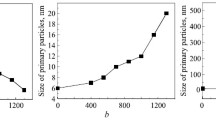
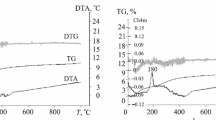
Nanocrystalline powders in the ZrO2−Y2O3−CeO2 system were produced by hydrothermal synthesis in an alkaline environment. The powder properties were studied by differential thermal analysis, Xray diffraction, electron microscopy, petrography, and BET. A low-temperature ZrO2-based cubic solid solution crystallized in the powders in hydrothermal conditions. The specific surface area of the powders was 81−110 m2/g. The lattice parameters of the ZrO2-based solid solution increased monotonically with higher CeO2 amount. The research results are needed for the microstructural design of composites in the ZrO2−Y2O3−CeO2 system with high resistance to low-temperature ageing.




Similar content being viewed by others
References
E.V. Dudnik, A.V. Shevchenko, A.K. Ruban, V.P. Red’ko, and L.M. Lopato, “Microstructural design of ZrO2–Y2O3–CeO2–Al2O3 materials,” Powder Metall. Met. Ceram., 49, Nos. 9–10, 528–536 (2011).
E.V. Dudnik, S.N. Lakiza, Ya.S. Tishchenko, A.K. Ruban, V.P. Red’ko, A.V. Shevchenko, and L.M. Lopato, “Phase diagrams of refractory oxide systems and microstructural design of materials,” Powder Metall. Met. Ceram., 53, Nos. 5–6, 303–311 (2014).
S. Sōmiya and T. Akiba, “Hydrothermal zirconia powders: A Bibliography,” J. Eur. Ceram. Soc., 19, No. 1, 81–87 (1999).
A.V. Shevchenko, “Hydrothermal techniques in materials science,” in: Inorganic Materials Science. Fundamentals of Materials Science [in Russian], Kyiv (2008), Vol. 2, pp. 272–281.
O. Vasylkiv and Y. Sakka, “Hydroxide synthesis, colloidal processing and sintering of nano-size 3Y-TZP powder,” Scripta Mater., 44, 2219–2223 (2001).
O. Vasylkiv, Y. Sakka, and V. Skorokhod, “Low-temperature processing and mechanical properties of zirconia and zirconia-alumina nano-ceramic,” J. Am. Ceram. Soc., 86, No. 2, 299–304 (2003).
O. Vasylkiv and Y. Sakka, “Synthesis and colloidal processing of zirconia nano-powder,” J. Am. Ceram. Soc., 84, No. 11, 2489–2494 (2001).
E.V. Dudnik, “Modern methods for hydrothermal synthesis of ZrO2-based nanocrystalline powders,” Powder Metall. Met. Ceram., 48, Nos. 3–4, 238–248 (2009).
Z. Yanjie, Z. Yingying, C. Yanning, C. Chongqi, L. Xingyi, and Z. Qi, “Low-temperature water–gas shift reaction over Au/ZrO2 catalysts using hydrothermally synthesized zirconia as supports,” Chin. J. Catal., 33, 230–236 (2012).
Yu.M. Fedenko, T.A. Dontsova, and I.M. Astrelin, “Turbidimetric method for estimating the sizes of nanoparticles in ZrO2 white sols,” Nauk. Vist. NTUU KPI, Issue 1, 155–158 (2012).
M. Taguchi, T. Nakane, A. Matsushita, Y. Sakka, T. Uchikoshi, T. Funazukuri, and T. Naka, “One-pot synthesis of monoclinic ZrO2 nanocrystals under subcritical hydrothermal conditions,” J. Supercrit. Fluids, 85, 57–61 (2014).
E.V. Dudnik and A.V. Shevchenko, “Variation in properties of ZrO2–Y2O3–CeO2–Al2O3 powders during thermal treatment at 400 to 1300°C,” Powder Metall. Met. Ceram., 49, Nos. 3–4, 125–134 (2010).
A. Behbahani, S. Rowshanzamir, and A. Esmaeilifar, “Hydrothermal synthesis of zirconia nanoparticles from commercial zirconia,” Procedia Eng., 42, 908–917 (2012).
L.M. Rudkovskaya, R.N. Pshenichny, T.V. Pavlenko, and A.A. Omelchuk, “Nanostructured zirconium dioxide synthesized hydrothermally from zirconium concentrate decomposition products,” Nanosyst. Nanomater. Nanotekhnol., 10, No. 2, 351–360 (2012).
R. Espinoza-Gonzáleza, E. Mosquera, I. Moglia, R. Villarroel, and V.M. Fuenzalida, “Hydrothermal growth and characterization of zirconia nanostructures on non-stoichiometric zirconium oxide,” Ceram. Int. (2014), URL: [https://doi.org/10.1016/j.ceramint.2014.07.034].
Ch.V. Reddy, I.N. Reddy, J. Shim, D. Kim, and K. Yoo, “Synthesis and structural, optical, photocatalytic, and electrochemical properties of undoped and yttrium-doped tetragonal ZrO2 nanoparticles,” Ceram. Int. (2018), URL: [https://doi.org/10.1016/j.ceramint.2018.04.020].
G. Growth, L. Fei, L. Yanhuai, S. Zhongxiao, X. Kewei, M. Dayan, G. Bo, and C. Hon, “Characteristics of hydrothermally prepared yttria stabilized zirconia nanocrystals during calcination,” Rare Met. Mater. Eng., 46, No. 4, 899–905 (2017).
C. Duran, K. Sato, Y. Hotta, H. Göçmez, and K. Watari, “Ball milling assisted hydrothermal synthesis of ZrO2 nanopowders,” Ceram. Int., 41, 5588–5593 (2015).
A.O. Hongmin, L.U. Xiangsheng, Z. He, Z. Jing, H. Xiaowei, F. Zongyu, and X. Hong, “Preparation of scandia stabilized zirconia powder using microwave-hydrothermal method,” J. Rare Earths, 33, No. 7, 746–751 (2015).
J. Liao, Q. Wang, L. Nie, W. You, and J. Chen, “Single red upconversion and near-infrared downconversion luminescence properties of cubic ZrO2:Y3+–Yb3+–Er3+ nanophosphors via microwave hydrothermal synthesis,” Opt. Mater., 62, 479–484 (2016).
M. Asiltürk, E. Burunkaya, F. Sayilkan, N. Kiraz, and E. Arpaç, “Structural and optical properties of thin films prepared from surface modified ZrO2,” J. Non-Cryst. Solids, 357, 206–210 (2011).
X. Gan, Z. Yu, K. Yuan, C. Xu, X. Wang, L. Zhu, G. Zhang, and D. Xu, “Preparation of a CeO2-nanoparticle thermal radiation shield coating on ZrO2 fibers via a hydrothermal method,” Ceram. Int. (2017), URL: [https://doi.org/10.1016/j.ceramint.2017.07.161. 2017].
L.J. Zhao, W. Zai, M.H. Wong, and H.C. Man, “Hydrothermal synthesis of Ag-ZrO2/r-GO coating on CoCrMo substrate,” Mater. Lett., 228, 314–317 (2018).
Zh. Song, C. Duan, M. Shi, S. Li, and Y. Guan, “One-step preparation of ZrO2/SiO2 microspheres and modification with d-fructose 1, 6-bisphosphate as stationary phase for hydrophilicinteraction chromatography,” J. Chromatogr. (2017), URL: [https://doi.org/10.1016/j.chroma.2017.09.046].
O. Vasylkiv, Y. Sakka, Y. Maeda, and V.V. Skorokhod, “Nano-engineering of zirconia-noble metals composites,” J. Eur. Ceram. Soc., 24, No. 2, 469–473 (2004).
O. Vasylkiv, Y. Sakka, Y. Maeda, and V.V. Skorokhod, “Sonochemical preparation and properties of Pt–3Y-TZP nano-composites,” J. Am. Ceram. Soc., 88, No. 3, 639–644 (2005).
O.V. Almiasheva, A.Yu. Postnov, N.V. Maltseva, and E.A. Vlasov, “Thermally stable catalyst based on ZrO2–Al2O3 nanocomposite for hydrogen oxidation,” Nanosyst. Fiz. Khim. Mat., 3, No. 6, 75–82 (2012).
Zh. Song, P. Ning, Q. Zhang, H. Li, J. Zhang, Y. Wang, X. Liu, and Z. Huang, “Activity and hydrothermal stability of CeO2–ZrO2–WO3 for the selective catalytic reduction of NOx with NH3,” J. Environ. Sci. (2015), URL: [https://doi.org/10.1016/j.jes.2015.06.010].
P. Ning, Z. Song, H.Li, Q. Zhang, X. Liu, J. Zhang, X. Tang, and Z. Huang, “Selective catalytic reduction of NO with NH3 over CeO2–ZrO2–WO3 catalysts prepared by different methods,” Appl. Surf. Sci. (2015), URL: [https://doi.org/10.1016/j.apsusc.2015.01.118].
A.I. Ramos-Guerra, I. Martínez-Merlín, and C. Falcony, “The role of the stabilizing agent on the structural and luminescent properties of hydrothermally synthesized ZrO2: Tb3+phosphors,” Ceram. Int. (2018), URL: [https://doi.org/10.1016/j.ceramint.2018.04.216].
M.A.I. Mohamed, V.-D. Dao, S.Y. Ahmed, M.M. Hamouda, A.Y. Mohamed, Y.K. Muhammad, H.-S. Choi, and A.M.B. Nasser, “Physicochemical and photo-electrochemical characterization of novel N-doped nanocomposite ZrO2/TiO2 photoanode towards technology of dye-sensitized solar cells,” Mater. Charact. (2017), URL: [doi: https://doi.org/10.1016/j.matchar.2017.03.014].
A.V. Shevchenko, E.V. Dudnik, V.V. Tsukrenko, A.K. Ruban, V.P. Red’ko, and L.M. Lopato, “Microstructural design of bioinert composites in the ZrO2–Y2O3–CeO2–Al2O3–CoC system,” Powder Metall. Met. Ceram., 51, Nos. 11–12, 724–733 (2012).
A.V. Shevchenko, E.V. Dudnik, A.K. Ruban, V.M. Vereschaka, V.P. Red’ko, and L.M. Lopato, “Hydrothermal synthesis of nanocrystalline powders in the ZrO2−Y2O3−CeO2 system,” Powder Metall. Met. Ceram., 46, Nos. 1–2, 18–24 (2007).
I.M. Vasserman, Chemical Solution Deposition [in Russian], Leningrad (1980), p. 208.
O.Yu. Kurapova, V.G. Konakov, S.N. Golubev, and V.M. Ushakov, “Interaction between the synthesis procedure, phase formation, and particle size of precursor powders for ceramics with final composition 9CaO91ZrO2,” Nov. Ogneupory, No. 4, 47–52 (2014).
O.Yu. Kurapova, V.G. Konakov, S.N. Golubev, and V.M. Ushakov, “Phase formation and stability of solid solutions in nanosized zirconia dioxide precursors produced by cryochemical methods,” Vest. SPb Gos. Univ. Ser. 4: Fiz. Khim., 3(61), 296–310 (2016).
Acknowledgments
The authors are grateful to V.M. Pavlikov, PhD in Chemical Sciences, for the differential thermal analysis and to L.D. Bilash for determining the powders’ specific surface area.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkova Metallurgiya, Vol. 58, Nos. 3–4 (526), pp. 3–12, 2019.
Rights and permissions
About this article
Cite this article
Marek, I.O., Ruban, O.K., Redko, V.P. et al. Physicochemical Properties of Hydrothermal Nanocrystalline ZrO2–Y2O3–CeO2 Powders. Powder Metall Met Ceram 58, 125–132 (2019). https://doi.org/10.1007/s11106-019-00055-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-019-00055-2