Abstract
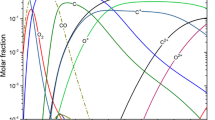
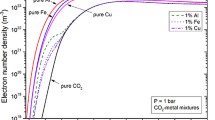
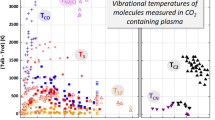
It has become increasingly clear that deviations from local thermodynamic equilibrium occur in thermal plasmas. This paper is devoted to investigating the non-equilibrium characteristics of CO2 thermal plasmas, which have wide application in industry. A two-temperature chemical kinetic model with a comprehensive chemical system is developed to calculate the non-equilibrium characteristics of CO2 thermal plasmas for a wide temperature range, from 12,000 to 500 K, at atmospheric pressure. The non-equilibrium results are compared to the equilibrium composition obtained by Gibbs free energy minimization, and significant deviations are found at lower temperatures. Based on the dependence of molar fractions on temperature, the dominant species are determined in three temperature ranges. The dominant reactions are then obtained by considering their contribution to the generation and loss of the dominant species. Using the dominant species and reactions, the full model is simplified into three simpler models and the accuracy of the simplified models is evaluated. It is shown that this approach greatly reduces the number of species and reactions considered, while showing good agreement with the full model, with a root-mean-square error of no more than 4 %. Thus, the complicated physicochemical processes in non-equilibrium CO2 thermal plasmas can be characterized by relatively few species and reactions. It is suggested that the two-temperature chemical kinetic model developed in this paper can be applied to the full range of pressures that occur in arc welding, arc quenching and other industrial applications. In addition, the simplified methods can be applied in multi-dimensional models to reduce the chemical complexity and computing time while capturing the main physicochemical processes in non-equilibrium CO2 thermal plasmas.






Similar content being viewed by others
References
Li TQ, Wu CS (2015) Int J Adv Manuf Technol 78(1–4):593–602
Liu ZM, Liu YK, Wu CS, Luo Z (2015) Weld J 94(6):196S–202S
Bidajwala RC, Trivedi MMA (2015) Int J Innov Res Sci Technol 1(7):147–149
Ranga Janardhana G, Senthil Kumar M, Dhanasekar B (2015) Appl Mech Mater 719:46–49
Schlegel N, Ebert S, Mauer G, Vassen R (2015) J Thermal Spray Technol 24(1–2):144–151
Sokołowski P, Łatka L, Pawłowski L, Ambroziak A, Kozerski S, Nait-Ali B (2015) Surf Coat Technol 268:147–152
Rong MZ, Zhong LL, Cressault Y, Gleizes A, Wang XH, Chen F, Zheng H (2014) J Phys D Appl Phys 47(49):495202
Zhong LL, Wang XH, Yang AJ, Liu DX, Wu Y, Rong MZ (2014) Predominant particles in SF6-Cu mixture at temperatures of 300–50,000 K. In: 20th International conference on gas discharges and their applications Orléans, France
Rat V, Murphy AB, Aubreton J, Elchinger MF, Fauchais P (2008) J Phys D Appl Phys 41(18):183001
Wang X, Zhong L, Rong M, Yang A, Liu D, Wu Y, Miao S (2015) J Phys D Appl Phys 48(15):155205
Wang X, Zhong L, Cressault Y, Gleizes A, Rong M (2014) J Phys D Appl Phys 47(49):495201
Coufal O (1998) J Phys D Appl Phys 31(16):2025
Murphy AB, Arundell CJ (1994) Plasma Chem Plasma Process 14(4):451–490
Cressault Y, Gleizes A, Riquel G (2012) J Phys D Appl Phys 45(26):265202
Girard R, Belhaouari JB, Gonzalez JJ, Gleizes A (1999) J Phys D Appl Phys 32(22):2890
Amakawa T, Jenista J, Heberlein J, Pfender E (1998) J Phys D Appl Phys 31(20):2826–2834
Tanaka M, Ushio M (1999) J Phys D Appl Phys 32(10):906–912
Almeida RMS, Belinov MS, Naidis GV (2000) J Phys D Appl Phys 33(8):960–967
Li H-P, Belinov MS (2007) J Phys D Appl Phys 40(7):2010–2017
Yang G, Heberlein J (2007) Plasma Sources Sci Technol 16(3):529–542
Wang WZ, Rong MZ, Yan JD, Murphy AB, Spencer JW (2011) Phys Plasma 18(11):113502
Tendero C, Tixier C, Tristant P, Desmaison J, Leprince P (2006) Spectrochim Acta Part B Atom Spectrosc 61(1):2–30
Kim IS, Son JS, Kim IG, Kim JY, Kim OS (2003) J Mater Process Technol 136(1):139–145
Wilhelm G, Gött G, Schöpp H, Uhrlandt D (2010) J Phys D Appl Phys 43(43):434004
Kang MJ, Rhee S (2001) Sci Technol Welding Joining 6(2):94–102
Tokihiko K, Rinsei I, Koichi Y, Yoshinori H (2009) Sci Technol Weld Join 14(8):740–746
Lu S, Fujii H, Nogi K (2008) J Mater Sci 43(13):4583–4591
Stoller PC, Seeger M, Iordanidis A, Naidis GV (2013) IEEE Trans Plasma Sci 41(8):2359–2369
LTA 72D1 CO2 High Voltage Circuit Breaker www.ABB.com/highvoltage
Rat V, André P, Aubreton J, Elchinger MF, Fauchais P, Lefort A (2001) J Phys D Appl Phys 34(14):2191
Borge E (1995) Thèse Université Paul Sabatier, Toulouse No 2051
André P, Aubreton J, Elchinger MF, Fauchais P, Lefort A (2001) Plasma Chem Plasma Process 21(1):83–105
Zhong L, Yang A, Wang X, Liu D, Wu Y, Rong M (2014) Phys Plasmas 21(5):053506
Sun H, Rong M, Wu Y, Chen Z, Yang F, Murphy AB, Zhang H (2015) J Phys D Appl Phys 48(5):055201
Yang A, Liu Y, Sun B, Wang X, Cressault Y, Zhong L, Niu C (2015) J Phys D Appl Phys 48(49):495202
Chen Z, Niu C, Zhang H, Sun H, Wu Y, Yang F, Xu Z (2015) In: 3rd international conference on electric power equipment—switching technology (ICEPE-ST), pp 36–39
Li X, Guo X, Zhao H, Jia S, Murphy AB (2015) J Appl Phys 117(14):143302
Yang A, Wang X, Rong M, Liu D, Iza F, Kong MG (2011) Phys Plasma 18(11):113503
Liu DX, Yang AJ, Wang XH, Rong MZ, Iza F, Kong MG (2012) J Phys D Appl Phys 45(30):305205
Yang A, Rong M, Wang X, Liu D, Kong MG (2013) J Phys D Appl Phys 46(41):415201
Yang A, Liu D, Rong M, Wang X, Kong MG (2014) Phys Plasma 21(8):083501
Brand KP, Kopainsky J (1978) Appl Phys 16(4):425–432
Bartlová M, Coufal O (2002) J Phys D Appl Phys 35(23):3065
Adamec L, Coufal O (1999) J Phys D Appl Phys 32(14):1702
Coll I, Casanovas AM, Vial L, Gleizes A, Casanovas J (2000) J Phys D Appl Phys 33(3):221
Coufal O, Sezemský P (2001) J Phys D Appl Phys 34(14):2174
Wang X, Gao Q, Fu Y, Yang A, Rong M, Wu Y, Niu C, Murphy AB (2016) J Phys D Appl Phys 49(10):105502
Inada Y, Matsuoka S, Kumada A, Ikeda H, Hidaka K (2014) J Phys D Appl Phys 47(17):175201
Kozák T, Bogaerts A (2014) Plasma Sources Sci Technol 23(4):045004
Aerts R, Somers W, Bogaerts A (2015) ChemSusChem 8(4):702–716
Aerts R, Martens T, Bogaerts A (2012) J Phys Chem C 116(44):23257–23273
Cenian A, Chernukho A, Borodin V, Śliwiński G (1994) Contrib Plasma Phys 34(1):25–37
Beuthe TG, Chang JS (1997) Jpn J Appl Phys 36(7S):4997
Wu Y, Chen Z, Cressault Y, Murphy AB, Guo A, Liu Z, Sun H (2015) J Phys D Appl Phys 48(41):415205
Yang F, Chen Z, Wu Y, Rong M, Guo A, Liu Z, Wang C (2015) Phys Plasma 22(10):103508
Gurvich LV, Veyts IV, Alcock CB (1989) Thermodynamic properties of individual substances. Hemisphere, New York
Capitelli M, Colonna G, D’Angola A (2012) Fundamental aspects of plasma chemical physics. statistical thermodynamics. Springer, New York
Coufal O, Sezemský P, Živný O (2005) J Phys D Appl Phys 38(8):1265
Coufal O, Živný O (2011) Eur Phys J D 61(1):131–151
André P, Aubreton J, Elchinger MF, Fauchais P, Lefort A (1999) Ann N Y Acad Sci 891(1):81–89
Kee RJ, Rupley FM, Miller JA (1989) Chemkin-II: a Fortran chemical kinetics package for the analysis of gas-phase chemical kinetics. Sandia National Labs, Livermore
Gordon MH, Kruger CH (1993) Plasma Chem Plasma Process 13(3):365–378
Janev RK, Reiter D (2002) Forschungszentrum Jülich Zentralbibliothek
Janev RK, Murakami I, Kato T, Wang JG (2001) Cross sections and rate coefficients for electron-impact ionization of hydrocarbon molecules. National Inst for Fusion Sci Toki Gifu (Japan)
Kieffer LJ, Dunn GH (1966) Rev Modern Phys 38(1):1
Cunningham AJ, Hobson RM (1972) J Phys B Atom Mol Phys 5(12):2320
Eliasson B, Hirth M, Kogelschatz U (1987) J Phys D Appl Phys 20(11):1421
Chang JS, Hobson RM, Ichikawa Y, Kaneda T (1983) Atomic and molecular processes in an ionized gas. Tokyo Denki University Press, Tokyo
Woodall J, Agúndez M, Markwick-Kemper AJ, Millar TJ (2007) Astron Astrophys 466(3):1197–1204
Teulet P, Gonzalez JJ, Mercado-Cabrera A, Cressault Y, Gleizes A (2009) J Phys D Appl Phys 42(17):175201
Chang JS, Masuda S (1988) Pure Appl Chem 60(5):645–650
Liu DX, Rong MZ, Wang XH, Iza F, Kong MG, Bruggeman P (2010) Plasma Process Polym 7(9–10):846–865
Matejcik S, Kiendler A, Cicman P, Skalny J, Stampfli P, Illenberger E, Märk TD (1997) Plasma Sources Sci Technol 6(2):140
Maksimov AI, Polak LS, Sergienko AF, Slovetskii DI (1979) High Energy Chem 13(4):311–316
Ono S, Teii S (1984) J Phys D Appl Phys 17(10):1999
Dorai R (2002) Modeling of atmospheric pressure plasma processing of gases and surfaces. a Ph.D. thesis, University of Illinois at Urbana-Champaign 265
Gudmundsson JT, Thorsteinsson EG (2007) Plasma Sources Sci Technol 16(2):399
Kruse T, Roth P (1997) J Phys Chem A 101(11):2138–2146
Acknowledgments
This work was supported by National Key Basic Research Program of China (973 Program) (2015CB251001), National Natural Science Foundation of China (No. 51521065), China Postdoctoral Science Foundation (2015M572558), the Fundamental Research Funds for the Central Universities, Natural Science Basis Research Plan in Shaanxi Province of China (2016JQ5089), the State Key Laboratory of Electrical Insulation and Power Equipment (No. EIPE16307).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Qingqing Gao and Aijun Yang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Gao, Q., Yang, A., Wang, X. et al. Determination of the Dominant Species and Reactions in Non-equilibrium CO2 Thermal Plasmas with a Two-Temperature Chemical Kinetic Model. Plasma Chem Plasma Process 36, 1301–1323 (2016). https://doi.org/10.1007/s11090-016-9719-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-016-9719-0