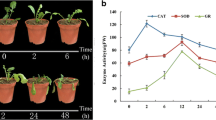
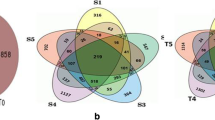
Abstract
Saccharina japonica is a cold-temperate species; it often suffers heat stress during cultivation in temperate and subtropical zone in China. In this study we investigated the response of this alga to heat stress on transcriptomic level. A total of 947 genes (1.32 %) were identified as different expression genes (DEGs), out of which 548 and 399 genes were respectively up- and down-regulated by the heat stress. Among the 138 enriched gene ontology (GO) terms, “cell part”, “binding” and “cellular process” ranked as the first three GO terms that contained the most DEGs. A total of 47 pathways involved 119 DEGs were enriched. Over half of these DEGs were involved in “protein processing in endoplasmic reticulum”, “metabolic pathway” and “biosynthesis of secondary metabolites”. Manual classification of DEGs indicated that 155 DEGs were divided into nine groups, including heat shock protein, antioxidant system, protein synthesis and degradation, and so on. The results indicated that the heat stress triggered complex response of S. japonica; in turn, S. japonica adjusted its physiological and metabolic processes to adapt to and survive the heat stress. The identified DEGs will shed light on the heat tolerance mechanism of S. japonica and benefit future breeding of variety with heat tolerance.


Similar content being viewed by others
References
Arner E, Holmgren A (2000) Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem 267:6102–6109
Asada K (1992) Ascorbate peroxidase—a hydrogen peroxide-scavenging enzyme in plants. Physiol Plant 852:235–241
Bartsch I, Wiencke C, Bischof K et al (2008) The genus Laminaria sensu lato: recent insights and developments. Eur J Phycol 43:1–86
Block MD, Verduyn C, Brouwer DD, Cornelissen M (2005) Poly(ADP-ribose) polymerase in plants affects energy homeostasis, cell death and stress tolerance. Plant J 41:95–106
Breeman AM, Pakker H (1994) Temperature ecotypes in seaweeds: adaptive significance and biogeographic implications. Bot Mar 37:171–180
Collén J, Guisle-Marsollier I, Leger J, Boyen C (2007) Response of the transcriptome of the intertidal red seaweed Chondrus crispus to controlled and natural stresses. New Phytol 176:45–55
Conesa A, Götz S, García-Gómez J, Terol J, Talón M, Robles M (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21:3674–3676
Deng YY, Yao JT, Wang XL, Guo H, Duan D (2012) Transcriptome sequencing and comparative analysis of Saccharina japonica (Laminariales, Phaeophyceae) under blue light induction. Plos One 7:e39704
Dittami S, Scornet D, Petit J et al (2009) Global expression analysis of the brown alga Ectocarpus siliculosus (Phaeophyceae) reveals large-scale reprogramming of the transcriptome in response to abiotic stress. Genome Biol 10:R66
Fu WD, Yao JT, Wang X, Liu F, Fu G, Duan D (2009) Molecular cloning and expression analysis of a Hsp70 gene from Laminaria japonica (Laminariaceae, Phaeophyta). Mar Biotechnol 11:738–74
Fu WD, Shuai L, Yao JT, Zheng B, Zhong M, Duan D (2011) Molecul ar cloning and expression analysis of a cytosolic Hsp70 gene from Ulva pertusa (Ulvophyceae, Chlorophyta). J Appl Phycol 23:681–690
Gao X, Endo H, Taniguchi K, Agatsuma Y (2013) Genetic differentiation of high-temperature tolerance in the kelp Undaria pinnatifida sporophytes from geographically separated populations along the Pacific coast of Japan. J Appl Phycol 25:567–574
Goldberg AL (2003) Protein degradation and protection against misfolded or damaged proteins. Nature 426:895–899
Hayes SA, Dice JF (1996) Roles of molecular chaperones in protein degradation. J Cell Biol 132:255–258
Heinrich S, Valentin K, Frickenhaus S, John U, Wienke C (2012) Transcriptomic analysis of acclimation to temperature and light stress in Saccharina latissima (Phaeophyceae). Plos One 7/8:e44342
Hwang Y, Jung G, Jin E (2008) Transcriptome analysis of acclimatory responses to thermal stress in Antarctic algae. Biochem Biophys Res Commun 367:635–641
Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T, Yamanishi Y (2008) KEGG for linking genomes to life and the environment. Nucl Acid Res 36/Database issue:D480–484
Kirst G, Wiencke C (1995) Ecophysiology of polar algae. J Phycol 31:181–199
Knight H, Knight MR (2001) Abiotic stress signaling pathways: specificity and cross-talk. Trends Plant Sci 6:262–267
Lane CE, Mayes C, Druehl LD, Saunders GW (2006) A multi-gene molecular investigation of the kelp (Laminariales, Phaeophyceae) supports substantial taxonomic reorganization. J Phycol 42:493–512
Langer T (2000) AAA proteases: cellular machines for degrading membrane proteins. Trends Biochem Sci 25:247–251
Li R, Yu C, Li Y, Lam TW, Yiu SM, Kristiansen K, Wang J (2009) SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics 25:1966–1967
Liu F, Pang SJ (2010) Performances of growth, photochemical efficiency, and stress tolerance of young sporophytes from seven populations of Saccharina japonica (Phaeophyta) under short-term heat stress. J Appl Phycol 22:221–229
Luikenhuis S, Perrone G, Dawes IW, Grant CM (1998) The yeast Saccharomyces cerevisiae contains two glutaredoxin genes that are required for protection against reactive oxygen species. Mol Biol Cell 9:1081–1091
Michel G, Tonon T, Scornet D, Cock JM, Kloareg B (2010) The cell wall polysaccharide metabolism of the brown alga Ectocarpus siliculosus. Insights into the evolution of extracellular matrix polysaccharides in Eukaryotes. New Phytol 188:82–97
Moroney JV, Bartlett SG, Samuelsson G (2001) Carbonic anhydrases in plants and algae. Plant Cell Environ 24:141–153
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5:621–628
Noctor G (2006) Metabolic signaling in defense and stress: the central roles of soluble redox couples. Plant Cell Environ 29:409–425
Pang SJ, Jin ZH, Sun JZ, Gao SQ (2007) Temperature tolerance of young sporophytes from two populations of Laminaria japonica revealed by chlorophyll fluorescence measurements and short-term growth and survival performances in tank culture. Aquaculture 262:493–503
Pearson GA, Hoarau G, Lago-Leston A, Coyer JA, Kube M, Reinhardt R, Henckel K, Serrão ET, Corre E, Olsen JL (2010) An expressed sequence tag analysis of the intertidal brown seaweeds Fucus serratus (L.) and F. vesiculosus (L.) (Heterokontophyta, Phaeophyceae) in response to abiotic stressors. Mar Biotechnol 12:195–213
Rizhsky L, Liang H, Mittler R (2002) The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol 130:1143–1151
Roeder V, Collén J, Rousvoal S, Corre E, Leblanc C, Boyen C (2005) Identification of stress gene transcripts in Laminaria digitata (Phaeophyceae) protoplast cultures by expressed sequence tag analysis. J Phycol 41:1227–1235
Strange RC, Spiteri MA, Ramachandran S, Fryer AA (2001) Glutathione-S-transferase family of enzymes. Mut Res 482:21–26
Teo SS, Ho CL, Teoh S, Rahmin RA, Phang SM (2009) Transcriptomic analysis of Gracilaria changii (Rhodophyta) in response to hyper- and hypoosmotic stress. J Phycol 45:1093–1099
Tiwari BS, Belenghi B, Levine A (2002) Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol 128:1271–1281
Tseng CK (2001) Algal biotechnology industries and research activities in China. J Appl Phycol 13:375–380
Tseng CK, Chang CF (1960) An analysis of the nature of marine algal flora. Oceanol Limnol Sinica 3:177–187
van Wijk SJ, Timmers HT (2010) The family of ubiquitin-conjugating enzymes (E2s): deciding between life and death of proteins. FASEB J 24:4981–4993
Vayda ME, Yuan ML (1994) The heat shock response of an Antarctic alga is evident at 5 °C. Plant Mol Biol 24:229–233
Wang WX, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9:244–252
Wang Y, Yu QY, Tang XX, Wang L (2009) Calcium pretreatment increases thermotolerance of Laminaria japonica sporophytes. Prog Nat Sci 19:435–442
Wang WJ, Wang FJ, Sun XT, Liu FL, Liang ZR (2013) Comparison of transcriptome under red and blue light culture of Saccharina japonica (Phaeophyceae). Planta 237:1123–1133
Xuan H, Tong SM, Hou HS (2011) Effects of high temperature stress on growth and physiology of gametophytes of Laminaria japonica. Tianjing Agric Sci 17/2:5–8 (in Chinese with English abstract)
Yao JT, Fu WD, Wang XL, Duan D (2009) Improved RNA Isolation for Laminaria japonica Aresch (Laminariaceae, Phaeophyta). J Appl Phycol 21:233–238
Ye J, Fang L, Zheng H, Zhang Y, Chen J, Zhang Z, Wang J, Li S, Li R, Bolund L, Wang J (2006) WEGO: a web tool for plotting GO annotations. Nucl Acid Res 34:W293–297
Zhang L, Cui CJ, Li XJ, Zhang Z, Luo S, Liang G, Liu Y, Yang G (2013) Effect of temperature on the development of Saccharina japonica gametophytes. J Appl Phycol 25:261–267
Zheng Q, Wang XJ (2008) GOEAST: a web-based software toolkit for gene ontology enrichment analysis. Nucl Acid Res 36:W358–W363
Acknowledgments
The authors are grateful to the anonymous reviewers for their constructive comments on this paper. This research was funded by the National Natural Science Foundation of China (no. 41306176; 30901095), the 863 Hi-Tech Research and Development Program of China (2012AA10A406), the Natural Science Foundation of Shandong province (ZR2012CQ030), and the Development Program of Science and Technology of Shandong Province (2013GGF01028).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Full list of differently expressed genes (DEGs) between control and heat stress group. (Excle) (XLSM 151 kb)
Table S2
Full list of GO terms produced by GO enrichment analysis for the DEGs. (Excle) (XLSM 17 kb)
Table S3
Full list of pathways produced by pathway enrichment analysis with KEGG annotation for the DEGs. (Excle) (XLSM 12 kb)
Rights and permissions
About this article
Cite this article
Liu, F., Wang, W., Sun, X. et al. RNA-Seq revealed complex response to heat stress on transcriptomic level in Saccharina japonica (Laminariales, Phaeophyta). J Appl Phycol 26, 1585–1596 (2014). https://doi.org/10.1007/s10811-013-0188-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-013-0188-z