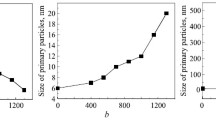
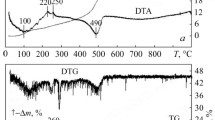
The properties of nanocrystalline powders of compositions (mol.%) 97 ZrO2–Y2O3, 95 ZrO2–3 Y2O3–2 CeO2, 92.5 ZrO2–2.5 Y2O3–5 CeO2, 90 ZrO2–2 Y2O3–8 CeO2, and 88 ZrO2–12 CeO2 were studied. The powders were produced by hydrothermal synthesis in an alkaline environment from a coprecipitated hydroxide mixture with a residual moisture of 15–20%. The powder properties were determined by X-ray diffraction (XRD), electron microscopy, BET, and petrography. Metastable F-ZrO2 was found to form in the hydrothermally synthesized powders. According to XRD, the F-ZrO2 → T-ZrO2 phase transformation began at 700°C and finished at 850–1000°C. The crystal optical characteristics of the powders indicate that the F-ZrO2 → T-ZrO2 phase transformation started at 400°C. The variations in F-ZrO2 and T-ZrO2 unit cell volumes are associated with lattice distortions under the action of different mechanisms in costabilization of the zirconia-based solid solution and with the ratio of Y2O3 and CeO2 in the solid solution. The tetragonality of the powders increases in the ZrO2 costabilization. The transformation strengthening mechanism for ceramics based on ZrO2 (Y2O3, CeO2) solid solutions becomes more effective with the formation of T-ZrO2, whose capability to the T-ZrO2 → M-ZrO2 phase transformation increases. The morphology of the powders varies topologically continuously, and the sizes of their primary particles hardly increase up to 1150°C. The variation in the specific surface area (from 153 to 2 m2/g) of the powders is determined by the F-ZrO2 → T-ZrO2 phase transformation and their sintering activity above 1000°C.





Similar content being viewed by others
References
J. Chevalier, Al. Liens, H. Reveron, F. Zhang, P. Reynaud, Th. Douillard, L. Preiss, V. Sergo, V. Lughi, M. Swain, and N. Courtois, “Forty years after the promise of “ceramic steel”: zirconia-based composites with a metal-like mechanical behavior,” J. Am. Ceram. Soc., 103, 1482–1513 (2020), https://doi.org/10.1111/jace.16903.
E.V. Dudnik, S.N. Lakiza, Ya.S. Tishchenko, A.K. Ruban, V.P. Redko, A.V. Shevchenko, and L.M. Lopato, “Phase diagrams of refractory oxide systems and microstructural design of materials,” Powder Metall. Met. Ceram., 53, No. 5–6, 303–311 (2014).
J. Lin and J. Duh, “Correlation of mechanical properties and composition in tetragonal CeO2–Y2O3–ZrO2 ceramic system,” Mater. Chem. Phys., 78, 246–252 (2002), https://doi.org/10.1111/j.1151-2916.1988.tb07528.x.
B. Bastide, P. Canale, and P. Odier, “Characterization of a new ternary Ce–Y–tetragonal zirconia,” J. Eur. Ceram. Soc., 5, 289–293 (1989).
R. Annamalai, N. Nagaraju, K.D. Agrawal, and A. Muthuchamy, “Effect of heating mode on sinterability of YSZ + CeO2 ceramic,” Metals, 8, 189 (2018).
J.-D. Lin and J.G. Duh, “Mechanical properties and resistance to hydrothermal aging of ceria-and yttria-doped tetragonal zirconia ceramics,” Mater. Chem. Phys., 77, 808–818 (2003).
J. Lin and J. Duh, “Fracture toughness and hardness of ceria-and yttria-doped tetragonal zirconia ceramics,” Mater. Chem. Phys., 78, 253–261 (2003).
X. Huang, A. Zakurdaev, and D. Wang, “Microstructure and phase transformation of zirconia-based ternary oxides for thermal barrier coating applications,” J. Mater. Sci., 43, 2631–2641 (2008).
E. Altuncu and F. Ustel, “Optimization of APS process parameters using a design of experiment for CSZ (Y2O3–CeO2 stabilized zirconia) coatings,” in: 13th Int. Conf. Plasma Surface Engineering in Germany, No. 10–14, 295–298 (2012).
M. Hajizadeh-Oghaz, R. Shoja Razavi, and A. Ghasemi, “Synthesis and characterization of ceria–yttria co-stabilized zirconia (CYSZ) nanoparticles by sol-gel process for thermal barrier coatings (TBCs) applications,” J. Sol-Gel Sci. Technol., 74, 603–612 (2015).
X.-J. Jin, “Martensitic transformation in zirconia containing ceramics and its applications,” Curr. Opin. Solid State Mater. Sci., 9, 313–318 (2005).
A.A. Bukaemskiy, D. Barrier, and G. Modolo, “Physical properties of 8 mol.% ceria doped yttria stabilised zirconia powder and ceramic and their behavior during annealing and sintering,” J. Eur. Ceram. Soc., 26, 1507–1515 (2006).
T.E. Konstantinova, I.A. Danilenko, and V.V. Tokii, “Zirconium dioxide nanopowders: production, analysis, and application,” Nanosyst. Nanomater. Nanotechnol., 2, Issue 2, 609–632 (2004).
Y.L. Zhang, X.J. Jin, Y.H. Rong, T.Y. Hsu (Xu Zuyao), D.Y. Jiang, and J.L. Shi, “On the t → m martensitic transformation in Ce–Y-TZP ceramics,” Acta Mater., 54, 1289–1295 (2006).
S.G. Huanga, J. Vleugelsa, L. Lib, O. Van der Biesta, and P.L. Wang, “Composition design and mechanical properties of mixed (Ce, Y)-TZP ceramics obtained from coated starting powders,” J. Eur. Ceram. Soc., 25, 3109–3115 (2005).
S. Sharma, N. Gokhale, R. Dayal, and R. Lal, “Synthesis, microstructure and mechanical properties of ceria stabilized tetragonal zirconia prepared by spray drying technique,” Bull. Mater. Sci., 25, No. 1, 15–20 (2002).
K. Suresh Kumar and T. Mathews, “Sol-gel synthesis and microwave assisted sintering of zirconia-ceria solid solution,” J. Alloys Compd., 391, No. 1, 177–180 (2005).
E.N. Makarova and I.V. Antsiferova, “Studying the solubility of ZrO2–Y2O3–CeO2–Al2O3 nanopowders in an aqueous environment at different pH values,” Izv. Vuz. Poroshk. Metall. Funkts. Pokr., No. 4, 11–16 (2016).
R. Feng, X. Yang, W. Ji, and C. Au, “Hydrothermal synthesis of stable mesoporous ZrO2–Y2O3 and CeO2–ZrO2–Y2O3 from simple inorganic salts and CTAB template in aqueous medium,” Mater. Chem. Phys., 107, 132–136 (2008).
W.S. Lee, S.W. Kim, B.H. Koo, and D.S. Bae, “Synthesis and microstructure of Y2O3-doped ZrO2–CeO2 composite nanoparticles by hydrothermal process,” Colloids Surf A: Physicochem. Eng. Aspects, 313, 100–104 (2008).
N.Yu. Koval’ko, M.V. Kalinina, T.P. Maslennikova, L.V. Morozova, S.V. Myakin, T.V. Khamova, M.Yu. Arsent’ev, and O.A. Shilova, “Comparative study of powders based on the ZrO2–Y2O3–CeO2 system obtained by various liquid phase methods of synthesis,” Glass Phys. Chem., 44, No. 5, 433–439 (2018).
S. Somiya and T. Akiba, “Hydrothermal zirconia powders: A Bibliography,” J. Eur. Ceram. Soc., 19, Issue 1, 81–87 (1999).
A.V. Shevchenko, “Hydrothermal technologies in materials science. Inorganic materials science,” in: Fundamentals of Materials Science [in Russian], Kyiv (2008), Vol. 2, pp. 272–281.
O. Vasylkiv and Y. Sakka, “Hydroxide synthesis, colloidal processing and sintering of nano-size 3Y-TZP powder,” Scr. Mater., 44, 2219–2223 (2001).
E.V. Dudnik, “Modern methods for hydrothermal synthesis of ZrO2-based nanocrystalline powders,” Powder Metall. Met. Ceram., 48, No. 3–4, 238–248 (2009).
Yu.M. Fedenko, T.A. Dontsova, and I.M. Astrelin, “Turbidimetric method for assessing the sizes of nanoparticles in white ZrO2 sols,” Nauk. Visti NTUU KPI, Issue 1, 155–158 (2012).
M. Taguchi, T. Nakane, A. Matsushita, Y. Sakka, T. Uchikoshi, T. Funazukuri, and T. Naka, “One-pot synthesis of monoclinic ZrO2 nanocrystals under subcritical hydrothermal conditions,” J. Supercrit. Fluids, 85, 57–61 (2014).
E.V. Dudnik and A.V. Shevchenko, “Variation in properties of ZrO2–Y2O3–CeO2–Al2O3 powders during thermal treatment at 400 to 1300°C,” Powder Metall. Met. Ceram., 49, No. 3–4, 125–134 (2010).
L.M. Rudkovskaya, R.N. Pshenichnyy, T.V. Pavlenko, and A.A. Omelchuk, “Nanostructured zirconium diozide synthesized by hydrothermal synthesis from zirconium concentrate decomposition products,” Nanosyst. Nanomater. Nanotechnol., 10, No. 2, 351–360 (2012).
Ch.V. Reddy, I.N. Reddy, J. Shim, D. Kim, and K. Yoo, “Synthesis and structural, optical, photocatalytic, and electrochemical properties of undoped and yttrium-doped tetragonal ZrO2 nanoparticles,” Ceram. Int., 44, No. 11, 12329–12339 (2018).
G. Growth, L. Fei, L. Yanhuai, S. Zhongxiao, X. Kewei, M. Dayan, G. Bo, and C. Hon, “Characteristics of hydrothermally prepared yttria stabilized zirconia nanocrystals during calcination,” Rare Met. Mater. Eng., 46, No. 4, 899–905 (2017).
C. Duran, K. Sato, Y. Hotta, and H. Göçmez, “Ball milling assisted hydrothermal synthesis of ZrO2 nanopowders,” Ceram. Int., 41, 5588–5593 (2015).
A.O. Hongmin, L.U. Xiangsheng, Z. He, Z. Jing, H. Xiaowei, F. Zongyu, and X. Hong, “Preparation of scandia stabilized zirconia powder using microwave-hydrothermal method,” J. Rare Earths, 33, No. 7, 746–751 (2015).
X. Gan, Z. Yu, K. Yuan, C. Xu, X. Wang, L. Zhu, G. Zhang, and D. Xu, “Preparation of a CeO2-nanoparticle thermal radiation shield coating on ZrO2 fibers via a hydrothermal method,” Ceram. Int. (2017), https://doi.org/10.1016/j.ceramint.2017.07. 161.
L.J. Zhao, W. Zai, M.H. Wong, and H.C. Man, “Hydrothermal synthesis of Ag–ZrO2/r–GO coating on CoCrMo substrate,” Mater. Lett., 228, 314–317 (2018).
A. Behbahani, S. Rowshanzamir, and A. Esmaeilifar, “Hydrothermal synthesis of zirconia nanoparticles from commercial zirconia,” Proc. Eng., 42, 908–917 (2012).
O. Vasylkiv, Y. Sakka, Y. Maeda, and V.V. Skorokhod, “Nano-engineering of zirconia-noble metals composites,” J. Eur. Ceram. Soc., 24, No. 2, 469–473 (2004).
A.I. Ramos-Guerra, I. Martínez-Merlín, and C. Falcony, “The role of the stabilizing agent on the structural and luminescent properties of hydrothermally synthesized ZrO2: Tb3+ phosphors,” Ceram. Int. (2018), [https://doi.org/10.1016/j.ceramint.2018.04.216].
A.V. Shechenko, E.V. Dudnik, V.V. Tsukrenko, V.P. Red’ko, and L.M. Lopato, “Microstructural design of bioinert composites in the ZrO2–Y2O3–CeO2–Al2O3–CoO system,” Powder Metall. Met. Ceram., 51, No. 11–12, 724–733 (2013).
E.V. Dudnik, A.V. Shevchenko, A.K. Ruban, V.P. Red’ko, and L.M. Lopato, “Microstructural design of ZrO2–Y2O3–CeO2–Al2O3 materials,” Powder Metall. Met. Ceram., 49, No. 9–10, 528–536 (2011).
A.V. Shevchenko, E.V. Dudnik, A.K. Ruban, V.M. Vereshchaka, V.P. Red’ko, and L.M. Lopato, “Hydrothermal synthesis of nanocrystalline powders in the ZrO2–Y2O3–CeO2 system,” Powder Metall. Met. Ceram., 46, No. 1–2, 18–24 (2007).
I.O. Marek, O.K. Ruban, V.P. Red’ko, M.I. Danilenko, and O.V. Dudnik, “Physicochemical properties of hydrothermal nanocrystalline ZrO2–Y2O3–CeO2 powders,” Powder Metall. Met. Ceram., 58, No. 3–4, 125–132 (2019).
R.D. Shannon, “Revised effective ionic-radii and systematic studies of interatomic distances in halides and chalcogenides,” Acta Crystallogr., 32, 751–767 (1976).
G.Ya. Akimov, E.V. Chaika, V.M. Timchenko, G.A. Marinin, and V.V. Burkhovetskii, “Wear of magnesia- and ceria-stabilized zirconia ceramics in friction against steel without a lubricant,” Ogneup. Tekh. Keram., 30, No. 5, 511–515 (2009).
O.V. Dudnik, I.O. Marek, O.K. Ruban, V.P. Red’ko, M.I. Danilenko, S.A. Kornii, and L.M. Melakh, “Effect of heat treatment on the structure and phase composition of the nanosized powder based on a ZrO2 solid solution,” Powder Metall. Met. Ceram., 59, No. 1–2, 1–8 (2020).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkova Metallurgiya, Vol. 60, Nos. 7–8 (540), pp. 3–15, 2021.
Rights and permissions
About this article
Cite this article
Marek, I.O., Dudnik, O.V., Korniy, S.A. et al. Effect of Heat Treatment in the Temperature Range 400−1300°C on the Properties of Nanocrystalline ZrO2−Y2O3−CeO2 Powders. Powder Metall Met Ceram 60, 385–395 (2021). https://doi.org/10.1007/s11106-021-00251-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-021-00251-z