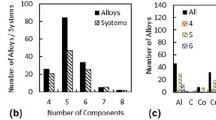
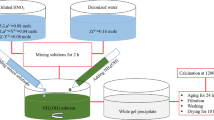
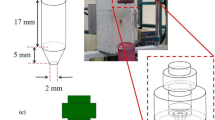
It is shown that the phase diagrams of refractory oxide systems based on ZrO2, HfO2, Al2O3, and rare earth oxides underlie the microstructural design of various high-performance materials. Process steps to produce coarse-grained ceramics in the HfO2–ZrO2–Y2O3, ZrO2–Y2O3–Sc2O3, HfO2–ZrO2–Sc2O3, Y2O3–Er2O3, Y2O3–ZrO2, Y2O3–HfO2, Y2O3–Al2O3, Y2O3–SiO2, and Y2O3–La2O3 systems to perform at temperatures up to 2200°C are designed. Process steps to produce high-performance fine-grained composites in the HfO2–ZrO2–Y2O3 (Ln2O3) (Ln–Dy, Ho, Er, Tm, Yb), ZrO2–Y2O3–Sc2O3, ZrO2–Y2O3–Sc2O3, Al2O3–Zr(Hf)O2–Ln(Y)2O3 (Ln–La, Nd, Sm, Gd, Er, Yb), and ZrO2–Y2O3–CeO2–Al2O3 systems are designed as well.






Similar content being viewed by others
References
A. V. Shevchenko, Phase Diagrams of Zirconium and Hafnium Oxides with Rare Earth Elements as Physicochemical Basis for New Materials [in Russian], ScD Thesis, Kiev (2007), p. 531.
I. V. Tananaev, V. B. Fedorov, I. D. Morokhov, et al., “Physicochemical fundamentals of metastable ultrafine substances and prospects of their application,” Izv. Akad. Nauk SSSR. Neorg. Mater., 20, No. 6, 1026–1033 (1984).
V. V. Skorokhod, “Hierarchy of structural levels and structural engineering of inorganic materials,” in: Inorganic Materials Science [in Russian], Naukova Dumka, Kiev (2008). Vol. 1, 339–357.
A. V. Shevchenko, L. M. Lopato, T. V. Obolonchik, et al., “Liquidus surface of the HfO2–ZrO2–Y2O3 system,” Izv. Akad. Nauk SSSR. Neorg. Mater., 23, No. 3, 452–455 (1987).
A. V. Shevchenko, L. M. Lopato, V. D. Tkachenko, and A. K. Ruban, “Interaction of hafnium and zirconium dioxides,” Izv. Akad. Nauk SSSR. Neorg. Mater., 23, No. 2, 259–263 (1987).
L. M. Lopato, V. P. Red’ko, G. I. Gerasimyuk, and A. V. Shevchenko, “Synthesis of some REE zirconates (hafnates),” Powder Metall. Met. Ceram., 29, No. 4, 318–320 (1990).
T. V. Obolonchik, L. M. Lopato, G. I. Gerasimyuk, and A. V. Shevchenko, “Interaction in the HfO2−ZrO2−Y2O3 system at 1250–1900°C,” Izv. Akad. Nauk SSSR. Neorg. Mater., 27, No. 11, 2345–2349 (1991).
N. A. Toropov, V. P. Borzakovskii, V. V. Lapin, and N. N. Kurtseva, Phase Diagrams of Silicate Systems: Handbook [in Russian], Nauka, Leningrad (1969), p. 435.
L. M. Lopato, B. S. Nigmanov, A. V. Shevchenko, and Z. A. Zaitseva, “Interaction between lanthanum and yttrium oxides,” Izv. Akad. Nauk SSSR. Neorg. Mater., 22, No. 5, 771–774 (1986).
I. M. Maister, L. M. Lopato, A. V. Shevchenko, and B. S. Nigmanov, “The Er2O3–Y2O3 system,” Izv. Akad. Nauk SSSR. Neorg. Mater., 20, No. 3, 446–448 (1984).
S. N. Lakiza, L. M. Lopato, and A. V. Shevchenko, “Interactions in the Al2O3–ZrO2–Y2O3 system,” Powder Metall. Met. Ceram., 33, No. 9–10, 486–490 (1994).
A. V. Shevchenko, L. M. Lopato, and I. E. Kiryakova, “Interaction of HfO2 with Y2O3, Ho2O3, Er2O3, Tm2O3, Yb2O3, and Lu2O3,” Izv. Akad. Nauk SSSR. Neorg. Mater., 20, No. 12, 1991–1996 (1984).
A. K. Ruban, A. V. Shevchenko, and L. M. Lopato, “Ceramic rods for precision casting of low-doped chromium,” in: Structural Chromium Alloys [in Russian], Naukova Dumka, Kiev (1986), pp. 104–109.
L. I. Lugin, L. M. Lopato, G. I. Gerasimyuk, and A. V. Shevchenko, “Phase relations in the La2O3–CaO and Ce2O3–CaO systems,” Ukr. Khim. Zh., 38, No. 2, 143–148 (1972).
L. M. Lopato, G. I. Gerasimyuk, A. V. Shevchenko, and S. G. Tresvyatskii, “Phase equilibria in the Dy2O3–CaO, Y2O3–CaO, and Yb2O3–CaO systems,” Izv. Akad. Nauk SSSR. Neorg. Mater., 9, No. 3, 157–162 (1973).
A. V. Shevchenko, L. M. Lopato, and G. I. Gerasimyuk, Refractory Material [in Russian], USSR Inventor’s Certificate 589235, IPC C0 4 B 35/20, No. 2182297/29-33; Bulletin No. 3; appl. October 17, 1975; publ. January 25 (1978), p. 7.
A. K. Ruban, A. V. Shevchenko, A. N. Rakitskii, et al., “Production of crucibles from rare earth oxides for melting of low-doped chromium in induction furnaces,” in: Structural Chromium Alloys [in Russian], Naukova Dumka, Kiev (1986), pp. 120–128.
A. N. Rakitskii, T. L. Kuznetsova, I. L. Yakimenko, et al., “Induction melting of low-doped chromium alloys in yttrium oxide crucibles,” in: Structural Chromium Alloys [in Russian], Naukova Dumka, Kiev (1986), pp. 115–120.
A. V. Shevchenko, A. K. Ruban, T. V. Obolonchik, et al., “Production of shell molds for shaped casting of low-doped chromium,” in: Structural Chromium Alloys [in Russian], Naukova Dumka, Kiev (1988), pp. 110–114.
A. V. Zyrin, V. P. Red’ko, L. M. Lopato, and A. V. Shevchenko, “Ordered phases in the ZrO2–Sc2O3 and HfO2–Sc2O3 systems,” Izv. Akad. Nauk SSSR. Neorg. Mater., 23, No. 8, 1326–1329 (1987).
A. V. Zyrin, A. V. Shevchenko, and L. M. Lopato, “Electrical properties of phases in the ZrO2–Y2O3–La2O3 system,” Powder Metall. Met. Ceram., 39, No. 3–4, 146–150 (2000).
A. V. Shevchenko, I. M. Maister, L. M. Lopato, and V. D. Tkachenko, “Interaction in the HfO2–ZrO2–Sc2O3 system at 1700–2850°C,” Izv. Akad. Nauk SSSR. Neorg. Mater., 25, No. 6, 989–993 (1989).
A. V. Shevchenko, I. M. Maister, and L. M. Lopato, “Interaction in the HfO2–Sc2O3 and ZrO2–Sc2O3 systems at high temperatures,” Izv. Akad. Nauk SSSR. Neorg. Mater., 23, No. 8, 1320–1324 (1987).
I. M. Maister, L. M. Lopato, Z. A. Zaitseva, and A. V. Shevchenko, “Interaction in the ZrO2–Y2O3–Sc2O3 system at 1300–1900°C,” Izv. Akad. Nauk SSSR. Neorg. Mater., 27, No. 11, 2337–2340 (1991).
A. V. Shevchenko, L. M. Lopato, and I. M. Maister, “Liquidus surface of the ZrO2–Y2O3–Sc2O3 system,” Izv. Akad. Nauk SSSR. Neorg. Mater., 27, No. 12, 2673–2676 (1991).
A. V. Shevchenko, V. A. Dubok, E. V. Dudnik, et al., “Transparent ceramics based on yttrium subgroup lanthanide, yttrium, and scandium oxides,” Powder Metall. Met. Ceram., 49, No. 9–10, 537–545 (2010).
S. M. Lakiza and L. M. Lopato, “Stable and metastable phase relations in the system alumina–zirconia–yttria,” J. Am. Ceram. Soc., 80, No. 4, 893–902 (1997).
S. M. Lakiza, “Directionally solidified eutectics in the Al2O3–ZrO2–Ln2O3 systems,” Teor. Prakt. Metall., No. 4–5, 126–127 (2006).
S. M. Lakiza, J. S. Tyschenko, and L. M. Lopato, “Phase diagrams of the systems Al2O3–Zr(Hf)O2–Ln(Y)2O3 as a scientific base for creating new high-temperature structural ceramics,” in: Proc. Symp. J. Functional and Structural Ceramics and Ceram. Matrix Composites (September 15–19, 2008), Warsaw (2008), pp. 58–69.
S. M. Lakiza, J. S. Tyschenko, and L. M. Lopato, “Phase diagram of the Al2O3–HfO2–Y2O3 system,” J. Eur. Ceram. Soc., 31, 1285–1291 (2011).
J. S. Tyschenko, S. M. Lakiza, and L. M. Lopato, “Phase diagram of the Al2O3–HfO2–Er2O3 system,” J. Am. Ceram. Soc., 95, 4015–4020 (2012).
E. V. Dudnik, Physicochemical Fundamentals for Developing ZrO2-Based Materials [in Russian], ScD Thesis, Kiev (2010), p. 403.
A. V. Shevchenko, E. V. Dudnik, A. K. Ruban, et al., “Hydrothermal synthesis of nanocrystalline powders in the ZrO2–Y2O3–CeO2 system,” Powder Metall. Met. Ceram., 46, No. 1–2, 18–24 (2007).
E. V. Dudnik, A. V. Shevchenko, A. K. Ruban, et al., “Microstructural design of ZrO2–Y2O3–CeO2–Al2O3 materials,” Powder Metall. Met. Ceram., 49, No. 9–10, 528–536 (2010).
A. V. Shevchenko, E. V. Dudnik, V. V. Tsukrenko, et al., “Microstructural design of bioinert composites in the ZrO2–Y2O3–CeO2–Al2O3–CoC system,” Powder Metall. Met. Ceram., 51, No. 11–12, 724–733 (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
*Deceased.
Translated from Poroshkovaya Metallurgiya, Vol. 53, No. 5–6 (497), pp. 67–78, 2014.
Rights and permissions
About this article
Cite this article
Dudnik, E.V., Lakiza, S.N., Tishchenko, Y.S. et al. Phase Diagrams of Refractory Oxide Systems and Microstructural Design of Materials. Powder Metall Met Ceram 53, 303–311 (2014). https://doi.org/10.1007/s11106-014-9617-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-014-9617-z