.5194
Fickers P, Benetti PH, Waché Y et al (2005) Hydrophobic substrate utilisation by the yeast Yarrowia lipolytica, and its potential applications. FEMS Yeast Res 5:527–543. https://doi.org/10.1016/J.FEMSYR.2004.09.004
Fitzherbert EB, Struebig MJ, Morel A et al (2008) How will oil palm expansion affect biodiversity? Trends Ecol Evol 23:538–545. https://doi.org/10.1016/J.TREE.2008.06.012
Fontanille P, Kumar V, Christophe G et al (2012) Bioconversion of volatile fatty acids into lipids by the oleaginous yeast Yarrowia lipolytica. Biores Technol 114:443–449. https://doi.org/10.1016/J.BIORTECH.2012.02.091
Gao R, Li Z, Zhou X et al (2017) Oleaginous yeast Yarrowia lipolytica culture with synthetic and food waste-derived volatile fatty acids for lipid production. Biotechnol Biofuels 10:1–15. https://doi.org/10.1186/S13068-017-0942-6/TABLES/6
Gao R, Li Z, Zhou X et al (2020) Enhanced lipid production by Yarrowia lipolytica cultured with synthetic and waste-derived high-content volatile fatty acids under alkaline conditions. Biotechnol Biofuels 13:1–16. https://doi.org/10.1186/S13068-019-1645-Y/TABLES/5
García-Franco A, Godoy P, de la Torre J et al (2021) United Nations sustainability development goals approached from the side of the biological production of fuels. Microb Biotechnol 14:1871–1877. https://doi.org/10.1111/1751-7915.13912
Giakoumis EG (2018) Analysis of 22 vegetable oils’ physico-chemical properties and fatty acid composition on a statistical basis, and correlation with the degree of unsaturation. Renew Energy 126:403–419. https://doi.org/10.1016/J.RENENE.2018.03.057
GlobalPetrolPrices.com (2023) Diesel prices around the world, 28-Aug-2023. In: GlobalPetrolPrices.com. https://www.globalpetrolprices.com/diesel_prices/. Accessed 30 Aug 2023
Gonçalves FAG, Colen G, Takahashi JA (2014) Yarrowia lipolytica and its multiple applications in the biotechnological industry. The Sci World J. https://doi.org/10.1155/2014/476207
Gorte O, Hollenbach R, Papachristou I et al (2020) Evaluation of downstream processing, extraction, and quantification strategies for single cell oil produced by the oleaginous yeasts Saitozyma podzolica DSM 27192 and Apiotrichum porosum DSM 27194. Front Bioeng Biotechnol 8:355
Gottardi D, Siroli L, Vannini L et al (2021) Recovery and valorization of agri-food wastes and by-products using the non-conventional yeast Yarrowia lipolytica. Trends Food Sci Technol 115:74–86. https://doi.org/10.1016/J.TIFS.2021.06.025
Gropoșilă-Constantinescu D, Popa O, Mărgărit G, Visan L (2015) Production of microbial lipids by Yarrowia lipolytica. Roman Biotechnol Lett 20:10937
Höök M, Tang X (2013) Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 52:797–809. https://doi.org/10.1016/J.ENPOL.2012.10.046
Horincar G, Horincar VB, Gottardi D, Bahrim G (2017) Tailoring the potential of Yarrowia lipolytica for bioconversion of raw palm fat for antimicrobials production. LWT 80:335–340. https://doi.org/10.1016/J.LWT.2017.02.026
Iglesias-Iglesias R, Campanaro S, Treu L et al (2019) Valorization of sewage sludge for volatile fatty acids production and role of microbiome on acidogenic fermentation. Biores Technol 291:121817. https://doi.org/10.1016/J.BIORTECH.2019.121817
Iglesias-Iglesias R, Fernandez-Feal MM, del Kennes C, Veiga MC (2021) Valorization of agro-industrial wastes to produce volatile fatty acids: combined effect of substrate/inoculum ratio and initial alkalinity. Environ Technol 42:3889–3899. https://doi.org/10.1080/09593330.2020.1743370
Issariyakul T, Dalai AK (2014) Biodiesel from vegetable oils. Renew Sustain Energy Rev 31:446–471. https://doi.org/10.1016/J.RSER.2013.11.001
Jahurul MHA, Zaidul ISM, Norulaini NAN et al (2013) Cocoa butter fats and possibilities of substitution in food products concerning cocoa varieties, alternative sources, extraction methods, composition, and characteristics. J Food Eng 117:467–476. https://doi.org/10.1016/J.JFOODENG.2012.09.024
Jianzhong H, Qiaoqin S, Xiaolan Z et al (1998) Studies on the breeding of Mortierella isabellina mutant high producing lipid and its fermentation conditions. Wei Sheng Wu Xue Tong Bao 25:187–191
Katre G, Raskar S, Zinjarde S et al (2018) Optimization of the in situ transesterification step for biodiesel production using biomass of Yarrowia lipolytica NCIM 3589 grown on waste cooking oil. Energy 142:944–952. https://doi.org/10.1016/J.ENERGY.2017.10.082
Kennes C (2023) The grand challenge of water, waste, wastewater and emissions engineering and valorization. Front Environ Eng 2:1149950. https://doi.org/10.3389/FENVE.2023.1149950
Kuttiraja M, Douha A, Valéro JR, Tyagi RD (2016) Elucidating the Effect of Glycerol Concentration and C/N Ratio on Lipid Production Using Yarrowia lipolytica SKY7. Appl Biochem Biotechnol 180:1586–1600. https://doi.org/10.1007/S12010-016-2189-2
Lagoa-Costa B, Kennes C, Veiga MC (2020) Cheese whey fermentation into volatile fatty acids in an anaerobic sequencing batch reactor. Biores Technol 308:123226. https://doi.org/10.1016/J.BIORTECH.2020.123226
Lagoa-Costa B, Kennes C, Veiga MC (2022) Influence of feedstock mix ratio on microbial dynamics during acidogenic fermentation for polyhydroxyalkanoates production. J Environ Manage 303:114132. https://doi.org/10.1016/J.JENVMAN.2021.114132
Larroude M, Celinska E, Back A et al (2018) A synthetic biology approach to transform Yarrowia lipolytica into a competitive biotechnological producer of β-carotene. Biotechnol Bioeng 115:464–472. https://doi.org/10.1002/BIT.26473
Leong WH, Lim JW, Lam MK et al (2018) Third generation biofuels: a nutritional perspective in enhancing microbial lipid production. Renew Sustain Energy Rev 91:950–961. https://doi.org/10.1016/J.RSER.2018.04.066
Li Y, Zhao Z (Kent), Bai F (2007) High-density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed-batch culture. Enzyme Microb Technol. 41:312–317. https://doi.org/10.1016/j.enzmictec.2007.02.008
Liu HH, Ji XJ, Huang H (2015a) Biotechnological applications of Yarrowia lipolytica: past, present and future. Biotechnol Adv 33:1522–1546. https://doi.org/10.1016/J.BIOTECHADV.2015.07.010
Liu Y, Wang Y, Liu H, Zhang A (2015b) Enhanced lipid production with undetoxified corncob hydrolysate by Rhodotorula glutinis using a high cell density culture strategy. Biores Technol 180:32–39. https://doi.org/10.1016/j.biortech.2014.12.093
Llamas M, Magdalena JA, González-Fernández C, Tomás-Pejó E (2020a) Volatile fatty acids as novel building blocks for oil-based chemistry via oleaginous yeast fermentation. Biotechnol Bioeng 117:238–250. https://doi.org/10.1002/BIT.27180
Llamas M, Tomás-Pejó E, González-Fernández C, Greses S (2020b) Volatile fatty acids from organic wastes as novel low-cost carbon source for Yarrowia lipolytica. New Biotechnol 56:123–129. https://doi.org/10.1016/J.NBT.2020.01.002
Lopes M, Gomes AS, Silva CM, Belo I (2018) Microbial lipids and added value metabolites production by Yarrowia lipolytica from pork lard. J Biotechnol 265:76–85. https://doi.org/10.1016/J.JBIOTEC.2017.11.007
Lopes M, Miranda SM, Costa AR et al (2022) Yarrowia lipolytica as a biorefinery platform for effluents and solid wastes valorization–challenges and opportunities. Crit Rev Biotechnol 42:163–183. https://doi.org/10.1080/07388551.2021.1931016
Lv PJ, Qiang S, Liu L et al (2020) Dissolved-oxygen feedback control fermentation for enhancing β-carotene in engineered Yarrowia lipolytica. Sci Rep 10:1–11. https://doi.org/10.1038/S41598-020-74074-0
Madani M, Enshaeieh M, Abdoli A (2017) Single cell oil and its application for biodiesel production. Process Saf Environ Prot 111:747–756. https://doi.org/10.1016/J.PSEP.2017.08.027
Madzak C (2015) Yarrowia lipolytica: recent achievements in heterologous protein expression and pathway engineering. Appl Microbiol Biotechnol 99:4559–4577. https://doi.org/10.1007/S00253-015-6624-Z
Madzak C (2018) Engineering Yarrowia lipolytica for use in biotechnological applications: a review of major achievements and recent innovations. Mol Biotechnol 60:621–635. https://doi.org/10.1007/S12033-018-0093-4
Magdouli S, Brar SK, Blais JF (2018) Morphology and rheological behaviour of Yarrowia lipolytica: Impact of dissolved oxygen level on cell growth and lipid composition. Process Biochem 65:1–10. https://doi.org/10.1016/j.procbio.2017.10.021
Matthäus F, Ketelhot M, Gatter M, Barth G (2014) Production of lycopene in the non-carotenoid-producing yeast Yarrowia lipolytica. Appl Environ Microbiol 80:1660–1669. https://doi.org/10.1128/AEM.03167-13
Morales-Palomo S, González-Fernández C, Tomás-Pejó E (2022) Prevailing acid determines the efficiency of oleaginous fermentation from volatile fatty acids. J Environ Chem Eng 10:107354. https://doi.org/10.1016/J.JECE.2022.107354
Nambou K, Zhao C, Wei L et al (2014) Designing of a “cheap to run” fermentation platform for an enhanced production of single cell oil from Yarrowia lipolytica DSM3286 as a potential feedstock for biodiesel. Biores Technol 173:324–333. https://doi.org/10.1016/J.BIORTECH.2014.09.096
Naveira-Pazos C, Veiga MC, Kennes C (2022) Accumulation of lipids by the oleaginous yeast Yarrowia lipolytica grown on carboxylic acids simulating syngas and carbon dioxide fermentation. Biores Technol 360:127649. https://doi.org/10.1016/J.BIORTECH.2022.127649
Naylor RL, Higgins MM (2017) The political economy of biodiesel in an era of low oil prices. Renew Sustain Energy Rev 77:695–705. https://doi.org/10.1016/j.rser.2017.04.026
Neuling U, Kaltschmitt M (2018) Techno-economic and environmental analysis of aviation biofuels. Fuel Process Technol 171:54–69. https://doi.org/10.1016/J.FUPROC.2017.09.022
Nicaud JM (2012) Yarrowia lipolytica. Yeast (chichester, England) 29:409–418. https://doi.org/10.1002/YEA.2921
Nogueira JP, da Silva Souza IHS, Andrade JKS, Narain N (2022) Status of research on lactones used as aroma: a bibliometric review. Food Biosci 50:102004. https://doi.org/10.1016/j.fbio.2022.102004
OECD/FAO (2022) OCDE‑FAO Perspectivas Agrícolas 2022‑2031. https://doi.org/10.1787/820EF1BB-ES
Olkiewicz M, Torres CM, Jiménez L et al (2016) Scale-up and economic analysis of biodiesel production from municipal primary sewage sludge. Bioresour Technol 214:122–131. https://doi.org/10.1016/j.biortech.2016.04.098
Pagot Y, Endrizzi A, Nicaud J-M, Belin J-M (1997) Utilization of an auxotrophic strain of the yeast Yarrowia lipolytica to improve γ-decalactone production yields. Lett Appl Microbiol 25:113–116. https://doi.org/10.1046/j.1472-765X.1997.00182.x
Palyzová A, Spížek J, Vítová M, Řezanka T (2022) Biosynthesis of polyunsaturated fatty acids by metabolic engineering of yeast Yarrowia lipolytica. Stud Nat Prod Chem 74:197–223. https://doi.org/10.1016/B978-0-323-91099-6.00007-4
Papanikolaou S, Aggelis G (2011) Lipids of oleaginous yeasts. Part II: technology and potential applications. Eur J Lipid Sci Technol 113:1052–1073. https://doi.org/10.1002/EJLT.201100015
Papanikolaou S, Chevalot I, Komaitis M et al (2001) Kinetic profile of the cellular lipid composition in an oleaginous Yarrowia lipolytica capable of producing a cocoa-butter substitute from industrial fats. Antonie Van Leeuwenhoek 80:215–224. https://doi.org/10.1023/A:1013083211405/METRICS
Papanikolaou S, Chevalot I, Komaitis M et al (2002) Single cell oil production by Yarrowia lipolytica growing on an industrial derivative of animal fat in batch cultures. Appl Microbiol Biotechnol 58:308–312. https://doi.org/10.1007/S00253-001-0897-0
Papanikolaou S, Muniglia L, Chevalot I et al (2003) Accumulation of a cocoa-butter-like lipid by Yarrowia lipolytica cultivated on agro-industrial residues. Curr Microbiol 46:124–130. https://doi.org/10.1007/S00284-002-3833-3
Park YK, Nicaud JM (2020) Screening a genomic library for genes involved in propionate tolerance in Yarrowia lipolytica. Yeast (chichester, England) 37:131–140. https://doi.org/10.1002/YEA.3431
Park GW, Son S, Moon M et al (2021a) Volatile fatty acids from lipid-extracted yeast provide additional feedstock for microbial lipid production. Catalysts 11:1009. https://doi.org/10.3390/catal11081009
Park YK, González-Fernández C, Robles-Iglesias R et al (2021b) Bioproducts generation from carboxylate platforms by the non-conventional yeast Yarrowia lipolytica. FEMS Yeast Res 21:foab047. https://doi.org/10.1093/FEMSYR/FOAB047
Patel A, Matsakas L (2019) A comparative study on de novo and ex novo lipid fermentation by oleaginous yeast using glucose and sonicated waste cooking oil. Ultrason Sonochem 52:364–374. https://doi.org/10.1016/J.ULTSONCH.2018.12.010
Pearlson M, Wollersheim C, Hileman J (2013) A techno-economic review of hydroprocessed renewable esters and fatty acids for jet fuel production. Biofuels, Bioprod Biorefin 7:89–96. https://doi.org/10.1002/bbb.1378
Pereira AS, Miranda SM, Lopes M, Belo I (2021) Factors affecting microbial lipids production by Yarrowia lipolytica strains from volatile fatty acids: Effect of co-substrates, operation mode and oxygen. J Biotechnol 331:37–47. https://doi.org/10.1016/J.JBIOTEC.2021.02.014
Poli JS, da Silva MAN, Siqueira EP et al (2014) Microbial lipid produced by Yarrowia lipolytica QU21 using industrial waste: a potential feedstock for biodiesel production. Biores Technol 161:320–326. https://doi.org/10.1016/J.BIORTECH.2014.03.083
Priya DPS, Verma Y et al (2022) Biofuels: an alternative to conventional fuel and energy source. Mater Today Proc 48:1178–1184. https://doi.org/10.1016/j.matpr.2021.08.227
Probst KV, Schulte LR, Durrett TP et al (2016) Oleaginous yeast: a value-added platform for renewable oils. Crit Rev Biotechnol 36:942–955. https://doi.org/10.3109/07388551.2015.1064855
Rabenhorst J, Gatfield I (2002) Method of producing γ-decalactone using Yarrowia lipolytica strain HR 145 (DSM 12397)
Rakicka M, Lazar Z, Dulermo T et al (2015) Lipid production by the oleaginous yeast Yarrowia lipolytica using industrial by-products under different culture conditions. Biotechnol Biofuels 8:1–10. https://doi.org/10.1186/S13068-015-0286-Z/TABLES/3
Rene ER, Veiga MC, Kennes C (2022) Recent trends in biogenic gas. Waste Wastewater Ferment Ferment 8:347. https://doi.org/10.3390/FERMENTATION8080347
Richardson JW, Johnson MD, Outlaw JL (2012) Economic comparison of open pond raceways to photo bio-reactors for profitable production of algae for transportation fuels in the Southwest. Algal Res 1:93–100. https://doi.org/10.1016/j.algal.2012.04.001
Rigouin C, Gueroult M, Croux C et al (2017) Production of medium chain fatty acids by Yarrowia lipolytica: combining molecular design and TALEN to engineer the fatty acid synthase. ACS Synth Biol 6:1870–1879. https://doi.org/10.1021/ACSSYNBIO.7B00034/SUPPL_FILE/SB7B00034_SI_001.PDF
Robles-Iglesias R, Veiga MC, Kennes C (2021) Carbon dioxide bioconversion into single cell oils (lipids) in two reactors inoculated with Acetobacterium woodii and Rhodosporidium toruloides. J CO2 Utili 52:101668. https://doi.org/10.1016/J.JCOU.2021.101668
Robles-Iglesias R, Naveira-Pazos C, Fernández-Blanco C et al (2023) Factors affecting the optimisation and scale-up of lipid accumulation in oleaginous yeasts for sustainable biofuels production. Renew Sustain Energy Rev 171:113043. https://doi.org/10.1016/j.rser.2022.113043
Rodrigues G, Pais C (2000) The influence of acetic and other weak carboxylic acids on growth and cellular death of the yeast Yarrowia lipolytica. Food Technol Biotechnol 38:27–32
Ruiz-Herrera J, Sentandreu R (2002) Different effectors of dimorphism in Yarrowia lipolytica. Arch Microbiol 178:477–483. https://doi.org/10.1007/s00203-002-0478-3
Sabra W, Bommareddy RR, Maheshwari G et al (2017) Substrates and oxygen dependent citric acid production by Yarrowia lipolytica: insights through transcriptome and fluxome analyses. Microb Cell Fact 16:1–14. https://doi.org/10.1186/S12934-017-0690-0/FIGURES/6
Sae-ngae S, Cheirsilp B, Louhasakul Y et al (2020) Techno-economic analysis and environmental impact of biovalorization of agro-industrial wastes for biodiesel feedstocks by oleaginous yeasts. Sustain Environ Res 30:11. https://doi.org/10.1186/s42834-020-00052-w
Schrader J, Etschmann MMW, Sell D et al (2004) Applied biocatalysis for the synthesis of natural flavour compounds – current industrial processes and future prospects. Biotech Lett 26:463–472. https://doi.org/10.1023/B:BILE.0000019576.80594.0e
Sekova VY, Isakova EP, Deryabina YI (2015) Biotechnological applications of the extremophilic yeast Yarrowia lipolytica (Review). Prikl Biokhim Mikrobiol 51:290–304. https://doi.org/10.7868/S0555109915030150
Seo YH, Lee IG, Han J (2013) Cultivation and lipid production of yeast Cryptococcus curvatus using pretreated waste active sludge supernatant. Bioresour Technol 135:304–308. https://doi.org/10.1016/j.biortech.2012.10.024
Sestric R, Munch G, Cicek N et al (2014) Growth and neutral lipid synthesis by Yarrowia lipolytica on various carbon substrates under nutrient-sufficient and nutrient-limited conditions. Biores Technol 164:41–46. https://doi.org/10.1016/J.BIORTECH.2014.04.016
Sia CB, Kansedo J, Tan YH, Lee KT (2020) Evaluation on biodiesel cold flow properties, oxidative stability and enhancement strategies: a review. Biocatal Agric Biotechnol 24:101514. https://doi.org/10.1016/J.BCAB.2020.101514
Skarlis Str, Kondili E, Kaldellis JK (2012) Small-scale biodiesel production economics: a case study focus on Crete Island. J Clean Prod 20:20–26. https://doi.org/10.1016/j.jclepro.2011.08.011
Smith PC, Ngothai Y, Dzuy Nguyen Q, O’Neill BK (2010) Improving the low-temperature properties of biodiesel: methods and consequences. Renew Energy 35:1145–1151. https://doi.org/10.1016/J.RENENE.2009.12.007
Snopek P, Nowak D, Zieniuk B, Fabiszewska A (2021) Aeration and stirring in Yarrowia lipolytica lipase biosynthesis during batch cultures with waste fish oil as a carbon source. Fermentation 7:88. https://doi.org/10.3390/FERMENTATION7020088
Spier F, Buffon JG, Burkert CAV (2015) Bioconversion of raw glycerol generated from the synthesis of biodiesel by different oleaginous yeasts: lipid content and fatty acid profile of biomass. Indian J Microbiol 55:415–422. https://doi.org/10.1007/s12088-015-0533-9
Taherzadeh MJ, Niklasson C, Lidén G (1997) Acetic acid-friend or foe in anaerobic batch conversion of glucose to ethanol by Saccharomyces cerevisiae? Chem Eng Sci 52:2653–2659. https://doi.org/10.1016/S0009-2509(97)00080-8
Timoumi A, Guillouet SE, Molina-Jouve C et al (2018) Impacts of environmental conditions on product formation and morphology of Yarrowia lipolytica. Appl Microbiol Biotechnol 102:3831–3848. https://doi.org/10.1007/S00253-018-8870-3
Tomás-Pejó E, González-Fernández C, Greses S et al (2023) Production of short chain fatty acids (SCFAs) as chemicals or substrates for microbes to obtain biochemicals. Biotechnol Biofuels 16:96. https://doi.org/10.1186/s13068-023-02349-5
Tsigie YA, Wang C-Y, Truong C-T, Ju Y-H (2011) Lipid production from Yarrowia lipolytica Po1g grown in sugarcane bagasse hydrolysate. Bioresour Technol 102:9216–9222. https://doi.org/10.1016/j.biortech.2011.06.047
United Nations Development Programme (2023) Sustainable Development Goals. In: UNDP. https://www.undp.org/sustainable-development-goals. Accessed 23 Aug 2023
Unrean P, Khajeeram S, Champreda V (2017) Combining metabolic evolution and systematic fed-batch optimization for efficient single-cell oil production from sugarcane bagasse. Renew Energy 111:295–306. https://doi.org/10.1016/J.RENENE.2017.04.018
Vilela A (2022) Nuevo estudio: Los biocombustibles son 130% más costosos que los combustibles fósiles. In: H2 Bus. News. https://h2businessnews.com/nuevo-estudio-los-biocombustibles-son-130-mas-costosos-que-los-combustibles-fosiles/. Accessed 24 Aug 2023
Waché Y, Aguedo M, LeDall M-T et al (2002) Optimization of Yarrowia lipolytica’s β-oxidation pathway for γ-decalactone production. J Mol Catal B Enzym 19–20:347–351. https://doi.org/10.1016/S1381-1177(02)00185-6
Waché Y, Aguedo M, Nicaud J-M, Belin J-M (2003) Catabolism of hydroxyacids and biotechnological production of lactones by Yarrowia lipolytica. Appl Microbiol Biotechnol 61:393–404. https://doi.org/10.1007/s00253-002-1207-1
Whiffin F, Santomauro F, Chuck CJ (2016) Toward a microbial palm oil substitute: oleaginous yeasts cultured on lignocellulose. Biofuels, Bioprod Biorefin 10:316–334. https://doi.org/10.1002/BBB.1641
Wierzchowska K, Zieniuk B, Nowak D, Nowak D (2021) Phosphorus and nitrogen limitation as a part of the strategy to stimulate microbial lipid biosynthesis. Appl Sci (switzerland) 11:11819. https://doi.org/10.3390/APP112411819
Wu S, Zhao X, Shen H et al (2011) Microbial lipid production by Rhodosporidium toruloides under sulfate-limited conditions. Biores Technol 102:1803–1807. https://doi.org/10.1016/j.biortech.2010.09.033
Ye Z, Sun T, Hao H et al (2021) Optimising nutrients in the culture medium of Rhodosporidium toruloides enhances lipids production. AMB Express 11:149. https://doi.org/10.1186/s13568-021-01313-6
Yook SD, Kim J, Woo HM et al (2019) Efficient lipid extraction from the oleaginous yeast Yarrowia lipolytica using switchable solvents. Renew Energy 132:61–67. https://doi.org/10.1016/J.RENENE.2018.07.129
Zhao C, Gu D, Nambou K et al (2015) Metabolome analysis and pathway abundance profiling of Yarrowia lipolytica cultivated on different carbon sources. J Biotechnol 206:42–51. https://doi.org/10.1016/J.JBIOTEC.2015.04.005
Zhao Y, Liu S, Li J et al (2021) Advances in the application of Yarrowia lipolytica as a microbial cell factory. Shipin Kexue/food Sci 42:388–400. https://doi.org/10.7506/SPKX1002-6630-20200802-027
Zhu Q, Jackson EN (2015) Metabolic engineering of Yarrowia lipolytica for industrial applications. Curr Opin Biotechnol 36:65–72. https://doi.org/10.1016/J.COPBIO.2015.08.010
Acknowledgements
Our research on yeast biorefinery is partly funded by the Spanish Ministry of Science and Innovation and European FEDER funds (PID2020-117805RB-I00 and TED2021-130055B-I00). RRI thanks the Ministry of Science and Innovation for financial support of his doctoral research (E-15-2019-0344365). CFB (ED481A-2020/028) thanks Xunta de Galicia for financing her doctoral research. The authors, belonging to the BIOENGIN group, thank Xunta de Galicia for financial support to Competitive Reference Research Groups (ED431C 2021/55). Funding for open access charge covered by Universidade da Coruña/CISUG.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Naveira-Pazos, C., Robles-Iglesias, R., Fernández-Blanco, C. et al. State-of-the-art in the accumulation of lipids and other bioproducts from sustainable sources by Yarrowia lipolytica. Rev Environ Sci Biotechnol 22, 1131–1158 (2023). https://doi.org/10.1007/s11157-023-09670-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-023-09670-3