Abstract
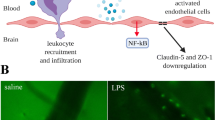
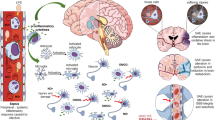
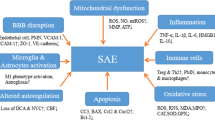
Sepsis-associated encephalopathy is a common neurological complication of sepsis and is responsible for higher mortality and poorer long-term outcomes in septic patients. Sepsis-associated encephalopathy symptoms can range from mild delirium to deep coma, which occurs in up to 70% of patients in intensive care units. The pathological changes in the brain associated with sepsis include cerebral ischaemia, cerebral haemorrhage, abscess and progressive multifocal necrotic leukoencephalopathy. Several mechanisms are involved in the pathogenesis of sepsis-associated encephalopathy, such as blood–brain barrier dysfunction, cerebral blood flow impairment, glial cell activation, leukocyte transmigration, and neurotransmitter disturbances. These events are interrelated and influence each other, therefore they do not act as independent factors. This review is focused on new evidence showing the pathological process of sepsis-associated encephalopathy.


Similar content being viewed by others
Data availability
All authors declares that all data support their published claims and comply with field standards.
Abbreviations
- SAE:
-
Sepsis-associated encephalopathy
- CNS:
-
Cerebral blood flow
- CSF:
-
Cerebrospinal fluid
- BBB:
-
Blood–brain barrier
- LPS:
-
Lipopolysaccharide
- GABA:
-
Gamma-aminobutyric acid
- AChE:
-
Acetylcholinesterase
- 5-HIIA:
-
5-Hydroxyindoleacetic acid
References
Singer M, Deutschman CS, Seymour CW et al (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315(8):801–810
Fleischmann C, Scherag A, Adhikari NK et al (2016) Assessment of global incidence and mortality of hospital-treated sepsis. current estimates and limitations. Am J Respir Crit Care Med 193(3):259–272
Schuler A, Wulf DA, Lu Y et al (2018) The impact of acute organ dysfunction on long-term survival in sepsis. Crit Care Med 46(6):843–849
Chung HY, Wickel J, Brunkhorst FM et al (2020) Sepsis-associated encephalopathy: from delirium to dementia? J Clin Med 9(3):703
Kikuchi DS, Campos ACP, Qu H et al (2019) Poldip2 mediates blood–brain barrier disruption in a model of sepsis-associated encephalopathy. J Neuroinflamm 16(1):241
Kuperberg SJ, Wadgaonkar R (2017) Sepsis-associated encephalopathy: the blood–brain barrier and the sphingolipid rheostat. Front Immunol 8:597
Zhao L, Wang Y, Ge Z et al (2021) Mechanical learning for prediction of sepsis-associated encephalopathy. Front Comput Neurosci 15:739265
Ai ML, Huang L, Feng Q et al (2019) The clinical significance of transcranial Doppler in early diagnosis of sepsis-associated encephalopathy. Zhonghua nei ke za zhi 58(11):814–818
Shulyatnikova T, Verkhratsky A (2020) Astroglia in sepsis associated encephalopathy. Neurochem Res 45(1):83–99
Ren C, Yao RQ, Zhang H et al (2020) Sepsis-associated encephalopathy: a vicious cycle of immunosuppression. J Neuroinflamm 17(1):14
Czempik PF, Pluta MP, Krzych LJ (2020) Sepsis-associated brain dysfunction: a review of current literature. Int J Environ Res Public Health 17(16):5852
Keaney J, Campbell M (2015) The dynamic blood–brain barrier. FEBS J 282(21):4067–4079
Jin X, Liu J, Liu W (2014) Early ischemic blood brain barrier damage: a potential indicator for hemorrhagic transformation following tissue plasminogen activator (tPA) thrombolysis? Curr Neurovasc Res 11(3):254–262
Nishioku T, Dohgu S, Takata F et al (2009) Detachment of brain pericytes from the basal lamina is involved in disruption of the blood–brain barrier caused by lipopolysaccharide-induced sepsis in mice. Cell Mol Neurobiol 29(3):309–316
Andreasen AS, Krabbe KS, Krogh-Madsen R et al (2008) Human endotoxemia as a model of systemic inflammation. Curr Med Chem 15(17):1697–1705
Erikson K, Tuominen H, Vakkala M et al (2020) Brain tight junction protein expression in sepsis in an autopsy series. Crit Care 24(1):385
Guo F, Tang J, Zhou Z et al (2012) GEF-H1-RhoA signaling pathway mediates LPS-induced NF-kappaB transactivation and IL-8 synthesis in endothelial cells. Mol Immunol 50(1–2):98–107
Weighardt H, Holzmann B (2007) Role of Toll-like receptor responses for sepsis pathogenesis. Immunobiology 212(9–10):715–722
Ni Y, Teng T, Li R et al (2017) TNFalpha alters occludin and cerebral endothelial permeability: role of p38MAPK. PLoS ONE 12(2):e0170346
Sankowski R, Mader S, Valdes-Ferrer SI (2015) Systemic inflammation and the brain: novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front Cell Neurosci 9:28
Nwafor DC, Brichacek AL, Mohammad AS et al (2019) Targeting the blood–brain barrier to prevent sepsis-associated cognitive impairment. J Cent Nerv Syst Dis 11:1179573519840652
Haileselassie B, Joshi AU, Minhas PS et al (2020) Mitochondrial dysfunction mediated through dynamin-related protein 1 (Drp1) propagates impairment in blood–brain barrier in septic encephalopathy. J Neuroinflamm 17(1):36
Vutukuri R, Brunkhorst R, Kestner RI et al (2018) Alteration of sphingolipid metabolism as a putative mechanism underlying LPS-induced BBB disruption. J Neurochem 144(2):172–185
Qin LH, Huang W, Mo XA et al (2015) LPS induces occludin dysregulation in cerebral microvascular endothelial cells via MAPK signaling and augmenting MMP-2 levels. Oxid Med Cell Longev. 2015:120641
Hu Y, Bi Y, Yao D et al (2019) Omi/HtrA2 protease associated cell apoptosis participates in blood–brain barrier dysfunction. Front Mol Neurosci 12:48
Dahl RH, Berg RMG, Taudorf S et al (2018) A reassessment of the blood–brain barrier transport of large neutral amino acids during acute systemic inflammation in humans. Clin Physiol Funct Imaging 38(4):656–662
Hughes CG, Patel MB, Pandharipande PP (2012) Pathophysiology of acute brain dysfunction: what’s the cause of all this confusion? Curr Opin Crit Care 18(5):518–526
Griton M, Dhaya I, Nicolas R et al (2020) Experimental sepsis-associated encephalopathy is accompanied by altered cerebral blood perfusion and water diffusion and related to changes in cyclooxygenase-2 expression and glial cell morphology but not to blood-brain barrier breakdown. Brain Behav Immun 83:200–213
Willie CK, Tzeng YC, Fisher JA et al (2014) Integrative regulation of human brain blood flow. J Physiol 592(5):841–859
Taccone FS, Su F, Pierrakos C et al (2010) Cerebral microcirculation is impaired during sepsis: an experimental study. Crit Care 14(4):R140
Ferlini L, Su F, Creteur J et al (2020) Cerebral autoregulation and neurovascular coupling are progressively impaired during septic shock: an experimental study. Intensive Care Med Exp 8(1):44
Taccone FS, Su F, De Deyne C et al (2014) Sepsis is associated with altered cerebral microcirculation and tissue hypoxia in experimental peritonitis. Crit Care Med 42(2):e114–e122
Faraci FM, Taugher RJ, Lynch C et al (2019) Acid-sensing ion channels: novel mediators of cerebral vascular responses. Circ Res 125(10):907–920
Hoiland RL, Fisher JA, Ainslie PN (2019) Regulation of the cerebral circulation by arterial carbon dioxide. Compr Physiol 9(3):1101–1154
Taccone FS, Castanares-Zapatero D, Peres-Bota D et al (2010) Cerebral autoregulation is influenced by carbon dioxide levels in patients with septic shock. Neurocrit Care 12(1):35–42
Zhou Q, Cao B, Niu L et al (2010) Effects of permissive hypercapnia on transient global cerebral ischemia-reperfusion injury in rats. Anesthesiology 112(2):288–297
Van Der Kleij LA, De Vis JB, De Bresser J et al (2020) Arterial CO2 pressure changes during hypercapnia are associated with changes in brain parenchymal volume. Eur Radiol Exp 4(1):17
Bothwell SW, Janigro D, Patabendige A (2019) Cerebrospinal fluid dynamics and intracranial pressure elevation in neurological diseases. Fluids Barriers CNS 16(1):9
Zhao Z, Nelson AR, Betsholtz C et al (2015) Establishment and dysfunction of the blood–brain barrier. Cell 163(5):1064–1078
Aguzzi A, Barres BA, Bennett ML (2013) Microglia: scapegoat, saboteur, or something else? Science 339(6116):156–161
Da Fonseca AC, Matias D, Garcia C et al (2014) The impact of microglial activation on blood–brain barrier in brain diseases. Front Cell Neurosci 8:362
Moraes CA, Zaverucha-Do-Valle C, Fleurance R et al (2021) Neuroinflammation in sepsis: molecular pathways of microglia activation. Pharmaceuticals (Basel) 14(5):416
Hickman S, Izzy S, Sen P et al (2018) Microglia in neurodegeneration. Nat Neurosci 21(10):1359–1369
Melief J, Koning N, Schuurman KG et al (2012) Phenotyping primary human microglia: tight regulation of LPS responsiveness. Glia 60(10):1506–1517
Heming N, Mazeraud A, Verdonk F et al (2017) Neuroanatomy of sepsis-associated encephalopathy. Crit Care 21(1):65
Michels M, Steckert AV, Quevedo J et al (2015) Mechanisms of long-term cognitive dysfunction of sepsis: from blood-borne leukocytes to glial cells. Intensive Care Med Exp 3(1):30
Kaushal V, Schlichter LC (2008) Mechanisms of microglia-mediated neurotoxicity in a new model of the stroke penumbra. J Neurosci 28(9):2221–2230
Michels M, Danieslki LG, Vieira A et al (2015) CD40–CD40 ligand pathway is a major component of acute neuroinflammation and contributes to long-term cognitive dysfunction after sepsis. Mol Med 21:219–226
Danielski LG, Giustina AD, Badawy M et al (2018) Brain barrier breakdown as a cause and consequence of neuroinflammation in sepsis. Mol Neurobiol 55(2):1045–1053
Han Q, Lin Q, Huang P et al (2017) Microglia-derived IL-1beta contributes to axon development disorders and synaptic deficit through p38-MAPK signal pathway in septic neonatal rats. J Neuroinflamm 14(1):52
Kacimi R, Giffard RG, Yenari MA (2011) Endotoxin-activated microglia injure brain derived endothelial cells via NF-kappaB, JAK-STAT and JNK stress kinase pathways. J Inflamm (Lond) 8:7
Michels M, Vieira AS, Vuolo F et al (2015) The role of microglia activation in the development of sepsis-induced long-term cognitive impairment. Brain Behav Immun 43:54–59
Zhao YZ, Gao ZY, Ma LQ et al (2017) Research on biogenesis of mitochondria in astrocytes in sepsis-associated encephalopathy models. Eur Rev Med Pharmacol Sci 21(17):3924–3934
Chen XL, Wang Y, Peng WW et al (2018) Effects of interleukin-6 and IL-6/AMPK signaling pathway on mitochondrial biogenesis and astrocytes viability under experimental septic condition. Int Immunopharmacol 59:287–294
Korcok J, Wu F, Tyml K et al (2002) Sepsis inhibits reduction of dehydroascorbic acid and accumulation of ascorbate in astroglial cultures: intracellular ascorbate depletion increases nitric oxide synthase induction and glutamate uptake inhibition. J Neurochem 81(1):185–193
Hasegawa-Ishii S, Inaba M, Umegaki H et al (2016) Endotoxemia-induced cytokine-mediated responses of hippocampal astrocytes transmitted by cells of the brain-immune interface. Sci Rep 6:25457
Parajuli B, Horiuchi H, Mizuno T et al (2015) CCL11 enhances excitotoxic neuronal death by producing reactive oxygen species in microglia. Glia 63(12):2274–2284
Villeda SA, Luo J, Mosher KI et al (2011) The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477(7362):90–94
Fernandes A, Silva RF, Falcao AS et al (2004) Cytokine production, glutamate release and cell death in rat cultured astrocytes treated with unconjugated bilirubin and LPS. J Neuroimmunol 153(1–2):64–75
Montoya A, Elgueta D, Campos J et al (2019) Dopamine receptor D3 signalling in astrocytes promotes neuroinflammation. J Neuroinflamm 16(1):258
Rossaint J, Margraf A, Zarbock A (2018) Role of platelets in leukocyte recruitment and resolution of inflammation. Front Immunol 9:2712
Giles JA, Greenhalgh AD, Denes A et al (2018) Neutrophil infiltration to the brain is platelet-dependent, and is reversed by blockade of platelet GPIbalpha. Immunology 154(2):322–328
Margraf A, Ley K, Zarbock A (2019) Neutrophil recruitment: from model systems to tissue-specific patterns. Trends Immunol 40(7):613–634
Peng X, Luo Z, He S et al (2021) Blood–brain barrier disruption by lipopolysaccharide and sepsis-associated encephalopathy. Front Cell Infect Microbiol 11:768108
Wu F, Chen X, Zhai L et al (2020) CXCR2 antagonist attenuates neutrophil transmigration into brain in a murine model of LPS induced neuroinflammation. Biochem Biophys Res Commun 529(3):839–845
Wu F, Zhao Y, Jiao T et al (2015) CXCR2 is essential for cerebral endothelial activation and leukocyte recruitment during neuroinflammation. J Neuroinflamm 12:98
Zhou H, Andonegui G, Wong CH et al (2009) Role of endothelial TLR4 for neutrophil recruitment into central nervous system microvessels in systemic inflammation. J Immunol 183(8):5244–5250
Zarbato GF, De Souza Goldim MP, Giustina AD et al (2018) Dimethyl fumarate limits neuroinflammation and oxidative stress and improves cognitive impairment after polymicrobial sepsis. Neurotox Res 34(3):418–430
Jin R, Yang G, Li G (2010) Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 87(5):779–789
Krizbai IA, Bauer H, Bresgen N et al (2005) Effect of oxidative stress on the junctional proteins of cultured cerebral endothelial cells. Cell Mol Neurobiol 25(1):129–139
Turner RJ, Sharp FR (2016) Implications of MMP9 for blood brain barrier disruption and hemorrhagic transformation following ischemic stroke. Front Cell Neurosci 10:56
Kenne E, Erlandsson A, Lindbom L et al (2012) Neutrophil depletion reduces edema formation and tissue loss following traumatic brain injury in mice. J Neuroinflamm 9:17
Andonegui G, Zelinski EL, Schubert CL et al (2018) Targeting inflammatory monocytes in sepsis-associated encephalopathy and long-term cognitive impairment. JCI Insight 3(9):1–8
Van Gool WA, Van De Beek D, Eikelenboom P (2010) Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet 375(9716):773–775
Zaghloul N, Addorisio ME, Silverman HA et al (2017) Forebrain cholinergic dysfunction and systemic and brain inflammation in murine sepsis survivors. Front Immunol 8:1673
Erbas O, Taskiran D (2014) Sepsis-induced changes in behavioral stereotypy in rats; involvement of tumor necrosis factor-alpha, oxidative stress, and dopamine turnover. J Surg Res 186(1):262–268
Freund HR, Muggia-Sullam M, Lafrance R et al (1986) Regional brain amino acid and neurotransmitter derangements during abdominal sepsis and septic encephalopathy in the rat. The effect of amino acid infusions. Arch Surg 121(2):209–216
Gyoneva S, Traynelis SF (2013) Norepinephrine modulates the motility of resting and activated microglia via different adrenergic receptors. J Biol Chem 288(21):15291–15302
Leon A, Lepouse C, Floch T et al (2006) Brain injury during severe sepsis. Ann Fr Anesth Reanim 25(8):863–867
Acharya S, Kim KM (2021) Roles of the functional interaction between brain cholinergic and dopaminergic systems in the pathogenesis and treatment of schizophrenia and Parkinson’s disease. Int J Mol Sci 22(9):1–8
Bugiani O (2021) Why is delirium more frequent in the elderly? Neurol Sci 42(8):3491–3503
Pavlov VA, Wang H, Czura CJ et al (2003) The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med 9(5–8):125–134
Santos-Junior NN, Catalao CHR, Costa LHA et al (2018) Experimental sepsis induces sustained inflammation and acetylcholinesterase activity impairment in the hypothalamus. J Neuroimmunol 324:143–148
Hofer S, Eisenbach C, Lukic IK et al (2008) Pharmacologic cholinesterase inhibition improves survival in experimental sepsis. Crit Care Med 36(2):404–408
Zivkovic AR, Sedlaczek O, Von Haken R et al (2015) Muscarinic M1 receptors modulate endotoxemia-induced loss of synaptic plasticity. Acta Neuropathol Commun 3:67
Van Eijk MM, Roes KC, Honing ML et al (2010) Effect of rivastigmine as an adjunct to usual care with haloperidol on duration of delirium and mortality in critically ill patients: a multicentre, double-blind, placebo-controlled randomised trial. Lancet 376(9755):1829–1837
Tomasi CD, Salluh J, Soares M et al (2015) Baseline acetylcholinesterase activity and serotonin plasma levels are not associated with delirium in critically ill patients. Rev Bras Ter Intensiva 27(2):170–177
Liu A, Ding S (2019) Anti-inflammatory effects of dopamine in lipopolysaccharide (LPS)-stimulated RAW264.7 cells via inhibiting NLRP3 inflammasome activation. Ann Clin Lab Sci 49(3):353–360
Rangel-Barajas C, Coronel I, Floran B (2015) Dopamine receptors and neurodegeneration. Aging Dis 6(5):349–368
Bissonette GB, Roesch MR (2016) Development and function of the midbrain dopamine system: what we know and what we need to. Genes Brain Behav 15(1):62–73
Sommer BR, Wise LC, Kraemer HC (2002) Is dopamine administration possibly a risk factor for delirium? Crit Care Med 30(7):1508–1511
Freund HR, Muggia-Sullam M, Peiser J et al (1985) Brain neurotransmitter profile is deranged during sepsis and septic encephalopathy in the rat. J Surg Res 38(3):267–271
Shimizu I, Adachi N, Liu K et al (1999) Sepsis facilitates brain serotonin activity and impairs learning ability in rats. Brain Res 830(1):94–100
Nolan RA, Reeb KL, Rong Y et al (2020) Dopamine activates NF-kappaB and primes the NLRP3 inflammasome in primary human macrophages. Brain Behav Immun Health 2:17
Li F, Zhang B, Duan S et al (2020) Small dose of L-dopa/benserazide hydrochloride improved sepsis-induced neuroinflammation and long-term cognitive dysfunction in sepsis mice. Brain Res 1737:146780
Beis D, Holzwarth K, Flinders M et al (2015) Brain serotonin deficiency leads to social communication deficits in mice. Biol Lett 11(3):1–8
Bengtsson F, Bugge M, Hansson L et al (1987) Serotonin metabolism in the central nervous system following sepsis or portacaval shunt in the rat. J Surg Res 43(5):420–429
O’dell TJ, Connor SA, Guglietta R et al (2015) beta-Adrenergic receptor signaling and modulation of long-term potentiation in the mammalian hippocampus. Learn Mem 22(9):461–471
Xu H, Rajsombath MM, Weikop P et al (2018) Enriched environment enhances beta-adrenergic signaling to prevent microglia inflammation by amyloid-beta. EMBO Mol Med 10(9):1–7
Manczak EM, Dougherty B, Chen E (2019) Parental depressive symptoms potentiate the effect of youth negative mood symptoms on gene expression in children with asthma. J Abnorm Child Psychol 47(1):99–108
Zong MM, Zhou ZQ, Ji MH et al (2019) Activation of beta2-adrenoceptor attenuates sepsis-induced hippocampus-dependent cognitive impairments by reversing neuroinflammation and synaptic abnormalities. Front Cell Neurosci 13:293
Obata K (2013) Synaptic inhibition and gamma-aminobutyric acid in the mammalian central nervous system. Proc Jpn Acad Ser B 89(4):139–156
Sallam MY, El-Gowilly SM, Abdel-Galil AG et al (2016) Central GABAA receptors are involved in inflammatory and cardiovascular consequences of endotoxemia in conscious rats. Naunyn Schmiedebergs Arch Pharmacol 389(3):279–288
Dadsetan S, Balzano T, Forteza J et al (2016) Infliximab reduces peripheral inflammation, neuroinflammation, and extracellular GABA in the cerebellum and improves learning and motor coordination in rats with hepatic encephalopathy. J Neuroinflamm 13(1):245
Serantes R, Arnalich F, Figueroa M et al (2006) Interleukin-1beta enhances GABAA receptor cell-surface expression by a phosphatidylinositol 3-kinase/Akt pathway: relevance to sepsis-associated encephalopathy. J Biol Chem 281(21):14632–14643
Wang DS, Zurek AA, Lecker I et al (2012) Memory deficits induced by inflammation are regulated by alpha5-subunit-containing GABAA receptors. Cell Rep 2(3):488–496
Acknowledgements
First and foremost, I would like to show my deepest gratitude to my supervisor, Professor Wenlan Liu, a kind, responsible and resourceful scholar, who has provided me with valuable guidance in every stage of scientific research. In addition, I would like to thank the anonymous reviewers for their helpful remarks.
Funding
The work was supported by Guangdong Innovation Platform of Translational Research for Cerebrovascular Diseases and grants from National Natural Science Foundation of China (Grant No. 81760227, 81873747), Guangdong Basic and Applied Basic Research Foundation (Grant No. 2019A1515010311) and Science and Technology Innovation Commission of Shenzhen Municipality (Grant Nos. GJHZ20190820115001765, JCYJ20180507184656626).
Author information
Authors and Affiliations
Contributions
KY had the idea for the article and drafted the work. JQC and TW performed the literature search, and YZ critically wrote and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or nonfinancial interests to disclose.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, K., Chen, J., Wang, T. et al. Pathogenesis of sepsis-associated encephalopathy: more than blood–brain barrier dysfunction. Mol Biol Rep 49, 10091–10099 (2022). https://doi.org/10.1007/s11033-022-07592-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07592-x