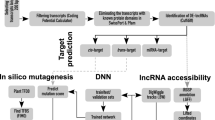
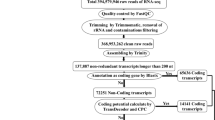
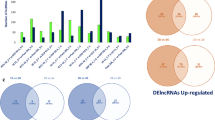
Abstract
Salinity adversely affects the yield and growth of rice (Oryza sativa L.) plants severely, particularly at reproductive stage. Long non-coding RNAs (lncRNAs) are key regulators of diverse molecular and cellular processes in plants. Till now, no systematic study has been reported for regulatory roles of lncRNAs in rice under salinity at reproductive stage. In this study, total 80 RNA-seq data of Horkuch (salt-tolerant) and IR-29 (salt-sensitive) genotypes of rice were used and found 1626 and 2208 transcripts as putative high confidence lncRNAs, among which 1529 and 2103 were found to be novel putative lncRNAs in root and leaf tissue respectively. In Horkuch and IR-29, 14 and 16 lncRNAs were differentially expressed in root tissue while 18 and 63 lncRNAs were differentially expressed in leaf tissue. Interaction analysis among the lncRNAs, miRNAs and corresponding mRNAs indicated that these modules are involved in different biochemical pathways e.g. phenyl propanoid pathway during salinity stress in rice. Interestingly, two differentially expressed lncRNAs such as TCONS_00008914 and TCONS_00008749 were found as putative target mimics of known rice miRNAs. This study indicates that lncRNAs are involved in salinity adaptation of rice at reproductive stage through certain biochemical pathways.





Similar content being viewed by others
References
Chowrasia S, Panda AK, Rawal HC, Kaur H, Mondal TK (2018) Identification of jumonjiC domain containing gene family among the Oryza species and their expression analysis in FL478, a salt tolerant rice genotype. Plant Physiol Biochem 130:43–53
Lutts S, Kinet JM, Bouharmont J (1995) Changes in plant response to NaCl during development of rice (Oryza sativa L.) varieties differing in salinity resistance. J Exp Bot 46:1843–1852
Ganie SA, Dey N, Mondal TK (2016) Promoter methylation regulates the abundance of osa-miR393a in contrasting rice genotypes under salinity stress. Funct Integr Genomics 16:1–11
Ganie SA, Mondal TK (2015) Genome-wide development of novel miRNA-based microsatellite markers of rice (Oryza sativa) for genotyping applications. Mol Breed 35:51–60
Gay F, Maraval B, Roques S, Gunata Z, Boulanger R, Audeber A, Mestres C (2010) Effect of salinity on yield and 2-acetyl-1-pyrroline content in the grains of three fragrant rice cultivars (Oryza sativa L.) in Camargue (France). Field Crops Res 117:154–160
Ahmadizadeh M, Vispo NA, Calapit-Palao CDO, Pangaan ID, Viña CD, Singh RK (2016) Reproductive stage salinity tolerance in rice: a complex trait to phenotype. Indian J Plant Physiol 21:528–536
Kung JT, Colognori D, Lee JT (2013) Long noncoding RNAs: past, present, and future. Genetics 193:651–669
Wang KC, Chang HY (2011) Molecular mechanisms of long noncoding RNAs. Mol Cell 43:904–914
Wilusz JE, Sunwoo H, Spector DL (2009) Long noncoding RNAs: functional surprises from the RNA world. Genes Dev 23:1494–1504
Faghihi MA, Wahlestedt C (2009) Regulatory roles of natural antisense transcripts. Nat Rev Mol Cell Biol 10:637–643
Bhatia G, Goyal N, Sharma S, Upadhyay SK, Singh K (2017) Present scenario of long non-coding RNAs in plants. Non coding RNA 3:16–23
Di C, Yuan J, Wu Y, Li J, Lin H, Hu L, Zhang T, Qi Y, Gerstein MB, Guo Y, Lu ZJ (2014) Characterization of stress-responsive lncRNAs in Arabidopsis thaliana by integrating expression, epigenetic and structural features. Plant J 80:848–861
Razzaque S, Haque T, Elias SM, Rahman MS, Biswas S, Schwartz S, Ismail AM, Walia H, Juenger TE, Seraj ZI (2017) Reproductive stage physiological and transcriptional responses to salinity stress in reciprocal populations derived from tolerant (Horkuch) and susceptible (IR29) rice. Sci Rep 7:46138
Kawahara Y, de la Bastide M, Hamilton JP, Kanamori H, McCombie WR, Ouyang S, Schwartz DC, Tanaka T, Wu J, Zhou S, Childs KL, Davidson RM, Lin H, Quesada-Ocampo L, Vaillancourt B, Sakai H, Lee SS, Kim J, Numa H, Itoh T, Buell CR, Matsumoto T (2013) Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice 6:4
Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, Van Baren MJ, Salzberg SL, Wold BJ, Pachter L (2010) Transcript assembly and quantification by RNA-seqreveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28:511–515
Kong L, Zhang Y, Ye Z, Liu X, Zhao S, Wei L, Gao G (2007) CPC: assess the protein- coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res 35:W345–W349
Lowe TM, Eddy SR (1997) tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res 25:955–964
Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, Couger MB, Eccles D, Li B, Lieber M, Macmanes MD, Ott M, Orvis J, Pochet N, Strozzi F, Weeks N, Westerman R, William T, Dewey CN, Henschel R, Leduc RD, Friedman N, Regev A (2013) De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc 8:1494–1512
Wucher V, Legeai F, Hédan B, Rizk G, Lagoutte L, Leeb T, Jagannathan V, Cadieu E, David A, Lohi H, Cirera S, Fredholm M, Botherel N, Leegwater PAJ, Le Béguec C, Fieten H, Johnson J, Alföldi J, André C, Lindblad-Toh K, Hitte C, Derrien T (2017) FEELnc: a tool for long non-coding RNA annotation and its application to the dog transcriptome. Nucleic Acids Res 45:e57
Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA (2009) Circos: an information aesthetic for comparative genomics. Genome Res 19:1639–1645
Sun X, Dong B, Yin L, Zhang R, Du W, Liu D, Shi N, Li A, Liang Y, Mao L (2013) PMTED: a plant microRNA target expression database. BMC Bioinform 14:174
Gurjar AKS, Panwar AS, Gupta R, Mantri SS (2016) PmiRExAt: plant miRNA expression atlas database and web applications. Database 2016:baw060
Dai X, Zhao PX (2011) psRNATarget: a plant small RNA target analysis server. Nucleic Acids Res 39:W155–W159
Mondal TK, Ganie SA, Rana MK, Sharma TR (2014) Genome-wide analysis of zinc transporter genes of maize (Zea mays). Plant Mol Biol Rep 32:605–616
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C T method. Nat Protoc 3:1101–1108
Rinn JL, Chang HY (2012) Genome regulation by long noncoding RNAs. Annu Rev Biochem 81:145–166
Zhang W, Han Z, Guo Q, Liu Y, Zheng Y, Wu F, Jin W (2014) Identification of maize long non-coding RNAs responsive to drought stress. PLoS ONE 9:e98958
Chen J, Quan M, Zhang D (2015) Genome-wide identification of novel long non- coding RNAs in Populus tomentosa tension wood, opposite wood and normal wood xylem by RNA-seq. Planta 241:125–143
Tang W, Zheng Y, Dong J, Yu J, Yue J, Liu F, Guo X, Huang S, Wisniewski M, Sun J, Niu X, Ding J, Liu J, Fei Z, Liu Y (2016) Comprehensive transcriptome profiling reveals long noncoding RNA expression and alternative splicing regulation during fruit development and ripening in kiwifruit (Actinidia chinensis). Front Plant Sci 7:335
Li L, Eichten SR, Shimizu R, Petsch K, Yeh CT, Wu W, Chettoor AM, Givan SA, Cole RA, Fowler JE, Evans MMS, Scanlon MJ, Yu J, Schnable PS, Timmermans MCP, Springer NM, Muehlbauer GJ (2014) Genome-wide discovery and characterization of maize long non-coding RNAs. Genome Biol 15:R40
Zhang YC, Liao JY, Li ZY, Yu Y, Zhang JP, Li QF, Qu LH, Shu WS, Chen YQ (2014) Genome-wide screening and functional analysis identify a large number of long noncoding RNAs involved in the sexual reproduction of rice. Genome Biol 15:512
Lekklar C, Pongpanich M, Suriya-Arunroj D, Chinpongpanich A, Tsai H, Comai L, Chadchawan S, Buaboocha T (2019) Genome-wide association study for salinity tolerance at the flowering stage in a panel of rice accessions from Thailand. BMC Genomics 20:76
Mustafiz A, Singh AK, Pareek A, Sopory SK, Singla-Pareek SL (2011) Genome-wide analysis of rice and Arabidopsis identifies two glyoxalase genes that are highly expressed in abiotic stresses. Funct Integr Genomics 11:293–305
Singh A, Giri J, Kapoor S, Tyagi AK, Pandey GK (2010) Protein phosphatase complement in rice: genome-wide identification and transcriptional analysis under abiotic stress conditions and reproductive development. BMC Genomics 11:1–18
Joshi RK, Megha S, Basu U, Rahman MH, Kav NNV (2016) Genome wide identification and functional prediction of long non-coding rnas responsive to Sclerotinia sclerotiorum Infection in Brassica napus. PLoS ONE 11:e0158784
Li S, Yu X, Lei N, Cheng Z, Zhao P, He Y, Wang W, Peng M (2017) Corrigendum: genome-wide identification and functional prediction of cold and/or drought-responsive lncRNAs in cassava. Sci Rep 7:45981
Li H, Wang Y, Chen M, Xiao P, Hu C, Zeng Z, Hu Z (2016) Genome-wide long non-coding RNA screening, identification and characterization in a model microorganism Chlamydomonas reinhardtii. Sci Rep 6:34109
Wang T, Liu M, Zhao M, Chen R, Zhang W (2015) Identification and characterization of long non-coding RNAs involved in osmotic and salt stress in Medicago truncatula using genome-wide high-throughput sequencing. BMC Plant Biol 15:131
Ganie SA, Pani DR, Mondal TK (2017) Genome-wide analysis of DUF221 domain-containing gene family in Oryza species and identification of its salinity stress-responsive members in rice. PLoS ONE 12:e0182469
Jangam AP, Pathak RR, Raghuram N (2016) Microarray analysis of rice d1 (RGA1) mutant reveals the potential role of G-protein alpha subunit in regulating multiple abiotic stresses such as drought, salinity, heat, and cold. Front Plant Sci 7:11
Chen X, Sun S, Liu F, Shen E, Liu L, Ye C, Xiao B, Timko MP, Zhu QH, Fan L, Cao P (2019) A transcriptomic profile of topping responsive non-coding RNAs in tobacco roots. (Nicotiana tabacum). BMC Genomics 20:1–14
Johnsson P, Ackley A, Vidarsdottir L, Lui WO, Corcoran M, Grandér D, Morris KV (2013) A pseudogene long-noncoding-RNA network regulates PTEN transcription and translation in human cells. Nat Struct Mol Biol 20:440–446
Gupta PK (2014) Competing endogenous RNA (ceRNA): a new class of RNA working as miRNA sponges. Curr Sci 106:823–830
Fan C, Hao Z, Yan J, Li G (2015) Genome-wide identification and functional analysis of lincRNAs acting as miRNA targets or decoys in maize. BMC Genomics 16:793
Mondal TK, Ganie SA, Debnath AB (2015) Identification of novel and conserved miRNAs from extreme halophyte, Oryza coarctata, a wild relative of rice. PLoS ONE 10:e0140675
Sharma A, Shahzad B, Rehman A, Bhardwaj R, Landi M, Zheng B (2019) Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 24:2452
Ahmed IM, Cao F, Han Y, Nadira UA, Zhang G, Wu F (2013) Differential changes in grain ultrastructure, amylase, protein and amino acid profiles between Tibetan wild and cultivated barleys under drought and salinity alone and combined stress. Food Chem 141:2743–2750
Rossi L, Borghi M, Francini A, Lin X, Xie DY, Sebastiani L (2016) Salt stress induces differential regulation of the phenylpropanoid pathway in Olea europaea cultivars Frantoio (salt-tolerant) and Leccino (salt-sensitive). J Plant Physiol 204:8–15
Valifard M, Mohsenzadeh S, Niazi A, Moghadam A (2015) Phenylalanine ammonia lyase isolation and functional analysis of phenylpropanoid pathway under salinity stress in’Salvia’species. Aust J Crop Sci 9:656–665
Dehghan S, Sadeghi M, Pöppel A, Fischer R, Lakes-Harlan R, Kavousi HR, Vilcinskas A, Rahnamaeian M (2014) Differential inductions of phenylalanine ammonia-lyase and chalcone synthase during wounding, salicylic acid treatment, and salinity stress in safflower, Carthamus tinctorius. Biosci Rep 34:e00114
Acknowledgements
The authors are grateful to Indian Council of Agricultural Research, New Delhi for financial Assistance and Director, ICAR-National Institute for Plant Biotechnology, New Delhi for providing the facility.
Funding
The research was funded by ICAR, New Delhi through in-house funding.
Author information
Authors and Affiliations
Contributions
TKM: conceived and designed the experiment, PJ did major data analysis and wrote paper, SH performed raw data analysis, HD helped in data analysis. JN performed wet lab validation of data and DSB, TKM and TRS helped in paper writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships or non-financial that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11033_2021_6246_MOESM1_ESM.tif
Supplementary information 1 Fig. S1 Sample correlation matrix of lncRNAs, A) Horkuch root, B) IR-29 root, C) Horkuch leaf, D) IR-29 leaf. (TIF 41541 kb)
11033_2021_6246_MOESM2_ESM.tif
Supplementary information 2 Fig S2 Principal component analysis of lncRNA in control and stress conditions, A) Horkuch root, B) Horkuch leaf, C) IR-29 root, D) IR-29 leaf. (TIF 101178 kb)
11033_2021_6246_MOESM3_ESM.tif
Supplementary information 3 Fig. S3: The heat maps of differentially expressed lncRNAs A) Horkuch root B) IR-29 root tissue, C) Horkuch leaf, D) IR-29 leaf tissue. (TIF 36136 kb)
11033_2021_6246_MOESM4_ESM.tif
Supplementary information 4 Fig. S4: Volcano plot showing Pairwise comparisons of transcript abundance in A) IR-29 root, B) IR-29 leaf, C) Horkuch leaf, D) Horkuch root. Differentially expressed lncRNAs with FDR <0.05 are red coloured dots while black coloured dot represents non-significant differentially expressed lncRNA (TIF 18037 kb)
11033_2021_6246_MOESM5_ESM.tif
Supplementary information 5 Fig.S5: MA plot of differentially expressed lncRNAs in leaf tissue of Horkuch A), IR-29 B) and root tissue of Horkuch C), IR-29 D). Differentially expressed lncRNAs with FDR <0.05 are coloured red. (TIF 18645 kb)
11033_2021_6246_MOESM6_ESM.tif
Supplementary information 6 Fig. S6 Distribution patterns of identified lncRNAs on chromosomes, A) Leaf tissue, B) Root tissue. (TIF 30594 kb)
11033_2021_6246_MOESM9_ESM.tif
Supplementary information 9 Fig. S9 Gene ontology annotation of mRNA present in lncRNA- miRNA-mRNA interaction module. (TIF 21930 kb)
Rights and permissions
About this article
Cite this article
Jain, P., Hussian, S., Nishad, J. et al. Identification and functional prediction of long non-coding RNAs of rice (Oryza sativa L.) at reproductive stage under salinity stress. Mol Biol Rep 48, 2261–2271 (2021). https://doi.org/10.1007/s11033-021-06246-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06246-8