Abstract
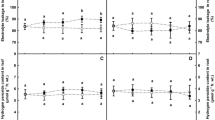
The experiment was conducted to investigate the formation of oxidative stress and the development of anti-oxidative enzymes in two barley genotypes differing in anoxia tolerance. Waterlogging led to significant reduction in root and shoot weight, green leaf area and tillers per plant, but tolerant Xiumai 3 was much less reduced than sensitive Gerdner. Malondialdehyde (MDA) content, an indicator of membrane lipid peroxidation, significantly increased in Gerdner when the plants were subjected to waterlogging, but remained little changed in Xiumai 3. Superoxide dismutase (SOD) activity was increased with waterlogging treatment and the sensitive cultivar had higher activity than the tolerant one during the experimental duration. At early stage of waterlogging treatment, both peroxidase (POD) and catalase (CAT) activities significantly increased in Xuimai 3, while obviously decreased in Gerdner. Moreover, both cultivars showed substantial increase in both POD and CAT with the progress of waterlogging exposure. Glutathione reductase (GR) activity was increased in both tolerant- and sensitive cultivars under waterlogging. It may be assumed from the current results that SOD activity appears to be not a constraining factor limiting the scavenging of ROS, and it is the change of POD and CAT activity under waterlogging that determine the status of oxidative stress. The difference between genotypes in waterlogging tolerance could be distinguished from the changed patterns of these enzymatic activities.





Similar content being viewed by others
References
Ahmed S, Nawata E, Hosokawa M, Domae Y, Sakuratani T (2002) Alterations in photosynthesis and some antioxidant enzymatic activities of mungbean subjected to waterlogging. Plant Sci 163:117–123
Armstrong W (1979) Aeration in higher plants. In: Woolhouse HW (ed) Advances in botanical research, vol 7. Academic, New York, pp 225–232
Asada K (1999) The water–water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Asada K, Takahashi M (1987) Production and scavenging of active oxygen in photosynthesis. In: Kyle DJ et al (eds) Photoinhibition. Elsevier, pp 227–287
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequence of minor changes in conditions. Annal Biochem 161:559–566
Bowler C, Slooten L, Vandenbranden S, Derycke R, Botterman J, Sybesma M, Van Montagu M, Inze D (1991) Manganese superoxide dismutase can reduce cellular damage mediated by oxygen radicals in transgenic plant. EMBO J 10:1723–1732
Broadbent P, Creissen GP, Kular B, Wellburn AR, Mullineaux P (1995) Oxidative stress responses in transgenic tobacco containing altered levels of glutathione reductase activity. Plant J 8:247–255
Crawford RMM (1978) Metabolic adaptation to anoxia. In: Hook DD, Crawford RMM (eds) Plant life in anaerobic environments. Ann Arbor Sci Publ, Ann Arbor, Michigan, pp 119–136
Creissen G, Firmin J, Fryer M, Kular B, Leyland N, Reynolds H, Pastori G, Wellburn F, Baker N, Wellburn A, Mullineaux P (1999) Elevated glutathione biosynthetic capacity in the chloroplasts of transgenic tobacco plants paradoxically causes increased oxidative stress. Plant Cell 11:1277–1292
Foyer C, Lelandais M, Kunert JJ (1994) Photooxidative stress in plants. Plant Physiol 92:696–717
Gossett DR, Millhollon EP, Lucas MC (1994) Antioxidant response to NaCl stress in salt-tolerant and salt-sensitive cultivars of cotton. Crop Sci 34:706–714
Hendry GAF, Thorpe PC, Merzlyak MN (1993) Stress indicators-lipid peroxidation. In: Hendry GAF, Grime JP (eds) Methods in comparative plant ecology. Chapman & Hall, London, pp 154–156
Hwang SY, Lin HW, Chem RH, Lo HF, Li F (1999) Reduction susceptibility to waterlogging together with highlight stress is related to increases in superoxide dismutase and catalase activities in sweet potato. Plant Growth Regul 27:167–172
Maehly PC, Chance M (1954) The assay of catalase and peroxidases. In: Gluck D (ed) Methods of biochemical analysis. Intersciences Publishers, New York, pp 357–424
Meloni DA, Oliva MO, Martinez CA, Cambraia J (2003) Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot 49:69–76
Noctor G, Foyer C (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Peters JL, Castillo FJ, Heath RH (1989) Alteration of extracellular enzymes in pinto bean leaves upon exposure to air pollution, ozone and sulfur dioxide. Plant Physiol 89:159–164
Richter C, Schweizer M (1997) Oxidative stress in mitochondria, in: Scandalios JG (ed) Oxidative stress and the molecular biology of antioxidant defense, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, Monograph Series, vol 34. pp 169–200
Schaedle M, Bassham JA (1977) Chloroplast glutathione reductase. Plant Physiol 59:1011–1012
Scandalias JG (1993) Oxygen stress and superoxide dismutase. Plant Physiol 101:7–12
Sgherri CLM, Pinzio C, Navari-Izzo F (1993) Chemical changes and O2 production in thylakoid membranes under water stress. Physiol Plant 87:211–216
Smirnoff J, Colombe SV (1988) Drought influences the activity if enzymes of the chloroplast hydrogen peroxide scavenging system. J Exp Bot 39:1097–1109
Wu FB, Zhang GP, Dominy P (2003) Four barley genotypes respond differently to cadmium: lipid peroxidation and activities of antioxidant capacity. Environ Exp Bot 50:67–77
Zhang J, Kirkham MB (1994) Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol 35:1259–1266
Zhang GP, Tanakamaru K, Abe J, Morita S (2006) Genotypic difference in the response of photosynthesis and soluble sugars to waterlogging in barley, Acta Physiologiae Plantarum (submitted for publication)
Acknowledgment
Authors are deeply indebted to Japan Society for the Promotion of Science for its financial support to this research program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Bartosz.
Rights and permissions
About this article
Cite this article
Zhang, G., Tanakamaru, K., Abe, J. et al. Influence of waterlogging on some anti-oxidative enzymatic activities of two barley genotypes differing in anoxia tolerance. Acta Physiol Plant 29, 171–176 (2007). https://doi.org/10.1007/s11738-006-0022-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-006-0022-1