Abstract
Background
Eleusine coracana (L.) Gaertn is a crucial C4 species renowned for its stress robustness and nutritional significance. Because of its adaptability traits, finger millet (ragi) is a storehouse of critical genomic resources for crop improvement. However, more knowledge about this crop’s molecular responses to heat stress needs to be gained.
Methods and results
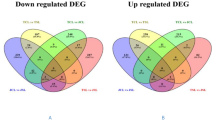
In the present study, a comparative RNA sequencing analysis was done in the leaf tissue of the finger millet, between the heat-sensitive (KJNS-46) and heat-tolerant (PES-110) cultivars of Ragi, in response to high temperatures. On average, each sample generated about 24 million reads. Interestingly, a comparison of transcriptomic profiling identified 684 transcripts which were significantly differentially expressed genes (DEGs) examined between the heat-stressed samples of both genotypes. The heat-induced change in the transcriptome was confirmed by qRT-PCR using a set of randomly selected genes. Pathway analysis and functional annotation analysis revealed the activation of various genes involved in response to stress specifically heat, oxidation-reduction process, water deprivation, and changes in heat shock protein (HSP) and transcription factors, calcium signaling, and kinase signaling. The basal regulatory genes, such as bZIP, were involved in response to heat stress, indicating that heat stress activates genes involved in housekeeping or related to basal regulatory processes. A substantial percentage of the DEGs belonged to proteins of unknown functions (PUFs), i.e., not yet characterized.
Conclusion
These findings highlight the importance of candidate genes, such as HSPs and pathways that can confer tolerance towards heat stress in ragi. These results will provide valuable information to improve the heat tolerance in heat-susceptible agronomically important varieties of ragi and other crops.




Similar content being viewed by others
Data availability
All the datasets used are online available.
References
Goron TL, Raizada MN (2015) Genetic diversity and genomic resources available for the small millet crops to accelerate a New Green Revolution. Front Plant Sci 6:157. https://doi.org/10.3389/fpls.2015.00157
Hittalmani S, Mahesh HB, Shirke MD et al (2017) Genome and transcriptome sequence of Finger millet (Eleusine coracana (L.) Gaertn.) Provides insights into drought tolerance and nutraceutical properties. BMC Genom 18:465
Keller I, Seehausen O (2012) Thermal adaptation and ecological speciation. Mol Ecol 21:782–799
Baniwal SK, Bharti K, Chan KY et al (2014) Heat stress response in plants: a complex game with chaperones and more than twenty heat stress transcription factors. J Biosci 29:471–487
Liu F, Wang W, Sun X et al (2013) RNA-Seq revealed complex response to heat stress on transcriptomic level in Saccharina Japonica (Laminariales, Phaeophyta). J Appl Phycol 26:1585–1596
Dittami SM, Scornet D, Petit JL et al (2009) Global expression analysis of the brown alga Ectocarpus siliculosus (Phaeophyceae) reveals large-scale reprogramming of the transcriptome in response to abiotic stress. Genome Biol 10:R66
Liu GT, Wang JF, Cramer G et al (2012) Transcriptomic analysis of grape (Vitis vinifera L.) leaves during and after recovery from heat stress. BMC Plant Biol 12:174
Li T, Xu X, Li Y et al (2015) Comparative transcriptome analysis reveals differential transcription in heat-susceptible and heat-tolerant pepper (Capsicum annum L.) cultivars under heat stress. J Plant Biol 58:411–424
Li YF, Wang Y, Tang Y et al (2016) Transcriptome analysis of heat stress response in switchgrass (Panicum virgatum L). BMC Plant Biol 13:153
Frank G, Pressman E, Ophir R et al (2009) Transcriptional profiling of maturing tomato (Solanum lycopersicum L.) microspores reveals the involvement of heat shock proteins, ROS scavengers, hormones, and sugars in the heat stress response. J Exp Bot 60:3891–3908
Juan Jin L, Yang D, Liu FX, Qing Hao (2021) Comparative transcriptome analysis uncovers different heat stress responses in heat resistant and heat-sensitive jujube cultivars. PLoS ONE 15:e0235763
Qi XH, Xu XW, Lin XJ (2012) Identification of differentially expressed genes in cucumber (Cucumis sativus L.) root under waterlogging stress by digital gene expression profile. Genomics 99:160–168
Yan J, Yu L, Xuan J et al (2016) De novo transcriptome sequencing and gene expression profiling of spinach (Spinacia oleracea L.) leaves under heat stress. Sci Rep 6:19473
Yasuhiro H, Yozo O, Fumiyoshi M et al (2015) Landscape of the lipidome and transcriptome under heat stress in Arabidopsis thaliana. Sci Rep 5:10533
Wang W, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9:244–252
Kumar A, Gaur VS, Goel A, Gupta AK (2015) De Novo Assembly and characterization of developing spikes transcriptome of Finger Millet (Eleusine coracana): a minor crop having Nutraceutical properties. Plant Mol Biol Rep 33:905–922
Gururani K, Kumar A, Tiwari A et al (2020) Transcriptome wide identification and characterization of regulatory genes involved in EAA metabolism and validation through expression analysis in different developmental stages of finger millet spikes. 3 Biotech 8:347
Rahman H, Jagadeeshselvam N, Valarmathi R et al (2014) Transcriptome analysis of salinity responsiveness in contrasting genotypes of finger millet (Eleusine coracana L.) through RNA-sequencing. Plant Mol Biol 85:485–503
Li J, Wang Y, Wang L et al (2021) Integration of transcriptomic and proteomic analyses for finger millet [Eleusine coracana (L.) Gaertn.] In response to drought stress. PLoS ONE 16:e0247181
Parvathi MS, Nataraja KN, Reddy YAN et al (2019) Transcriptome analysis of finger millet (Eleusine coracana (L.) Gaertn.) Reveals unique drought responsive genes. J Genet 98:46
Sun M, Huang D, Zhang A et al (2020) Transcriptome analysis of heat stress and drought stress in pearl millet based on Pacbio full-length transcriptome sequencing. BMC Plant Biol 20:323
Yogeesh LN, Naryanareddy AB, Nanjareddy YA, Gowda MVC (2016) High temperature tolerant genotypes of Finger Millet (Eleusine coracana L). Nat Env & Poll Tech 15:1293–1296
Mahajan MM, Goyal E, Singh AK et al (2017) Transcriptome dynamics provide insights into long-term salinity stress tolerance in Triticum aestivum Cv. Kharchia Local Plant Physiol Biochem 121:128–139
Grabherr MG, Haas BJ, Yassour M et al (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotech 29:644–652
Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10:R25
Goyal E, Amit SK, Singh RS et al (2016a) Transcriptome profiling of the salt-stress response in Triticum aestivum Cv. Kharchia Local Sci Rep 6:27752
Goyal E, Kumar AS, Singh RS (2016b) De novo transcriptome sequencing and analysis of Hydrilla verticillata (L.f.) Royle}. Plant Omics 9:270–228
Mahajan MM, Goyal E, Singh AK et al (2020) Shedding light on response of Triticum aestivum Cv. Kharchia Local roots to long-term salinity stress through transcriptome profiling. Plant Growth Regul 90:369–381
Singh IN, Paleg IG, Aspinall D (1973) Stress metabolism I. Nitrogen metabolism and growth in the barley plant during water stress. Aust J Biol Sci 26:45–56
Wang W, Lin Y, Teng F et al (2018) Comparative transcriptome analysis between heat tolerant and sensitive Pyropia haitanensis strains in response to high-temperature stress. Algal Res 29:104–112
Bharti K, Nover L (2002) Heat stress-induced signalling. In: Scheel D, Wasternack C (eds) Plant signal transduction: frontiers in molecular biology. Oxford University Press, Oxford, pp 74–115
Kotak S, Larkindale J, Lee U et al (2007) Complexity of the heat stress response in plants. Curr Opin Plant Biol 10:310–316
Queitsch C, Hong SW, Vierling E, Lindquist S (2000) Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell 12:479–492
Yamada K, Fukao Y, Hayashi M et al (2007) Cytosolic HSP90 regulates the heat shock response that is responsible for heat acclimation in Arabidopsis thaliana. J Biol Chem 282:37794–37804
Myouga F, Motohashi R, Kuromori T, Nagata N, Shinozaki K (2006) An Arabidopsis chloroplast-targeted Hsp101 homologue, APG6, has an essential role in chloroplast development as well as heat-stress response. Plant J 48:249–260
Lee U, Wie C, Escobar M et al (2005) Genetic analysis reveals domain interactions of Arabidopsis Hsp100/ClpB and cooperation with the small heat shock protein chaperone system. Plant Cell 17:559–571
Pressman E, Shaked R, Firon N (2007) Tomato (Lycopersicon esculentum) response to heat stress: focus on pollen grains. Plant Stress 1:216–227
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. EEB 61:199–223
Golldack D, Lu king I, Yang O (2011) Plant tolerance to drought and salinity: stress regulating transcription factors and their functional significance in the cellular transcriptional network. Plant Cell Rep 30:1383–1391
Jakoby M, Weisshaar B, Droge-Laser W (2002) bZIP transcription factors in Arabidopsis. Trends Plant Sci 7:106–111
Tran LSP, Nakashima K, Sakuma Y et al (2004) Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell 16:2481–2498
Guo W, Zhang J, Zhang N et al (2015) The wheat NAC transcription factor TaNAC2L is regulated at the transcriptional and post-translational levels and promotes heat stress tolerance in transgenic Arabidopsis. PLoS ONE 10:1–11
Sakuma Y, Maruyama K, Qin F et al (2006) Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heat-stress-responsive gene expression. Proc Natl Acad Sci USA 103:18822–18827
Miller G, Shulaev V, Mittler R (2008) Reactive oxygen signaling and abiotic stress. Physiol Plant 133:481–489
Schain NG, Dreni L, Lawas LM et al (2016) Genome-wide transcriptome analysis during Anthesis reveals New insights into the molecular basis of heat stress responses in tolerant and sensitive Rice varieties. Plant Cell Physiol 57:57–68
Prandl R, Hinderhofer K, Eggers-Schumacher G, Schoffl F (1998) HSF3, a new heat shock factor from Arabidopsis thaliana, derepresses the heat shock response and confers thermotolerance when overexpressed in transgenic plants. MolGen Genet 258:269–278
Acknowledgements
This financial support from the Department of Biotechnology, New Delhi, under the project “Phenomics and Genomics of Ragi (Eleusine coracana)” is acknowledged. The authors are grateful to Prof. Y.A. Nanja Reddy, Project Coordinator, AICP on Small Millets, UAS, GKVK Campus, Bangalore, for providing seeds of KJNS-46 and PES-110 and for their valuable suggestions.
Funding
This financial support is from the Department of Biotechnology, New Delhi, under the project “Phenomics and Genomics of Ragi (Eleusine coracana)”.
Author information
Authors and Affiliations
Contributions
E.G. grew the plants and carried out the experiment. E.G., A.K.M., and M.M.M. performed RNA isolation. K.K. conceived the study, designed the experiment, was associated with wet lab work, and coordinated the study. E.G., A.K.S., M.M.M, and K.K. were involved in result interpretation, analysis, and integration of results, besides associating with drafting and finalizing the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval
No ethical approval is needed.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig 1
: Physiological analysis under heat stress. Membrane Stability Index
Supplementary Fig. 2
: Transcriptome assembly information. (a) Length distribution of fully assembled transcripts. (b) Proteome analysis of total transcripts of E. coracana
Supplementary Table 1
: Primers used in the study
Supplementary Table 2
: FPKM value from the in-silico analysis
Supplementary Table 3
: GO classification of 684 differentially expressed unigenes
Supplementary Table 4
: KEGG classification of 684 differentially expressed unigenes
Supplementary Table 5
: Distribution of Transcription factor family of 684 differentially expressed unigenes
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goyal, E., Singh, A.K., Mahajan, M.M. et al. Comparative transcriptome profiling of contrasting finger millet (Eleusine coracana (L.) Gaertn) genotypes under heat stress. Mol Biol Rep 51, 283 (2024). https://doi.org/10.1007/s11033-024-09233-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09233-x