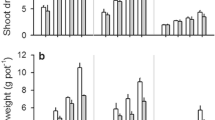
Abstract
Background and aims
Plant adaptation to waterlogged conditions requires a set of morphological and physiological/biochemical changes. The formation of aerenchyma is one of the most crucial adaptive traits for waterlogging tolerance. Enzymatic scavenging may also potentially contribute to waterlogging tolerance by providing detoxification of reactive oxygen species (ROS).
Methods
Changes of root porosity (as an indicator of aerenchyma formation) and activities in leaves of four major antioxidant enzymes, γ-amino butyric acid (GABA) and lactic acid contents in roots were evaluated in six barley genotypes contrasting in waterlogging tolerance.
Results
Soil waterlogging caused significant increases in adventitious root porosity in all genotypes. Waterlogging-tolerant genotypes showed not only significantly higher adventitious root porosity than sensitive genotypes but also much faster development of aerenchyma. The greatest difference in adventitious root porosity among genotypes was observed after 7 days of waterlogging treatment. At the same time, antioxidant enzyme activities in leaves, GABA and lactic acid contents in roots did not correlate with waterlogging tolerance.
Conclusions
A faster formation of aerenchyma in adventitious roots is one of the key factors for waterlogging tolerance in barley. This protocol is recommended to be applied in future studies to identify molecular markers linked to this trait using appropriate mapping populations.






Similar content being viewed by others
References
Aebi H, Packer L (1984) Methods in enzymology. Academic, New York
Arbona V, Hossain Z, López-Climent MF, Pérez-Clemente RM, Gómez-Cadenas A (2008) Antioxidant enzymatic activity is linked to waterlogging stress tolerance in citrus. Physiol Plant 132:452–466. doi:10.1111/j.1399-3054.2007.01029.x
Armstrong W (1979) Aeration in higher plants, vol 7. Advances in Botanical Research, London
Armstrong J, Armstrong W (1999) Phragmites die-back: toxic effects of propionic, butyric and caproic acids in relation to pH. New Phytol 142:201–217. doi:10.1046/j.1469-8137.1999.00395.x
Bai Q, Chai M, Gu Z, Cao X, Li Y, Liu K (2009) Effects of components in culture medium on glutamate decarboxylase activity and γ-aminobutyric acid accumulation in foxtail millet (Setaria italica L.) during germination. Food Chem 116:152–157. doi:10.1016/j.foodchem.2009.02.022
Bailey-Serres J, Chang R (2005) Sensing and signalling in response to oxygen deprivation in plants and other organisms. Ann Bot 96:507–518. doi:10.1093/aob/mci206
Bailey-Serres J, Voesenek LA (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59:313–339. doi:10.1146/annurev.arplant.59.032607.092752
Barrett-Lennard EG (2003) The interaction between waterlogging and salinity in higher plants: causes, consequences and implications. Plant Soil 253:35–54
Baxter A, Mittler R, Suzuki N (2014) ROS as key players in plant stress signalling. J Exp Bot 65:1229–1240. doi:10.1093/jxb/ert375
Beyer WF Jr, Fridovich I (1987) Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal Biochem 161:559–566. doi:10.1016/0003-2697(87)90489-1
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Broughton S, Zhou GF, Teakle LN, Matsuda R, Zhou MX, O’Leary AR, Colmer DT, Li CD (2015) Waterlogging tolerance is associated with root porosity in barley (Hordeum vulgare L.). Mol Breed 35:27. doi:10.1007/s11032-015-0243-3
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–775
Collaku A, Harrison SA (2005) Heritability of waterlogging tolerance in wheat. Crop Sci 45:722–727
Colmer TD (2003a) Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.). Ann Bot 91:301–309
Colmer TD (2003b) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ 26:17–36
Colmer TD, Greenway H (2011) Ion transport in seminal and adventitious roots of cereals during O2 deficiency. J Exp Bot 62:39–57. doi:10.1093/jxb/erq271
Colmer TD, Voesenek LACJ (2009) Flooding tolerance: suites of plant traits in variable environments. Funct Plant Biol 36:665–681
Damanik R, Maziah M, Ismail M, Ahmad S, Zain A (2010) Responses of the antioxidative enzymes in Malaysian rice (Oryza sativa L.) cultivars under submergence condition. Acta Physiol Plant 32:739–747. doi:10.1007/s11738-009-0456-3
de San P, Celedonio R, Abeledo LG, Miralles D (2014) Identifying the critical period for waterlogging on yield and its components in wheat and barley. Plant Soil 378:265–277. doi:10.1007/s11104-014-2028-6
Drew MC (1997) Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Biol 48:223–250
Drew MC, Saglio PH, Pradet A (1985) Larger adenylate energy charge and ATP/ADP ratios in aerenchymatous roots of Zea mays in anaerobic media as a consequence of improved internal oxygen transport. Planta 165:51–58. doi:10.1007/bf00392211
Evans DE (2003) Aerenchyma formation. New Phytol 161:35–49
Fan Y, Zhu M, Shabala S, Li CD, Johnson P, Zhou MX (2014) Antioxidant activity in salt-stressed barley leaves: evaluating time- and age-dependence and suitability for the use as a biochemical marker in breeding programs. J Agron Crop Sci 200:261–272. doi:10.1111/jac.12068
Felle HH (2005) pH regulation in anoxic plants. Ann Bot 96:519–532. doi:10.1093/aob/mci207
Ferner E, Rennenberg H, Kreuzwieser J (2012) Effect of flooding on C metabolism of flood-tolerant (Quercus robur) and non-tolerant (Fagus sylvatica) tree species. Tree Physiol 32:135–145. doi:10.1093/treephys/tps009
Garthwaite AJ, von Bothmer R, Colmer TD (2003) Diversity in root aeration traits associated with waterlogging tolerance in the genus Hordeum. Funct Plant Biol 30:875–889
Gibberd MR, Colmer TD, Cocks PS (1999) Root porosity and oxygen movement in waterlogging-tolerant Trifolium tomentosum and -intolerant Trifolium glomeratum. Plant Cell Environ 22:1161–1168. doi:10.1046/j.1365-3040.1999.00472.x
Gibbs J, Greenway H (2003) Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct Plant Biol 30:999–1036
Hossain Z, Lopez-Climent MF, Arbona V, Perez-Clemente RM, Gomez-Cadenas A (2009) Modulation of the antioxidant system in citrus under waterlogging and subsequent drainage. J Plant Physiol 166:1391–1404. doi:10.1016/j.jplph.2009.02.012
Huang X, Shabala S, Shabala L, Rengel Z, Wu X, Zhang G, Zhou M (2014) Linking waterlogging tolerance with Mn2+ toxicity: a case study for barley. Plant Biol. doi:10.1111/plb.12188
Jaeger C, Gessler A, Biller S, Rennenberg H, Kreuzwieser J (2009) Differences in C metabolism of ash species and provenances as a consequence of root oxygen deprivation by waterlogging. J Exp Bot. doi:10.1093/jxb/erp268
Khabaz-Saberi H, Setter TL, Waters I (2005) Waterlogging induces high to toxic concentrations of iron, aluminum, and manganese in wheat varieties on acidic soil. J Plant Nutr 29:899–911. doi:10.1080/01904160600649161
Kreuzwieser J, Fürniss S, Rennenberg H (2002) Impact of waterlogging on the N-metabolism of flood tolerant and non-tolerant tree species. Plant Cell Environ 25:1039–1049. doi:10.1046/j.1365-3040.2002.00886.x
Kreuzwieser J, Hauberg J, Howell KA, Carroll A, Rennenberg H, Millar AH, Whelan J (2009) Differential response of gray poplar leaves and roots underpins stress adaptation during hypoxia. Plant Physiol 149:461–473
Kumutha D, Ezhilmathi K, Sairam RK, Srivastava GC, Deshmukh PS, Meena RC (2009) Waterlogging induced oxidative stress and antioxidant activity in pigeonpea genotypes. Biol Plant 53:75–84. doi:10.1007/s10535-009-0011-5
Lee K-W, Chen PW, Yu S-M (2014) Metabolic adaptation to sugar/O2 deficiency for anaerobic germination and seedling growth in rice. Plant Cell Environ 37:2234–2244. doi:10.1111/pce.12311
Malik AI, Colmer TD, Lambers H, Schortemeyer M (2001) Changes in physiological and morphological traits of roots and shoots of wheat in response to different depths of waterlogging. Aust J Plant Physiol 28:1121–1131
Malik AI, English JP, Colmer TD (2009) Tolerance of Hordeum marinum accessions to O2 deficiency, salinity and these stresses combined. Ann Bot 103:237–248. doi:10.1093/aob/mcn142
Mano Y, Omori F (2008) Verification of QTL controlling root aerenchyma formation in a maize × teosinte “Zea nicaraguensis” advanced backcross population. Breed Sci 58:217–223
Mano Y, Omori F (2013) Relationship between constitutive root aerenchyma formation and flooding tolerance in Zea nicaraguensis. Plant Soil 370:1–14. doi:10.1007/s11104-013-1641-0
Mcdonald MP, Galwey NW, Colmer TD (2001) Waterlogging tolerance in the tribe Triticeae: the adventitious roots of Critesion marinum have a relatively high porosity and a barrier to radial oxygen loss. Plant Cell Environ 24:585–596
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Pang J, Zhou M, Mendham N, Shabala S (2004) Growth and physiological responses of six barley genotypes to waterlogging and subsequent recovery. Aust J Agric Res 55:895–906. doi:10.1071/ar03097
Pang J, Cuin T, Shabala L, Zhou M, Mendham N, Shabala S (2007a) Effect of secondary metabolites associated with anaerobic soil conditions on ion fluxes and electrophysiology in barley roots. Plant Physiol 145:266–276. doi:10.1104/pp. 107.102624
Pang J, Ross J, Zhou M, Mendham N, Shabala S (2007b) Amelioration of detrimental effects of waterlogging by foliar nutrient sprays in barley. Funct Plant Biol 34:221–227. doi:10.1071/fp06158
Raskin I (1983) A method for measuring leaf volume, density, thickness, and internal gas volume. HortScience 18:698–699
Ratcliffe RG (1997) In vivo NMR studies of the metabolic response of plant tissues to anoxia. Ann Bot 79:39–48
Reggiani R, Zaina S, Bertani A (1992) Plasmalemma ATPase in rice coleoptiles; stimulation by putrescine and polyamines. Phytochemistry 31:417–419. doi:10.1016/0031-9422(92)90009-F
Rivoal J, Hanson AD (1993) Evidence for a large and sustained glycolytic flux to lactate in anoxic roots of some members of the halophytic genus Limonium. Plant Physiol 101:553–560
Rocha M, Licausi F, Araújo WL, Nunes-Nesi A, Sodek L, Fernie AR, van Dongen JT (2010) Glycolysis and the tricarboxylic acid cycle are linked by alanine aminotransferase during hypoxia induced by waterlogging of Lotus japonicus. Plant Physiol 152:1501–1513
Sairam RK, Srivastava GC (2001) Water stress tolerance of wheat (Triticum aestivum L.): variations in hydrogen peroxide accumulation and antioxidant activity in tolerant and susceptible genotypes. J Agron Crop Sci 186:63–70. doi:10.1046/j.1439-037x.2001.00461.x
Sairam RK, Kumutha D, Ezhilmathi K, Chinnusamy V, Meena RC (2009) Waterlogging induced oxidative stress and antioxidant enzyme activities in pigeon pea. Biol Plant 53:493–504. doi:10.1007/s10535-009-0090-3
Sauter M (2013) Root responses to flooding. Curr Opin Plant Biol 16:282–286. doi:10.1016/j.pbi.2013.03.013
Setter TL, Waters I (2003) Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant Soil 253:1–34
Setter TL, Burgess P, Water I, Kuo J (1999) Genetic diversity of barley and wheat for waterlogging tolerance in Western Australia. In: Proceeding 9th Australian Barley Technical Symposium, Melbourne, Australia, 1999. Hindawi Publishing Corporation, Egypt
Shabala S (2011) Physiological and cellular aspects of phytotoxicity tolerance in plants: the role of membrane transporters and implications for crop breeding for waterlogging tolerance. New Phytol 190:289–298. doi:10.1111/j.1469-8137.2010.03575.x
Shabala S, Shabala L, Barcelo J, Poschenrieder C (2014) Membrane transporters mediating root signalling and adaptive responses to oxygen deprivation and soil flooding. Plant Cell Environ 37:2216–2233. doi:10.1111/pce.12339
Shimamura S, Yamamoto R, Nakamura T, Shimada S, Komatsu S (2010) Stem hypertrophic lenticels and secondary aerenchyma enable oxygen transport to roots of soybean in flooded soil. Ann Bot 106:277–284. doi:10.1093/aob/mcq123
Steffens B, Geske T, Sauter M (2010) Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytol 190:369–378. doi:10.1111/j.1469-8137.2010.03496.x
Striker GG, Casas C, Manzur ME, Ploschuk RA, Casal JJ (2014) Phenomic networks reveal largely independent root and shoot adjustment in waterlogged plants of Lotus japonicus. Plant Cell Environ 37:2278–2293. doi:10.1111/pce.12268
Takahashi H, Greenway H, Matsumura H, Tsutsumi N, Nakazono M (2014) Rice alcohol dehydrogenase 1 promotes survival and has a major impact on carbohydrate metabolism in the embryo and endosperm when seeds are germinated in partially oxygenated water. Ann Bot 113:851–859. doi:10.1093/aob/mct305
Tan W, Liu J, Dai T, Jing Q, Cao W, Jiang D (2008) Alterations in photosynthesis and antioxidant enzyme activity in winter wheat subjected to post-anthesis water-logging. Photosynthetica 46:21–27. doi:10.1007/s11099-008-0005-0
Tang B, Xu S-z, Zou X-l, Zheng Y-l, Qiu F-z (2010) Changes of antioxidative enzymes and lipid peroxidation in leaves and roots of waterlogging-tolerant and waterlogging-sensitive maize genotypes at seedling stage. Agricult Sci China 9:651–661. doi:10.1016/S1671-2927(09)60140-1
Teakle NL, Armstrong J, Barrett-Lennard EG, Colmer TD (2011) Aerenchymatous phellem in hypocotyl and roots enables O2 transport in Melilotus siculus. New Phytol 190:340–350. doi:10.1111/j.1469-8137.2011.03655.x
Thomas AL, Guerreiro SMC, Sodek L (2005) Aerenchyma formation and recovery from hypoxia of the flooded root system of nodulated soybean. Ann Bot 96:1191–1198. doi:10.1093/aob/mci272
Thomson CJ, Armstrong W, Waters I, Greenway H (1990) Aerenchyma formation and associated oxygen movement in seminal and nodal roots of wheat. Plant Cell Environ 13:395–403
Ushimaru T, Kanematsu S, Shibasaka M, Tsuji H (1999) Effect of hypoxia on the antioxidative enzymes in aerobically grown rice (Oryza sativa) seedlings. Physiol Plant 107:181–187. doi:10.1034/j.1399-3054.1999.100205.x
Ushimaru T, Kanematsu S, Katayama M, Tsuji H (2001) Antioxidative enzymes in seedlings of Nelumbo nucifera germinated under water. Physiol Plant 112:39–46. doi:10.1034/j.1399-3054.2001.1120106.x
Voesenek L, Bailey-Serres J (2013) Flooding tolerance: O2 sensing and survival strategies. Curr Opin Plant Biol 16:647–653. doi:10.1016/j.pbi.2013.06.008
Voesenek LACJ, Bailey-Serres J (2015) Flood adaptive traits and processes: an overview. New Phytol 206:57–73. doi:10.1111/nph.13209
Voesenek LACJ, Sasidharan R (2013) Ethylene—and oxygen signalling—drive plant survival during flooding. Plant Biol 15:426–435. doi:10.1111/plb.12014
Voesenek LACJ, Armstrong WO, Gemann GM, Colmer TD, McDonald MP (1999) A lack of aerenchyma and high rates of radial oxygen loss from the root base contribute to the waterlogging intolerance of Brassica napus. Funct Plant Biol 26:87–93. doi:10.1071/PP98086
Wang K, Jiang Y (2007) Antioxidant responses of creeping bentgrass roots to waterlogging. Crop Sci 47:232–238. doi:10.2135/cropsci2006.07.0498
Xia J-H, Saglio PH (1992) Lactic acid efflux as a mechanism of hypoxic acclimation of maize root tips to anoxia. Plant Physiol 100:40–46
Yamauchi T et al (2014) Ethylene and reactive oxygen species are involved in root aerenchyma formation and adaptation of wheat seedlings to oxygen-deficient conditions. J Exp Bot 65:261–273. doi:10.1093/jxb/ert371
Yan B, Dai Q, Liu X, Huang S, Wang Z (1996) Flooding-induced membrane damage, lipid oxidation and activated oxygen generation in corn leaves. Plant Soil 179:261–268. doi:10.1007/bf00009336
Yordanova RY, Christov KN, Popova LP (2004) Antioxidative enzymes in barley plants subjected to soil flooding. Environ Exp Bot 51:93–101. doi:10.1016/S0098-8472(03)00063-7
Yu Q, Rengel Z (1999) Waterlogging influences plant growth and activities of superoxide dismutases in narrow-leafed lupin and transgenic tobacco plants. J Plant Physiol 155:431–438. doi:10.1016/S0176-1617(99)80127-8
Zhang G, Tanakamaru K, Abe J, Morita S (2007) Influence of waterlogging on some anti-oxidative enzymatic activities of two barley genotypes differing in anoxia tolerance. Acta Physiol Plant 29:171–176. doi:10.1007/s11738-006-0022-1
Zhou M (2010) Improvement of plant waterlogging tolerance. In: Mancuso S, Shabala S (eds) Waterlogging signalling and tolerance in plants. Springer, Heidelberg, pp 267–285. doi:10.1007/978-3-642-10305-6_13
Zhou M (2011) Accurate phenotyping reveals better QTL for waterlogging tolerance in barley. Plant Breed 130:203–208. doi:10.1111/j.1439-0523.2010.01792.x
Zhou W, Zhao D, Lin X (1997) Effects of waterlogging on nitrogen accumulation and alleviation of waterlogging damage by application of nitrogen fertilizer and mixtalol in winter rape (Brassica napus L.). J Plant Growth Reg 16:47–53
Zhou MX, Li HB, Mendham NJ (2007) Combining ability of waterlogging tolerance in barley. Crop Sci 47:278–284. doi:10.2135/cropsci2006.02.0065
Zhou M, Johnson P, Zhou G, Li C, Lance R (2012) Quantitative trait loci for waterlogging tolerance in a barley cross of Franklin × YuYaoXiangTian Erleng and the relationship between waterlogging and salinity tolerance. Crop Sci 52:2082. doi:10.2135/cropsci2012.01.0008
Acknowledgments
This work was supported by the Australian Research Council Linkage grant (project LP120200516) and the Grains Research and Development Corporation (GRDC) of Australia. We are also grateful to Dr Lukasz Kotula for his technical help in root porosity measurement and Professor Tim Colmer of The University of Western Australia for providing many useful suggestions on a draft of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Guillermo Santa Maria.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Summary of two-factorial (days of measurement and genotypes) ANOVA analysis of changes in root porosity in brown sodosol soil. (DOC 36 kb)
Table S2
Correlation coefficients between waterlogging tolerance score and adventitious root porosity of waterlogging across 42 days waterlogging stress among six barley genotypes. Waterlogging tolerance score is based on plant healthiness score after 9 weeks waterlogging treatment in brown sodosol soil. (DOC 34 kb)
Figure S1
The appearance of two contrasting genotypes after 6 weeks waterlogging growing in brown sodosol soil: TAM407227 (waterlogging tolerance score = 9.5) and Franklin (waterlogging tolerance score = 1.5) (PDF 103 kb)
Figure S2
Adventitious root porosity of six different barley genotypes in aerobic conditions (potting mixture) after 7, 14, 21, 28, 35, 42 days of three-leaf stage growing barley, the same period of growing stages with measuring adventitious root porosity under waterlogging conditions (Fig. 3). Values are the means ± standard deviations of 3 replicates. Each replicate represents only green leaves from 3 single plants growing in different tanks. (PDF 111 kb)
Rights and permissions
About this article
Cite this article
Zhang, X., Shabala, S., Koutoulis, A. et al. Waterlogging tolerance in barley is associated with faster aerenchyma formation in adventitious roots. Plant Soil 394, 355–372 (2015). https://doi.org/10.1007/s11104-015-2536-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2536-z