Abstract
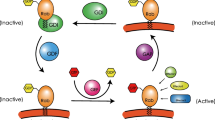
Small GTPases have been shown to play an important role in several cellular functions, including cytoskeletal remodeling, cell polarity, intracellular trafficking, cell-cycle, progression and lipid transformation. The Ras-associated binding (Rab) family of GTPases constitutes the largest family of GTPases and consists of almost 70 known members of small GTPases in humans, which are known to play an important role in the regulation of intracellular membrane trafficking, membrane identity, vesicle budding, uncoating, motility and fusion of membranes. Mutations in Rab genes can cause a wide range of inherited genetic diseases, ranging from neurodegenerative diseases, such as Parkinson’s disease (PD) and Alzheimer’s disease (AD) to immune dysregulation/deficiency syndromes, like Griscelli Syndrome Type II (GS-II) and hemophagocytic lymphohistiocytosis (HLH), as well as a variety of cancers. Here, we provide an extended overview of human Rabs, discussing their function and diseases related to Rabs and Rab effectors, as well as focusing on effects of (aberrant) Rab expression. We aim to underline their importance in health and the development of genetic and malignant diseases by assessing their role in cellular structure, regulation, function and biology and discuss the possible use of stem cell gene therapy, as well as targeting of Rabs in order to treat malignancies, but also to monitor recurrence of cancer and metastasis through the use of Rabs as biomarkers. Future research should shed further light on the roles of Rabs in the development of multifactorial diseases, such as diabetes and assess Rabs as a possible treatment target.


Similar content being viewed by others
Data availability
Not applicable.
References
Claing A (2013) Beta-arrestins: Modulators of small gtpase activation and function. Prog Mol Biol Transl Sci. https://doi.org/10.1016/B978-0-12-394440-5.00006-1
Song S, Cong W, Zhou S, Shi Y, Dai W, Zhang H, Wang X, He B, Zhang Q (2019) Small gtpases: structure, biological function and its interaction with nanoparticles. Asian J Pharm Sci. https://doi.org/10.1016/j.ajps.2018.06.004
Zhen Y, Stenmark H (2015) Cellular functions of rab gtpases at a glance. J Cell Sci. https://doi.org/10.1242/jcs.166074
Echard A, Opdam FJ, de Leeuw HJ, Jollivet F, Savelkoul P, Hendriks W, Voorberg J, Goud B, Fransen JA (2000) Alternative splicing of the human rab6a gene generates two close but functionally different isoforms. Mol Biol Cell. https://doi.org/10.1091/mbc.11.11.3819
Stenmark H, Olkkonen VM (2001) The rab gtpase family. Genome Biol. https://doi.org/10.1186/gb-2001-2-5-reviews3007
Stenmark H (2009) Rab gtpases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. https://doi.org/10.1038/nrm2728
Guadagno NA, Progida C (2019) Rab gtpases: switching to human diseases. Cells. https://doi.org/10.3390/cells8080909
Pylypenko O, Hammich H, Yu IM, Houdusse A (2018) Rab gtpases and their interacting protein partners: structural insights into Rab functional diversity. Small GTPases. https://doi.org/10.1080/215412481336191
Li G (2011) Rab gtpases, membrane trafficking and diseases. Curr Drug Targets. https://doi.org/10.2174/138945011795906561
Mitra S, Cheng KW, Mills GB (2011) Rab gtpases implicated in inherited and acquired disorders. Semin Cell Dev Biol. https://doi.org/10.1016/j.semcdb.2010.12.005
Corbeel L, Freson K (2008) Rab proteins and rab-associated proteins: Major actors in the mechanism of protein-trafficking disorders. Eur J Pediatr. https://doi.org/10.1007/s00431-008-0740-z
Klöpper TH, Kienle N, Fasshauer D, Munro S (2012) Untangling the evolution of rab g proteins: ımplications of a comprehensive genomic analysis. BMC Biol. https://doi.org/10.1186/1741-7007-10-71
Lee SH, Baek K, Dominguez R (2008) Large nucleotide-dependent conformational change in rab28. FEBS Lett. https://doi.org/10.1016/j.febslet.2008.11.008
Tsukuba T, Yamaguchi Y, Kadowaki T (2021) Large Rab gtpases: Novel membrane trafficking regulators with a calcium sensor and functional domains. Int J Mol Sci. https://doi.org/10.3390/ijms22147691
Srikanth S, Woo JS, Gwack Y (2017) A large Rab gtpase family in a small gtpase world. Small GTPases. https://doi.org/10.1080/215412481192921
Shirane M, Nakayama KI (2006) Protrudin induces neurite formation by directional membrane trafficking. J Sci. https://doi.org/10.1126/science.1134027
Egami Y, Taguchi T, Maekawa M, Arai H, Araki N (2014) Small gtpases and phosphoinositides in the regulatory mechanisms of macropinosome formation and maturation. Front Physiol. https://doi.org/10.3389/fphys.2014.00374
Kasmapour B, Cai L, Gutierrez MG (2013) Spatial distribution of phagolysosomes is independent of the regulation of lysosome position by rab34. Int J Biochem Cell Biol. https://doi.org/10.1016/j.biocel.2013.07.003
Kasmapour B, Gronow A, Bleck CK, Hong W, Gutierrez MG (2012) Size-dependent mechanism of cargo sorting during lysosome-phagosome fusion is controlled by rab34. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.1206811109
Prashar A, Schnettger L, Bernard EM, Gutierrez MG (2017) Rab gtpases in immunity and inflammation. Front Cell Infect Microbiol. https://doi.org/10.3389/fcimb.2017.00435
Seabra MC, Mules EH, Hume AN (2002) Rab gtpases, intracellular traffic and disease. Trends Mol Med. https://doi.org/10.1016/s1471-4914(01)02227-4
Bardin S, Miserey-Lenkei S, Hurbain I, Garcia-Castillo D, Raposo G, Goud B (2015) Phenotypic characterisation of rab6a knockout mouse embryonic fibroblasts. Biol Cell. https://doi.org/10.1111/boc.201400083
Borg M, Bakke O, Progida C (2014) A novel interaction between rab7b and actomyosin reveals a dual role in intracellular transport and cell migration. J Cell Sci. https://doi.org/10.1242/jcs.155861
Wu Q, Sun X, Yue W, Lu T, Ruan Y, Chen T, Zhang D (2016) Rab18, a protein associated with warburg micro syndrome, controls neuronal migration in the developing cerebral cortex. Mol Brain. https://doi.org/10.1186/s13041-016-0198-2
Hooper S, Gaggioli C, Sahai E (2010) A chemical biology screen reveals a role for rab21-mediated control of actomyosin contractility in fibroblast-driven cancer invasion. Br J Cancer. https://doi.org/10.1038/sj.bjc.6605469
Giridharan SS, Cai B, Naslavsky N, Caplan S (2012) Trafficking cascades mediated by rab35 and its membrane hub effector, mical-l1. Commun İntegr Biol. https://doi.org/10.4161/cib.20064
He YD, Liu DD, Xi DM, Yang LY, Tan YW, Liu Q, Mao HM, Deng WD (2010) Isolation, sequence identification and expression profile of three novel genes rab2a, rab3a and rab7a from black-boned sheep (Ovis aries). Mol Biol (Mosk) 44(1):20–27
Delfino L, Mason RP, Kyriacou CP, Giorgini F, Rosato E (2020) Rab8 promotes mutant htt aggregation, reduces neurodegeneration, and ameliorates behavioural alterations in a drosophila model of huntington’s disease. J Huntingtons Dis. https://doi.org/10.3233/JHD-200411
Yan T, Wang L, Gao J, Siedlak SL, Huntley ML, Termsarasab P, Perry G, Chen SG, Wang X (2018) Rab10 phosphorylation is a prominent pathological feature in alzheimer’s disease. J Alzheimers Dis. https://doi.org/10.3233/JAD-180023
Sakane A, Honda K, Sasaki T (2010) Rab13 regulates neurite outgrowth in pc12 cells through its effector protein, jrab/mical-l2. Mol Cell Biol. https://doi.org/10.1128/mcb.01067-09
Mamoor S (2022) Differential expression of rab17 in amyotrophic lateral sclerosis. OSF Preprints. https://doi.org/10.31219/osf.io/fmv6r
Wang L, Liang Z, Li G (2011) Rab22 controls ngf signaling and neurite outgrowth in pc12 cells. Mol Biol Cell. https://doi.org/10.1091/mbc.E11-03-0277
Sheehan P, Zhu M, Beskow A, Vollmer C, Waites CL (2016) Activity-dependent degradation of synaptic vesicle proteins requires rab35 and the escrt pathway. J Neurosci. https://doi.org/10.1523/jneurosci.0725-16.2016
Saraste J, Lahtinen U, Goud B (1995) Localization of the small gtp-binding protein rab1p to early compartments of the secretory pathway. J Cell Sci 108(4):1541–1552
Haas AK, Yoshimura S, Stephens DJ, Preisinger C, Fuchs E, Barr FA (2007) Analysis of gtpase-activating proteins: Rab1 and rab43 are key rabs required to maintain a functional golgi complex in human cells. J Cell Sci. https://doi.org/10.1242/jcs.014225
Zenner HL, Yoshimura S, Barr FA, Crump CM (2011) Analysis of rab gtpase-activating proteins indicates that rab1a/b and rab43 are important for herpes simplex virus 1 secondary envelopment. J Virol. https://doi.org/10.1128/jvi.00500-11
Liu X, Wang Z, Yang Y, Li Q, Zeng R, Kang J, Wu J (2016) Rab1a mediates proinsulin to insulin conversion in beta-cells by maintaining golgi stability through interactions with golgin-84. Protein Cell. https://doi.org/10.1007/s13238-016-0298-x
Jewett CE, Soh AWJ, Lin CH, Lu Q, Lencer E, Westlake CJ, Pearson CG, Prekeris R (2021) Rab19 directs cortical remodeling and membrane growth for primary ciliogenesis. Dev Cell. https://doi.org/10.1016/j.devcel.2020.12.003
Dejgaard SY, Murshid A, Erman A, Kizilay O, Verbich D, Lodge R, Dejgaard K, Ly-Hartig TB, Pepperkok R, Simpson JC, Presley JF (2008) Rab18 and rab43 have key roles in er-golgi trafficking. J Cell Sci. https://doi.org/10.1242/jcs.021808
Li C, Wei Z, Fan Y, Huang W, Su Y, Li H, Dong Z, Fukuda M, Khater M, Wu G (2017) The gtpase rab43 controls the anterograde er-golgi trafficking and sorting of gpcrs. Cell Rep. https://doi.org/10.1016/j.celrep.2017.10.011
Kelly EE, Giordano F, Horgan CP, Jollivet F, Raposo G, McCaffrey MW (2012) Rab30 is required for the morphological integrity of the golgi apparatus. Biol Cell. https://doi.org/10.1111/boc.201100080
Zulkefli KL, Mahmoud IS, Williamson NA, Gosavi PK, Houghton FJ, Gleeson PA (2021) A role for rab30 in retrograde trafficking and maintenance of endosome-tgn organization. Exp Cell Res. https://doi.org/10.1016/j.yexcr.2020.112442
Nakazawa H, Sada T, Toriyama M, Tago K, Sugiura T, Fukuda M, Inagaki N (2012) Rab33a mediates anterograde vesicular transport for membrane exocytosis and axon outgrowth. J Neurosci. https://doi.org/10.1523/JNEUROSCI.0989-12.2012
Jacobsen M, Repsilber D, Gutschmidt A, Neher A, Feldmann K, Mollenkopf HJ, Ziegler A, Kaufmann SH (2005) Ras-associated small gtpase 33a, a novel t cell factor, is down-regulated in patients with tuberculosis. J Infect Dis. https://doi.org/10.1086/444428
Morgan NE, Cutrona MB, Simpson JC (2019) Multitasking rab proteins in autophagy and membrane trafficking: a focus on rab33b. Int J Mol Sci. https://doi.org/10.3390/ijms20163916
Lin L, Shi Y, Wang M, Wang C, Zhu J, Zhang R (2019) Rab35/acap2 and rab35/rusc2 complex structures reveal molecular basis for effector recognition by rab35 gtpase. Structure (London). https://doi.org/10.1016/j.str.2019.02.008
Biesemann A, Gorontzi A, Barr F, Gerke V (2017) Rab35 protein regulates evoked exocytosis of endothelial weibel-palade bodies. J Biol Chem. https://doi.org/10.1074/jbc.M116.773333
Feng S, Knödler A, Ren J, Zhang J, Zhang X, Hong Y, Huang S, Peränen J, Guo W (2012) A rab8 guanine nucleotide exchange factor-effector interaction network regulates primary ciliogenesis. J Biol Chem. https://doi.org/10.1074/jbc.M111.333245
Hagemann N, Hou X, Goody RS, Itzen A, Erdmann KS (2012) Crystal structure of the rab binding domain of ocrl1 in complex with rab8 and functional implications of the ocrl1/rab8 module for lowe syndrome. Small GTPases. https://doi.org/10.4161/sgtp.19380
Stypulkowski E, Feng Q, Joseph I, Farrell V, Flores J, Yu S, Sakamori R, Sun J, Bandyopadhyay S, Das S, Dobrowolski R, Bonder EM, Chen MH, Gao N (2021) Rab8 attenuates wnt signaling and is required for mesenchymal differentiation into adipocytes. J Biol Chem. https://doi.org/10.1016/j.jbc.2021.100488
Huber LA, Dupree P, Dotti CG (1995) A deficiency of the small gtpase rab8 inhibits membrane traffic in developing neurons. Mol Cell Biol. https://doi.org/10.1128/MCB.15.2.918
Schluter OM, Schmitz F, Jahn R, Rosenmund C, Sudhof TC (2004) A complete genetic analysis of neuronal rab3 function. J Neurosci. https://doi.org/10.1523/JNEUROSCI.1610-04.2004
Yasuda T, Shibasaki T, Minami K, Takahashi H, Mizoguchi A, Uriu Y, Numata T, Mori Y, Miyazaki J, Miki T, Seino S (2010) Rim2alpha determines docking and priming states in insulin granule exocytosis. Cell Metab. https://doi.org/10.1016/j.cmet.2010.05.017
Coppola T, Frantz C, Perret-Menoud V, Gattesco S, Hirling H, Regazzi R (2002) Pancreatic beta-cell protein granuphilin binds rab3 and munc-18 and controls exocytosis. Mol Biol Cell. https://doi.org/10.1091/mbc.02-02-0025
Matsumoto M, Miki T, Shibasaki T, Kawaguchi M, Shinozaki H, Nio J, Saraya A, Koseki H, Miyazaki M, Iwanaga T, Seino S (2004) Noc2 is essential in normal regulation of exocytosis in endocrine and exocrine cells. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.0306709101
Cazares VA, Subramani A, Saldate JJ, Hoerauf W, Stuenkel EL (2014) Distinct actions of rab3 and rab27 gtpases on late stages of exocytosis of insulin. Traffic. https://doi.org/10.1111/tra.12182
Banworth MJ, Li G (2018) Consequences of Rab GTPase dysfunction in genetic or acquired human diseases. Small GTPases. https://doi.org/10.1080/215412481397833
Efergan A, Azouz NP, Klein O, Noguchi K, Rothenberg ME, Fukuda M, Sagi-Eisenberg R (2016) Rab12 regulates retrograde transport of mast cell secretory granules by interacting with the rilp-dynein complex. J İmmunol. https://doi.org/10.4049/jimmunol.1500731
Nokes RL, Fields IC, Collins RN, Fölsch H (2008) Rab13 regulates membrane trafficking between tgn and recycling endosomes in polarized epithelial cells. J Cell Biol. https://doi.org/10.1083/jcb.200802176
Ohira M, Oshitani N, Hosomi S, Watanabe K, Yamagami H, Tominaga K, Watanabe T, Fujiwara Y, Maeda K, Hirakawa K, Arakawa T (2009) Dislocation of rab13 and vasodilator-stimulated phosphoprotein in inactive colon epithelium in patients with crohn’s disease. Int J Mol Med. https://doi.org/10.3892/ijmm_00000300
Vieira OV (2018) Rab3a and rab10 are regulators of lysosome exocytosis and plasma membrane repair. Small GTPases https://doi.org/10.1080/21541248.2016.1235004
Tavana JP, Rosene M, Jensen NO, Ridge PG, Kauwe JS, Karch CM (2019) Rab10: an alzheimer’s disease resilience locus and potential drug target. Clin Interv Aging. https://doi.org/10.2147/CIA.S159148
Strick DJ, Elferink LA (2005) Rab15 effector protein: a novel protein for receptor recycling from the endocytic recycling compartment. Mol Biol Cell. https://doi.org/10.1091/mbc.e05-03-0204
Lu HF, Hung KS, Chu HW, Wong HS, Kim J, Kim MK, Choi BY, Tai YT, Ikegawa S, Cho EC, Chang WC (2019) Meta-analysis of genome-wide association studies identifies three loci associated with stiffness index of the calcaneus. Journal Bone Miner Res: Offic J Am Soc Bone Miner Res. https://doi.org/10.1002/jbmr.3703
Wong P, Iwasaki A (2017) Rab15 empowers dendritic cells to drive antiviral immunity. Sci Immunol. https://doi.org/10.1126/sciimmunol.aan6448
Zuk PA, Elferink LA (2000) Rab15 differentially regulates early endocytic trafficking. J Biol Chem. https://doi.org/10.1074/jbc.M000344200
Binotti B, Pavlos NJ, Riedel D, Wenzel D, Vorbrüggen G, Schalk AM, Kühnel K, Boyken J, Erck C, Martens H, Chua JJ, Jahn R (2015) The gtpase rab26 links synaptic vesicles to the autophagy pathway. Elife. https://doi.org/10.7554/eLife.05597
Jin RU, Mills JC (2014) Rab26 coordinates lysosome traffic and mitochondrial localization. J Cell Sci. https://doi.org/10.1242/jcs.138776
Masuda ES, Luo Y, Young C, Shen M, Rossi AB, Huang BC, Yu S, Bennett MK, Payan DG, Scheller RH (2000) Rab37 is a novel mast cell specific gtpase localized to secretory granules. FEBS Lett. https://doi.org/10.1016/s0014-5793(00)01288-6
Ljubicic S, Bezzi P, Brajkovic S, Nesca V, Guay C, Ohbayashi N, Fukuda M, Abderrhamani A, Regazzi R (2013) The gtpase rab37 participates in the control of insulin exocytosis. PLoS ONE. https://doi.org/10.1371/journal.pone.0068255
Wu SY, Wu HT, Wang YC, Chang CJ, Shan YS, Wu SR, Chiu YC, Hsu CL, Juan HF, Lan KY, Chu CW, Lee YR, Lan SH (2022) Liu HS (2022) Secretory autophagy promotes rab37-mediated insulin secretion under glucose stimulation both in vitro and in vivo. Autophagy. https://doi.org/10.1080/155486272123098
Sheng Y, Song Y, Li Z, Wang Y, Lin H, Cheng H, Zhou R (2018) Rab37 interacts directly with atg5 and promotes autophagosome formation via regulating atg5–12–16 complex assembly. Cell Death Differ. https://doi.org/10.1038/s41418-017-0023-1
Kimura T, Kaneko Y, Yamada S, Ishihara H, Senda T, Iwamatsu A, Niki I (2008) The gdp-dependent rab27a effector coronin 3 controls endocytosis of secretory membrane in insulin-secreting cell lines. J Cell Sci. https://doi.org/10.1242/jcs.030544
Bierings R, Hellen N, Kiskin N, Knipe L, Fonseca AV, Patel B, Meli A, Rose M, Hannah MJ, Carter T (2012) The interplay between the rab27a effectors slp4-a and myrip controls hormone-evoked weibel-palade body exocytosis. Blood. https://doi.org/10.1182/blood-2012-05-429936
Kuroda TS, Fukuda M, Ariga H, Mikoshiba K (2002) Synaptotagmin-like protein 5: A novel rab27a effector with c-terminal tandem c2 domains. Biochem Biophys Res Commun. https://doi.org/10.1016/s0006-291x(02)00320-0
Gross T, Wack G, Syhr KMJ, Tolmachova T, Seabra MC, Geisslinger G, Niederberger E, Schmidtko A, Kallenborn-Gerhardt W (2020) Rab27a contributes to the processing of inflammatory pain in mice. Cells. https://doi.org/10.3390/cells9061488
Matsunaga K, Taoka M, Isobe T, Izumi T (2017) Rab2a and rab27a cooperatively regulate the transition from granule maturation to exocytosis through the dual effector noc2. J Cell Sci. https://doi.org/10.1242/jcs.195479
Kimura T, Taniguchi S, Niki I (2010) Actin assembly controlled by gdp-rab27a is essential for endocytosis of the insulin secretory membrane. Arch Biochem Biophys. https://doi.org/10.1016/j.abb.2010.01.017
Jiang S, Shen D, Jia WJ, Han X, Shen N, Tao W, Gao X, Xue B, Li CJ (2016) Ggpps-mediated rab27a geranylgeranylation regulates beta cell dysfunction during type 2 diabetes development by affecting insulin granule docked pool formation. J Pathol. https://doi.org/10.1002/path.4652
Kasai K, Ohara-Imaizumi M, Takahashi N, Mizutani S, Zhao S, Kikuta T, Kasai H, Nagamatsu S, Gomi H, Izumi T (2005) Rab27a mediates the tight docking of insulin granules onto the plasma membrane during glucose stimulation. J Clin Invest. https://doi.org/10.1172/JCI22955
Gomi H, Mori K, Itohara S, Izumi T (2007) Rab27b is expressed in a wide range of exocytic cells and involved in the delivery of secretory granules near the plasma membrane. Mol Biol Cell. https://doi.org/10.1091/mbc.e07-05-0409
Starling GP, Yip YY, Sanger A, Morton PE, Eden ER, Dodding MP (2016) Folliculin directs the formation of a rab34-rilp complex to control the nutrient-dependent dynamic distribution of lysosomes. EMBO Rep. https://doi.org/10.15252/embr.201541382
Speight P, Silverman M (2005) Diacylglycerol-activated hmunc13 serves as an effector of the gtpase rab34. Traffic. https://doi.org/10.1111/j.1600-0854.2005.00321.x
Nottingham RM, Pusapati GV, Ganley IG, Barr FA, Lambright DG, Pfeffer SR (2012) Rutbc2 protein, a rab9a effector and gtpase-activating protein for rab36. J Biol Chem. https://doi.org/10.1074/jbc.M112.362558
Matsui T, Ohbayashi N, Fukuda M (2012) The rab interacting lysosomal protein (rilp) homology domain functions as a novel effector domain for small gtpase rab36: Rab36 regulates retrograde melanosome transport in melanocytes. J Biol Chem. https://doi.org/10.1074/jbc.M112.370544
Chen L, Hu J, Yun Y, Wang T (2010) Rab36 regulates the spatial distribution of late endosomes and lysosomes through a similar mechanism to rab34. Mol Membr Biol. https://doi.org/10.3109/09687680903417470
Tokuhisa M, Kadowaki T, Ogawa K, Yamaguchi Y, Kido MA, Gao W, Umeda M, Tsukuba T (2020) Expression and localisation of rab44 in immune-related cells change during cell differentiation and stimulation. Sci Rep. https://doi.org/10.1038/s41598-020-67638-7
Maruta Y, Fukuda M (2022) Large rab gtpase rab44 regulates microtubule-dependent retrograde melanosome transport in melanocytes. J Biol Chem. https://doi.org/10.1016/j.jbc.2022.102508
Jiang Y, Gruzieva O, Wang T, Forno E, Boutaoui N, Sun T, Merid SK, Acosta-Perez E, Kull I, Canino G, Anto JM, Bousquet J, Melen E, Chen W, Celedon JC (2019) Transcriptomics of atopy and atopic asthma in white blood cells from children and adolescents. Eur Respir J. https://doi.org/10.1183/13993003.00102-2019
Kadowaki T, Yamaguchi Y, Ogawa K, Tokuhisa M, Okamoto K, Tsukuba T (2021) Rab44 isoforms similarly promote lysosomal exocytosis, but exhibit differential localization in mast cells. FEBS Open Bio. https://doi.org/10.1002/2211-5463.13133
Longe C, Bratti M, Kurowska M, Vibhushan S, David P, Desmeure V, Huang JD, Fischer A, de Saint BG, Sepulveda FE, Blank U, Menasche G (2022) Rab44 regulates murine mast cell-driven anaphylaxis through kinesin-1-dependent secretory granule translocation. J Allergy Clin Immunol. https://doi.org/10.1016/j.jaci.2022.04.009
Yamaguchi Y, Sakai E, Okamoto K, Kajiya H, Okabe K, Naito M, Kadowaki T, Tsukuba T (2018) Rab44, a novel large rab gtpase, negatively regulates osteoclast differentiation by modulating intracellular calcium levels followed by nfatc1 activation. Cell Mol Life Sci. https://doi.org/10.1007/s00018-017-2607-9
Oshita H, Nishino R, Takano A, Fujitomo T, Aragaki M, Kato T, Akiyama H, Tsuchiya E, Kohno N, Nakamura Y, Daigo Y (2013) Rasef is a novel diagnostic biomarker and a therapeutic target for lung cancer. Mol Cancer Res. https://doi.org/10.1158/1541-7786.Mcr-12-0685-t
Miteva KT, Pedicini L, Wilson LA, Jayasinghe I, Slip RG, Marszalek K, Gaunt HJ, Bartoli F, Deivasigamani S, Sobradillo D, Beech DJ, McKeown L (2019) Rab46 integrates ca(2+) and histamine signaling to regulate selective cargo release from weibel-palade bodies. J Cell Biol. https://doi.org/10.1083/jcb.201810118
Dejgaard SY, Presley JF (2019) Rab18: New insights into the function of an essential protein. Cell Mol Life Sci. https://doi.org/10.1007/s00018-019-03050-3
Handley MT, Morris-Rosendahl DJ, Brown S, Macdonald F, Hardy C, Bem D, Carpanini SM, Borck G, Martorell L, Izzi C, Faravelli F, Accorsi P, Pinelli L, Basel-Vanagaite L, Peretz G, Abdel-Salam GM, Zaki MS, Jansen A, Mowat D, Glass I, Stewart H, Mancini G, Lederer D, Roscioli T, Giuliano F, Plomp AS, Rolfs A, Graham JM, Seemanova E, Poo P, Garcia-Cazorla A, Edery P, Jackson IJ, Maher ER, Aligianis IA (2013) Mutation spectrum in rab3gap1, rab3gap2, and rab18 and genotype-phenotype correlations in warburg micro syndrome and martsolf syndrome. Hum Mutat. https://doi.org/10.1002/humu.22296
Tan R, Wang W, Wang S, Wang Z, Sun L, He W, Fan R, Zhou Y, Xu X, Hong W, Wang T (2013) Small gtpase rab40c associates with lipid droplets and modulates the biogenesis of lipid droplets. PLoS ONE. https://doi.org/10.1371/journal.pone.0063213
Linklater ES, Duncan ED, Han KJ, Kaupinis A, Valius M, Lyons TR, Prekeris R (2021) Rab40-cullin5 complex regulates eplin and actin cytoskeleton dynamics during cell migration. J Cell Biol. https://doi.org/10.1083/jcb.202008060
Han KJ, Mikalayeva V, Gerber SA, Kettenbach AN, Skeberdis VA, Prekeris R (2022) Rab40c regulates focal adhesions and pp6 activity by controlling ankrd28 ubiquitylation. Life Sci Alliance. https://doi.org/10.26508/lsa.202101346
Bedoyan JK, Schaibley VM, Peng W, Bai Y, Mondal K, Shetty AC, Durham M, Micucci JA, Dhiraaj A, Skidmore JM, Kaplan JB, Skinner C, Schwartz CE, Antonellis A, Zwick ME, Cavalcoli JD, Li JZ, Martin DM (2012) Disruption of rab40al function leads to martin–probst syndrome, a rare x-linked multisystem neurodevelopmental human disorder. J Med Genet. https://doi.org/10.1136/jmedgenet-2011-100575
Zhu G, Zhai P, Liu J, Terzyan S, Li G, Zhang XC (2004) Structural basis of rab5-rabaptin5 interaction in endocytosis. Nat Struct Mol Biol. https://doi.org/10.1038/nsmb832
Xu W, Fang F, Ding J, Wu C (2018) Dysregulation of rab5-mediated endocytic pathways in alzheimer’s disease. Traffic. https://doi.org/10.1111/tra.12547
Nielsen E, Christoforidis S, Uttenweiler-Joseph S, Miaczynska M, Dewitte F, Wilm M, Hoflack B, Zerial M (2000) Rabenosyn-5, a novel rab5 effector, is complexed with hvps45 and recruited to endosomes through a fyve finger domain. J Cell Biol. https://doi.org/10.1083/jcb.151.3.601
Nehru V, Voytyuk O, Lennartsson J, Aspenström P (2013) Rhod binds the rab5 effector rabankyrin-5 and has a role in trafficking of the platelet-derived growth factor receptor. Traffic. https://doi.org/10.1111/tra.12121
Christoforidis S, McBride HM, Burgoyne RD, Zerial M (1999) The rab5 effector eea1 is a core component of endosome docking. Nature. https://doi.org/10.1038/17618
Guo X, Farías GG, Mattera R, Bonifacino JS (2016) Rab5 and its effector fhf contribute to neuronal polarity through dynein-dependent retrieval of somatodendritic proteins from the axon. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.1601844113
Kim S, Sato Y, Mohan PS, Peterhoff C, Pensalfini A, Rigoglioso A, Jiang Y, Nixon RA (2016) Evidence that the rab5 effector appl1 mediates app-βctf-induced dysfunction of endosomes in down syndrome and alzheimer’s disease. Mol Psychiatry. https://doi.org/10.1038/mp.2015.97
Bucci C, Lutcke A, Steele-Mortimer O, Olkkonen VM, Dupree P, Chiariello M, Bruni CB, Simons K, Zerial M (1995) Co-operative regulation of endocytosis by three rab5 isoforms. FEBS Lett. https://doi.org/10.1016/0014-5793(95)00477-q
Beaumont KA, Hamilton NA, Moores MT, Brown DL, Ohbayashi N, Cairncross O, Cook AL, Smith AG, Misaki R, Fukuda M, Taguchi T, Sturm RA, Stow JL (2011) The recycling endosome protein rab17 regulates melanocytic filopodia formation and melanosome trafficking. Traffic. https://doi.org/10.1111/j.1600-0854.2011.01172.x
Hunziker W, Peters PJ (1998) Rab17 localizes to recycling endosomes and regulates receptor-mediated transcytosis in epithelial cells. J Biol Chem. https://doi.org/10.1074/jbc.273.25.15734
Ono S, Otomo A, Murakoshi S, Mitsui S, Sato K, Fukuda M, Hadano S (2020) Als2, the small gtpase rab17-interacting protein, regulates maturation and sorting of rab17-associated endosomes. Biochem Biophys Res Commun. https://doi.org/10.1016/j.bbrc.2019.12.122
Jean S, Kiger AA (2016) Rab21 activity assay using gst-fused appl1. Bio-Protoc. https://doi.org/10.21769/BioProtoc.1738
Pellinen T, Tuomi S, Arjonen A, Wolf M, Edgren H, Meyer H, Grosse R, Kitzing T, Rantala JK, Kallioniemi O, Fässler R, Kallio M, Ivaska J (2008) Integrin trafficking regulated by rab21 is necessary for cytokinesis. Dev Cell. https://doi.org/10.1016/j.devcel.2008.08.001
Sun Z, Xie Y, Chen Y, Yang Q, Quan Z, Dai R, Qing H (2018) Rab21, a novel ps1 interactor, regulates gamma-secretase activity via ps1 subcellular distribution. Mol Neurobiol. https://doi.org/10.1007/s12035-017-0606-3
Zhu H, Liang Z, Li G (2009) Rabex-5 is a rab22 effector and mediates a rab22-rab5 signaling cascade in endocytosis. Mol Biol Cell. https://doi.org/10.1091/mbc.e09-06-0453
Johnson DL, Wayt J, Wilson JM, Donaldson JG (2017) Arf6 and rab22 mediate t cell conjugate formation by regulating clathrin-independent endosomal membrane trafficking. J Cell Sci. https://doi.org/10.1242/jcs.200477
Kauppi M, Simonsen A, Bremnes B, Vieira A, Callaghan J, Stenmark H, Olkkonen VM (2002) The small gtpase rab22 interacts with eea1 and controls endosomal membrane trafficking. J Cell Sci. https://doi.org/10.1242/jcs.115.5.899
Magadan JG, Barbieri MA, Mesa R, Stahl PD, Mayorga LS (2006) Rab22a regulates the sorting of transferrin to recycling endosomes. Mol Cell Biol. https://doi.org/10.1128/MCB.26.7.2595-2614.2006
Maldonado-Baez L, Donaldson JG (2013) Hook1, microtubules, and rab22: Mediators of selective sorting of clathrin-independent endocytic cargo proteins on endosomes. BioArchitecture. https://doi.org/10.4161/bioa.26638
Mizuta R, LaSalle JM, Cheng HL, Shinohara A, Ogawa H, Copeland N, Jenkins NA, Lalande M, Alt FW (1997) Rab22 and rab163/mouse brca2: Proteins that specifically interact with the rad51 protein. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.94.13.6927
Shakya S, Sharma P, Bhatt AM, Jani RA, Delevoye C, Setty SR (2018) Rab22a recruits bloc-1 and bloc-2 to promote the biogenesis of recycling endosomes. EMBO Rep. https://doi.org/10.15252/embr.201845918
Weigert R, Yeung AC, Li J, Donaldson JG (2004) Rab22a regulates the recycling of membrane proteins internalized independently of clathrin. Mol Biol Cell. https://doi.org/10.1091/mbc.e04-04-0342
Yeo JC, Wall AA, Luo L, Stow JL (2015) Rab31 and appl2 enhance fcγr-mediated phagocytosis through pi3k/akt signaling in macrophages. Mol Biol Cell. https://doi.org/10.1091/mbc.E14-10-1457
Wei D, Zhan W, Gao Y, Huang L, Gong R, Wang W, Zhang R, Wu Y, Gao S, Kang T (2021) Rab31 marks and controls an escrt-independent exosome pathway. Cell Res. https://doi.org/10.1038/s41422-020-00409-1
Yu H, Lin Y, Xu Y, Chen K, Wang Y, Fu L, Zhou H, Pi L, Che D, Qiu X, Gu X (2022) Association between rab31/rs9965664 polymorphism and immunoglobulin therapy resistance in patients with kawasaki disease. Front Cardiovasc Med. https://doi.org/10.3389/fcvm.2022.944508
Erdman RA, Shellenberger KE, Overmeyer JH, Maltese WA (2000) Rab24 is an atypical member of the rab gtpase family. Deficient gtpase activity, gdp dissociation inhibitor interaction, and prenylation of rab24 expressed in cultured cells. J Biol Chem. https://doi.org/10.1074/jbc.275.6.3848
Ylä-Anttila P, Eskelinen EL (2018) Roles for rab24 in autophagy and disease. Small GTPases. https://doi.org/10.1080/215412481317699
Igci M, Baysan M, Yigiter R, Ulasli M, Geyik S, Bayraktar R, Bozgeyik I, Bozgeyik E, Bayram A, Cakmak EA (2016) Gene expression profiles of autophagy-related genes in multiple sclerosis. Gene. https://doi.org/10.1016/j.gene.2016.04.042
Amaya C, Militello RD, Calligaris SD, Colombo MI (2016) Rab24 interacts with the rab7/rab interacting lysosomal protein complex to regulate endosomal degradation. Traffic. https://doi.org/10.1111/tra.12431
Pei G, Repnik U, Griffiths G, Gutierrez MG (2014) Identification of an immune-regulated phagosomal rab cascade in macrophages. J Cell Sci. https://doi.org/10.1242/jcs.144923
Schnettger L, Rodgers A, Repnik U, Lai RP, Pei G, Verdoes M, Wilkinson RJ, Young DB, Gutierrez MG (2017) A rab20-dependent membrane trafficking pathway controls M. tuberculosis replication by regulating phagosome spaciousness and integrity. Cell Host Microbe. https://doi.org/10.1016/j.chom.2017.04.004
Hackenbeck T, Huber R, Schietke R, Knaup KX, Monti J, Wu X, Klanke B, Frey B, Gaipl U, Wullich B, Ferbus D, Goubin G, Warnecke C, Eckardt KU, Wiesener MS (2011) The gtpase rab20 is a hif target with mitochondrial localization mediating apoptosis in hypoxia. Biochim et Biophys Acta. https://doi.org/10.1016/j.bbamcr.2010.10.019
Oguchi ME, Etoh K, Fukuda M (2018) Rab20, a novel rab small gtpase that negatively regulates neurite outgrowth of pc12 cells. Neurosci Lett. https://doi.org/10.1016/j.neulet.2017.10.056
Liu K, Xing R, Jian Y, Gao Z, Ma X, Sun X, Li Y, Xu M, Wang X, Jing Y, Guo W, Yang C (2017) Wdr91 is a rab7 effector required for neuronal development. J Cell Biol. https://doi.org/10.1083/jcb.201705151
Cai CZ, Yang C, Zhuang XX, Yuan NN, Wu MY, Tan JQ, Song JX, Cheung KH, Su H, Wang YT, Tang BS, Behrends C, Durairajan SSK, Yue Z, Li M (2020) Lu JH (2020) Nrbf2 is a rab7 effector required for autophagosome maturation and mediates the association of app-ctfs with active form of rab7 for degradation. Autophagy. https://doi.org/10.1080/155486271760623
Zhang M, Chen L, Wang S, Wang T (2009) Rab7: Roles in membrane trafficking and disease. Biosci Rep. https://doi.org/10.1042/bsr20090032
Harrison RE, Bucci C, Vieira OV, Schroer TA, Grinstein S (2003) Phagosomes fuse with late endosomes and/or lysosomes by extension of membrane protrusions along microtubules: Role of rab7 and rilp. Mol Cell Biol. https://doi.org/10.1128/MCB.23.18.6494-6506.2003
Li Z, Lai M, Li J, Yang D, Zhao M, Wang D, Sun Z, Wen P, Gou F, Dai Y, Ji Y, Zhao D, Qiao J, Yang L (2022) Rab7a gtpase is involved in mitophagosome formation and autophagosome-lysosome fusion in n2a cells treated with the prion protein fragment 106–126. Mol Neurobiol. https://doi.org/10.1007/s12035-022-03118-5
He D, Chen T, Yang M, Zhu X, Wang C, Cao X, Cai Z (2011) Small rab gtpase rab7b promotes megakaryocytic differentiation by enhancing il-6 production and stat3-gata-1 association. J Mol Med (Berl). https://doi.org/10.1007/s00109-010-0689-z
Walter M, Davies JP, Ioannou YA (2003) Telomerase immortalization upregulates rab9 expression and restores ldl cholesterol egress from niemann-pick c1 late endosomes. J Lipid Res. https://doi.org/10.1194/jlr.M200230-JLR200
Aivazian D, Serrano RL, Pfeffer S (2006) Tip47 is a key effector for rab9 localization. J Cell Biol. https://doi.org/10.1083/jcb.200510010
Díaz E, Schimmöller F, Pfeffer SR (1997) A novel rab9 effector required for endosome-to-tgn transport. J Cell Biol. https://doi.org/10.1083/jcb.138.2.283
Ganley IG, Pfeffer SR (2006) Cholesterol accumulation sequesters rab9 and disrupts late endosome function in npc1-deficient cells. J Biol Chem. https://doi.org/10.1074/jbc.M601679200
Yan H, Li WL, Xu JJ, Zhu SQ, Long X, Che JP (2013) D2 dopamine receptor antagonist raclopride induces non-canonical autophagy in cardiac myocytes. J Cell Biochem. https://doi.org/10.1002/jcb.24306
Jenkins D, Baynam G, De Catte L, Elcioglu N, Gabbett MT, Hudgins L, Hurst JA, Jehee FS, Oley C, Wilkie AO (2011) Carpenter syndrome: extended rab23 mutation spectrum and analysis of nonsense-mediated mrna decay. Hum Mutat. https://doi.org/10.1002/humu.21457
Onnis A, Finetti F, Patrussi L, Gottardo M, Cassioli C, Spanò S, Baldari CT (2015) The small gtpase rab29 is a common regulator of immune synapse assembly and ciliogenesis. Cell Death Differ. https://doi.org/10.1038/cdd.2015.17
Feng M, Hu X, Li N, Hu F, Chang F, Xu HF, Liu YJ (2018) Distinctive roles of rac1 and rab29 in lrrk2 mediated membrane trafficking and neurite outgrowth. J Biomed Res. https://doi.org/10.7555/jbr.31.20170039
MacLeod DA, Rhinn H, Kuwahara T, Zolin A, Di Paolo G, McCabe BD, Marder KS, Honig LS, Clark LN, Small SA, Abeliovich A (2013) Rab7l1 interacts with lrrk2 to modify intraneuronal protein sorting and parkinson’s disease risk. Neuron. https://doi.org/10.1016/j.neuron.2012.11.033
Nalls MA, Blauwendraat C, Vallerga CL, Heilbron K, Bandres-Ciga S, Chang D, Tan M, Kia DA, Noyce AJ, Xue A, Bras J, Young E, von Coelln R, Simon-Sanchez J, Schulte C, Sharma M, Krohn L, Pihlstrom L, Siitonen A, Iwaki H, Leonard H, Faghri F, Gibbs JR, Hernandez DG, Scholz SW, Botia JA, Martinez M, Corvol JC, Lesage S, Jankovic J, Shulman LM, Sutherland M, Tienari P, Majamaa K, Toft M, Andreassen OA, Bangale T, Brice A, Yang J, Gan-Or Z, Gasser T, Heutink P, Shulman JM, Wood NW, Hinds DA, Hardy JA, Morris HR, Gratten J, Visscher PM, Graham RR, Singleton AB, Me Research T, System Genomics of Parkinson’s Disease C, International Parkinson’s Disease Genomics C (2019) Identification of novel risk loci, causal insights, and heritable risk for parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol. https://doi.org/10.1016/S1474-4422(19)30320-5
Haile Y, Deng X, Ortiz-Sandoval C, Tahbaz N, Janowicz A, Lu JQ, Kerr BJ, Gutowski NJ, Holley JE, Eggleton P, Giuliani F, Simmen T (2017) Rab32 connects er stress to mitochondrial defects in multiple sclerosis. J Neuroinflammation. https://doi.org/10.1186/s12974-016-0788-z
Tamura K, Ohbayashi N, Maruta Y, Kanno E, Itoh T, Fukuda M (2009) Varp is a novel rab32/38-binding protein that regulates tyrp1 trafficking in melanocytes. Mol Biol Cell. https://doi.org/10.1091/mbc.e08-12-1161
Wasmeier C, Romao M, Plowright L, Bennett DC, Raposo G, Seabra MC (2006) Rab38 and rab32 control post-golgi trafficking of melanogenic enzymes. J Cell Biol. https://doi.org/10.1083/jcb.200606050
Gao Y, Wilson GR, Stephenson SEM, Bozaoglu K, Farrer MJ, Lockhart PJ (2018) The emerging role of rab gtpases in the pathogenesis of parkinson’s disease. Mov Disord. https://doi.org/10.1002/mds.27270
Gerondopoulos A, Langemeyer L, Liang JR, Linford A, Barr FA (2012) Bloc-3 mutated in hermansky-pudlak syndrome is a rab32/38 guanine nucleotide exchange factor. Curr Biol. https://doi.org/10.1016/j.cub.2012.09.020
Buffa L, Fuchs E, Pietropaolo M, Barr F, Solimena M (2008) Ica69 is a novel rab2 effector regulating er-golgi trafficking in insulinoma cells. Eur J Cell Biol. https://doi.org/10.1016/j.ejcb.2007.11.003
Short B, Preisinger C, Körner R, Kopajtich R, Byron O, Barr FA (2001) A grasp55-rab2 effector complex linking golgi structure to membrane traffic. J Cell Biol. https://doi.org/10.1083/jcb.200108079
White JA 2nd, Anderson E, Zimmerman K, Zheng KH, Rouhani R, Gunawardena S (2015) Huntingtin differentially regulates the axonal transport of a sub-set of rab-containing vesicles in vivo. Hum Mol Genet. https://doi.org/10.1093/hmg/ddv415
Sugawara T, Kano F, Murata M (2014) Rab2a is a pivotal switch protein that promotes either secretion or er-associated degradation of (pro)insulin in insulin-secreting cells. Sci Rep. https://doi.org/10.1038/srep06952
Morohoshi A, Miyata H, Oyama Y, Oura S, Noda T, Ikawa M (2021) Fam71f1 binds to rab2a and rab2b and is essential for acrosome formation and male fertility in mice. Development. https://doi.org/10.1242/dev.199644
Takahama M, Fukuda M, Ohbayashi N, Kozaki T, Misawa T, Okamoto T, Matsuura Y, Akira S, Saitoh T (2017) The rab2b-garil5 complex promotes cytosolic DNA-induced innate immune responses. Cell Rep. https://doi.org/10.1016/j.celrep.2017.08.085
Ni X, Ma Y, Cheng H, Jiang M, Guo L, Ji C, Gu S, Cao Y, Xie Y, Mao Y (2002) Molecular cloning and characterization of a novel human rab ( rab2b) gene. J Hum Genet. https://doi.org/10.1007/s100380200083
Aizawa M, Fukuda M (2015) Small gtpase rab2b and its specific binding protein golgi-associated rab2b interactor-like 4 (gari-l4) regulate golgi morphology. J Biol Chem. https://doi.org/10.1074/jbc.M115.669242
Chano T, Avnet S (2018) Rab39a: a rab small gtpase with a prominent role in cancer stemness. J Biochem. https://doi.org/10.1093/jb/mvy041
Seto S, Sugaya K, Tsujimura K, Nagata T, Horii T, Koide Y (2013) Rab39a interacts with phosphatidylinositol 3-kinase and negatively regulates autophagy induced by lipopolysaccharide stimulation in macrophages. PLoS ONE. https://doi.org/10.1371/journal.pone.0083324
Gambarte Tudela J, Buonfigli J, Lujan A, Alonso Bivou M, Cebrian I, Capmany A, Damiani MT (2019) Rab39a and rab39b display different intracellular distribution and function in sphingolipids and phospholipids transport. Int J Mol Sci. https://doi.org/10.3390/ijms20071688
Matsui T, Sakamaki Y, Nakashima S, Fukuda M (2022) Rab39 and its effector uaca regulate basolateral exosome release from polarized epithelial cells. Cell Rep. https://doi.org/10.1016/j.celrep.2022.110875
Cruz FM, Colbert JD, Rock KL (2020) The gtpase rab39a promotes phagosome maturation into mhc-i antigen-presenting compartments. EMBO J. https://doi.org/10.15252/embj.2019102020
Mignogna ML, Giannandrea M, Gurgone A, Fanelli F, Raimondi F, Mapelli L, Bassani S, Fang H, Van Anken E, Alessio M, Passafaro M, Gatti S, Esteban JA, Huganir R, D’Adamo P (2015) The intellectual disability protein rab39b selectively regulates glua2 trafficking to determine synaptic ampar composition. Nat Commun. https://doi.org/10.1038/ncomms7504
Giannandrea M, Bianchi V, Mignogna ML, Sirri A, Carrabino S, D’Elia E, Vecellio M, Russo S, Cogliati F, Larizza L, Ropers HH, Tzschach A, Kalscheuer V, Oehl-Jaschkowitz B, Skinner C, Schwartz CE, Gecz J, Van Esch H, Raynaud M, Chelly J, de Brouwer AP, Toniolo D, D’Adamo P (2010) Mutations in the small gtpase gene rab39b are responsible for x-linked mental retardation associated with autism, epilepsy, and macrocephaly. Am J Hum Genet. https://doi.org/10.1016/j.ajhg.2010.01.011
Wilson GR, Sim JC, McLean C, Giannandrea M, Galea CA, Riseley JR, Stephenson SE, Fitzpatrick E, Haas SA, Pope K, Hogan KJ, Gregg RG, Bromhead CJ, Wargowski DS, Lawrence CH, James PA, Churchyard A, Gao Y, Phelan DG, Gillies G, Salce N, Stanford L, Marsh AP, Mignogna ML, Hayflick SJ, Leventer RJ, Delatycki MB, Mellick GD, Kalscheuer VM, D’Adamo P, Bahlo M, Amor DJ, Lockhart PJ (2014) Mutations in rab39b cause x-linked intellectual disability and early-onset parkinson disease with α-synuclein pathology. Am J Hum Genet. https://doi.org/10.1016/j.ajhg.2014.10.015
Niu M, Zheng N, Wang Z, Gao Y, Luo X, Chen Z, Fu X, Wang Y, Wang T, Liu M, Yao T, Yao P, Meng J, Zhou Y, Ge Y, Wang Z, Ma Q, Xu H, Zhang YW (2020) Rab39b deficiency impairs learning and memory partially through compromising autophagy. Front Cell Dev Biol. https://doi.org/10.3389/fcell.2020.598622
Tsai YT (2013) Analysis of rab42 and rab40c as novel regulators of secretory membrane trafficking. Natural Sciences. Ruperto-Carola University of Heidelberg, Heidelberg
Fouraux MA, Deneka M, Ivan V, van der Heijden A, Raymackers J, van Suylekom D, van Venrooij WJ, van der Sluijs P, Pruijn GJ (2004) Rabip4’ is an effector of rab5 and rab4 and regulates transport through early endosomes. Mol Biol Cell. https://doi.org/10.1091/mbc.e03-05-0343
Mari M, Monzo P, Kaddai V, Keslair F, Gonzalez T, Le Marchand-Brustel Y, Cormont M (2006) The rab4 effector rabip4 plays a role in the endocytotic trafficking of glut 4 in 3t3-l1 adipocytes. J Cell Sci. https://doi.org/10.1242/jcs.02850
Yamamoto H, Koga H, Katoh Y, Takahashi S, Nakayama K, Shin HW (2010) Functional cross-talk between rab14 and rab4 through a dual effector, rufy1/rabip4. Mol Biol Cell. https://doi.org/10.1091/mbc.e10-01-0074
White JA 2nd, Krzystek TJ, Hoffmar-Glennon H, Thant C, Zimmerman K, Iacobucci G, Vail J, Thurston L, Rahman S, Gunawardena S (2020) Excess rab4 rescues synaptic and behavioral dysfunction caused by defective htt-rab4 axonal transport in huntington’s disease. Acta Neuropathol Commun. https://doi.org/10.1186/s40478-020-00964-z
Wang J, Deretic D (2015) The arf and rab11 effector fip3 acts synergistically with asap1 to direct rabin8 in ciliary receptor targeting. J Cell Sci. https://doi.org/10.1242/jcs.162925
Roland JT, Bryant DM, Datta A, Itzen A, Mostov KE, Goldenring JR (2011) Rab gtpase-myo5b complexes control membrane recycling and epithelial polarization. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.1010754108
Swiatecka-Urban A, Talebian L, Kanno E, Moreau-Marquis S, Coutermarsh B, Hansen K, Karlson KH, Barnaby R, Cheney RE, Langford GM, Fukuda M, Stanton BA (2007) Myosin vb is required for trafficking of the cystic fibrosis transmembrane conductance regulator in rab11a-specific apical recycling endosomes in polarized human airway epithelial cells. J Biol Chem. https://doi.org/10.1074/jbc.M608531200
Lock JG, Stow JL (2005) Rab11 in recycling endosomes regulates the sorting and basolateral transport of e-cadherin. Mol Biol Cell. https://doi.org/10.1091/mbc.e04-10-0867
Vijay S, Chiu M, Dacks JB, Roberts RC (2016) Exclusive expression of the rab11 effector sh3tc2 in schwann cells links integrin-α6 and myelin maintenance to charcot-marie-tooth disease type 4c. Biochem Biophys Acta. https://doi.org/10.1016/j.bbadis.2016.04.003
Xu CL, Wang JZ, Xia XP, Pan CW, Shao XX, Xia SL, Yang SX, Zheng B (2016) Rab11-fip2 promotes colorectal cancer migration and invasion by regulating pi3k/akt/mmp7 signaling pathway. Biochem Biophys Res Commun. https://doi.org/10.1016/j.bbrc.2016.01.031
Richards P, Didszun C, Campesan S, Simpson A, Horley B, Young KW, Glynn P, Cain K, Kyriacou CP, Giorgini F, Nicotera P (2011) Dendritic spine loss and neurodegeneration is rescued by rab11 in models of huntington’s disease. Cell Death Differ. https://doi.org/10.1038/cdd.2010.127
Li X, Valencia A, McClory H, Sapp E, Kegel KB, Difiglia M (2012) Deficient rab11 activity underlies glucose hypometabolism in primary neurons of huntington’s disease mice. Biochem Biophys Res Commun. https://doi.org/10.1016/j.bbrc.2012.04.070
Caswell PT, Spence HJ, Parsons M, White DP, Clark K, Cheng KW, Mills GB, Humphries MJ, Messent AJ, Anderson KI, McCaffrey MW, Ozanne BW, Norman JC (2007) Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3d microenvironments. Dev Cell. https://doi.org/10.1016/j.devcel.2007.08.012
Nam KT, Lee HJ, Smith JJ, Lapierre LA, Kamath VP, Chen X, Aronow BJ, Yeatman TJ, Bhartur SG, Calhoun BC, Condie B, Manley NR, Beauchamp RD, Coffey RJ, Goldenring JR (2010) Loss of rab25 promotes the development of intestinal neoplasia in mice and is associated with human colorectal adenocarcinomas. J Clin Invest. https://doi.org/10.1172/JCI40728
Lindsay AJ, McCaffrey MW (2015) Rab antibody characterization: comparison of rab14 antibodies. Methods Mol Biol (Clifton). https://doi.org/10.1007/978-1-4939-2569-8_13
Li Y, Liu H, Shao J, Xing G (2017) Mir-320a serves as a negative regulator in the progression of gastric cancer by targeting rab14. Mol Med Rep. https://doi.org/10.3892/mmr.2017.6937
Linford A, Yoshimura S, Nunes Bastos R, Langemeyer L, Gerondopoulos A, Rigden DJ, Barr FA (2012) Rab14 and its exchange factor fam116 link endocytic recycling and adherens junction stability in migrating cells. Dev Cell. https://doi.org/10.1016/j.devcel.2012.04.010
Wang M, Wang W, Ding J, Wang J, Zhang J (2020) Downregulation of rab17 promotes cell proliferation and invasion in non-small cell lung cancer through stat3/hif-1alpha/vegf signaling. Thoracic Cancer. https://doi.org/10.1111/1759-7714.13278
Bergbrede T, Chuky N, Schoebel S, Blankenfeldt W, Geyer M, Fuchs E, Goody RS, Barr F, Alexandrov K (2009) Biophysical analysis of the interaction of rab6a gtpase with its effector domains. J Biol Chem. https://doi.org/10.1074/jbc.M806003200
Fernandes H, Franklin E, Recacha R, Houdusse A, Goud B, Khan AR (2009) Structural aspects of rab6-effector complexes. Biochem Soc Trans. https://doi.org/10.1042/bst0371037
Matsuto M, Kano F, Murata M (2015) Reconstitution of the targeting of rab6a to the golgi apparatus in semi-intact hela cells: A role of bicd2 in stabilizing rab6a on golgi membranes and a concerted role of rab6a/bicd2 interactions in golgi-to-er retrograde transport. Biochim et Biophys Acta. https://doi.org/10.1016/j.bbamcr.2015.05.005
Pusapati GV, Luchetti G, Pfeffer SR (2012) Ric1-rgp1 complex is a guanine nucleotide exchange factor for the late golgi rab6a gtpase and an effector of the medial golgi rab33b gtpase. J Biol Chem. https://doi.org/10.1074/jbc.M112.414565
Liu S, Hunt L, Storrie B (2013) Rab41 is a novel regulator of golgi apparatus organization that is needed for er-to-golgi trafficking and cell growth. PLoS ONE. https://doi.org/10.1371/journal.pone.0071886
Liu S, Majeed W, Kudlyk T, Lupashin V, Storrie B (2016) Identification of rab41/6d effectors provides an explanation for the differential effects of rab41/6d and rab6a/a’ on golgi organization. Front Cell Dev Biol. https://doi.org/10.3389/fcell.2016.00013
Iarossi G, Marino V, Maltese PE, Colombo L, D’Esposito F, Manara E, Dhuli K, Modarelli AM, Cennamo G, Magli A, Dell’Orco D, Bertelli M (2020) Expanding the clinical and genetic spectrum of rab28-related cone-rod dystrophy: pathogenicity of novel variants in Italian families. Int J Mol Sci. https://doi.org/10.3390/ijms22010381
Blacque OE, Scheidel N, Kuhns S (2018) Rab gtpases in cilium formation and function. Small GTPases. https://doi.org/10.1080/215412481353847
Ohishi Y, Ammann S, Ziaee V, Strege K, Gross M, Amos CV, Shahrooei M, Ashournia P, Razaghian A, Griffiths GM, Ehl S, Fukuda M, Parvaneh N (2020) Griscelli syndrome type 2 sine albinism: Unraveling differential rab27a effector engagement. Front Immunol. https://doi.org/10.3389/fimmu.2020.612977
Menasche G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, Wulffraat N, Bianchi D, Fischer A, Le Deist F, de Saint BG (2000) Mutations in rab27a cause griscelli syndrome associated with haemophagocytic syndrome. Nat Genet. https://doi.org/10.1038/76024
Catz SD (2014) The role of rab27a in the regulation of neutrophil function. Cell Microbiol. https://doi.org/10.1111/cmi.12328
Cetica V, Hackmann Y, Grieve S, Sieni E, Ciambotti B, Coniglio ML, Pende D, Gilmour K, Romagnoli P, Griffiths GM, Arico M (2015) Patients with griscelli syndrome and normal pigmentation identify rab27a mutations that selectively disrupt munc13–4 binding. J Allergy Clin Immunol. https://doi.org/10.1016/j.jaci.2014.08.039
BasuRay S, Mukherjee S, Romero E, Wilson MC, Wandinger-Ness A (2010) Rab7 mutants associated with charcot-marie-tooth disease exhibit enhanced ngf-stimulated signaling. PLoS ONE. https://doi.org/10.1371/journal.pone.0015351
Verhoeven K, De Jonghe P, Coen K, Verpoorten N, Auer-Grumbach M, Kwon JM, FitzPatrick D, Schmedding E, De Vriendt E, Jacobs A, Van Gerwen V, Wagner K, Hartung HP, Timmerman V (2003) Mutations in the small gtp-ase late endosomal protein rab7 cause charcot-marie-tooth type 2b neuropathy. Am J Hum Genet. https://doi.org/10.1086/367847
Aligianis IA, Johnson CA, Gissen P, Chen D, Hampshire D, Hoffmann K, Maina EN, Morgan NV, Tee L, Morton J, Ainsworth JR, Horn D, Rosser E, Cole TR, Stolte-Dijkstra I, Fieggen K, Clayton-Smith J, Megarbane A, Shield JP, Newbury-Ecob R, Dobyns WB, Graham JM Jr, Kjaer KW, Warburg M, Bond J, Trembath RC, Harris LW, Takai Y, Mundlos S, Tannahill D, Woods CG, Maher ER (2005) Mutations of the catalytic subunit of rab3gap cause warburg micro syndrome. Nat Genet. https://doi.org/10.1038/ng1517
Aligianis IA, Morgan NV, Mione M, Johnson CA, Rosser E, Hennekam RC, Adams G, Trembath RC, Pilz DT, Stoodley N, Moore AT, Wilson S, Maher ER (2006) Mutation in rab3 gtpase-activating protein (rab3gap) noncatalytic subunit in a kindred with martsolf syndrome. Am J Hum Genet. https://doi.org/10.1086/502681
Fukui K, Sasaki T, Imazumi K, Matsuura Y, Nakanishi H, Takai Y (1997) Isolation and characterization of a gtpase activating protein specific for the rab3 subfamily of small g proteins. J Biol Chem. https://doi.org/10.1074/jbc.272.8.4655
Nagano F, Sasaki T, Fukui K, Asakura T, Imazumi K, Takai Y (1998) Molecular cloning and characterization of the noncatalytic subunit of the rab3 subfamily-specific gtpase-activating protein. J Biol Chem. https://doi.org/10.1074/jbc.273.38.24781
Ravikumar B, Imarisio S, Sarkar S, O’Kane CJ, Rubinsztein DC (2008) Rab5 modulates aggregation and toxicity of mutant huntingtin through macroautophagy in cell and fly models of huntington disease. J Cell Sci. https://doi.org/10.1242/jcs.025726
Gendaszewska-Darmach E, Garstka MA, Blazewska KM (2021) Targeting small gtpases and their prenylation in diabetes mellitus. J Med Chem. https://doi.org/10.1021/acs.jmedchem.1c00410
Ginsberg SD, Alldred MJ, Counts SE, Cataldo AM, Neve RL, Jiang Y, Wuu J, Chao MV, Mufson EJ, Nixon RA, Che S (2010) Microarray analysis of hippocampal ca1 neurons implicates early endosomal dysfunction during alzheimer’s disease progression. Biol Psychiatry. https://doi.org/10.1016/j.biopsych.2010.05.030
Ginsberg SD, Mufson EJ, Alldred MJ, Counts SE, Wuu J, Nixon RA, Che S (2011) Upregulation of select rab gtpases in cholinergic basal forebrain neurons in mild cognitive impairment and alzheimer’s disease. J Chem Neuroanat. https://doi.org/10.1016/j.jchemneu.2011.05.012
Soo KY, Halloran M, Sundaramoorthy V, Parakh S, Toth RP, Southam KA, McLean CA, Lock P, King A, Farg MA, Atkin JD (2015) Rab1-dependent er-golgi transport dysfunction is a common pathogenic mechanism in sod1, tdp-43 and fus-associated als. Acta Neuropathol. https://doi.org/10.1007/s00401-015-1468-2
Coune PG, Bensadoun JC, Aebischer P, Schneider BL (2011) Rab1a over-expression prevents golgi apparatus fragmentation and partially corrects motor deficits in an alpha-synuclein based rat model of parkinson’s disease. J Parkinsons Dis. https://doi.org/10.3233/JPD-2011-11058
Chung CY, Koprich JB, Hallett PJ, Isacson O (2009) Functional enhancement and protection of dopaminergic terminals by rab3b overexpression. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.0912193106
Seabra MC, Brown MS, Goldstein JL (1993) Retinal degeneration in choroideremia: deficiency of rab geranylgeranyl transferase. Science. https://doi.org/10.1126/science.8380507
Seabra MC, Ho YK, Anant JS (1995) Deficient geranylgeranylation of ram/rab27 in choroideremia. J Biol Chem. https://doi.org/10.1074/jbc.270.41.24420
Guney-Esken G, Aerts-Kaya F, (Methods in molecular biology, (2022) Generation and hematopoietic differentiation of mesenchymal stromal/stem cell-derived induced pluripotent stem cell lines for disease modeling of hematopoietic and immunological diseases. Methods Mol Biol (Clifton). https://doi.org/10.1007/7651_2021_452
Guney-Esken G, Erol OD, Pervin B, Gurhan Sevinc G, Onder T, Bilgic E, Korkusuz P, Gunel-Ozcan A, Uckan-Cetinkaya D, Aerts-Kaya F (2021) Development, characterization, and hematopoietic differentiation of griscelli syndrome type 2 induced pluripotent stem cells. Stem Cell Res Ther. https://doi.org/10.1186/s13287-021-02364-z
Wang Y, Sun H, Wang Z, Yang Z, Shi M, Yang J, Liu Y, Liu H, Zhang S, Shi C, Xu Y (2017) Generation of induced pluripotent stem cell line (zzui005-a) from a 21-year-old patient with a novel rab39b gene mutation in x-linked juvenile parkinsonism. Stem Cell Res. https://doi.org/10.1016/j.scr.2017.10.021
Erol OD, Seker ME, Aerts-Kaya F (2021) Correction of rab27a from griscelli syndrome type ii-derived mesenchymal stem cells. Human Gene Ther 32:19–20
Tolmachova T, Tolmachov OE, Barnard AR, de Silva SR, Lipinski DM, Walker NJ, Maclaren RE, Seabra MC (2013) Functional expression of rab escort protein 1 following aav2-mediated gene delivery in the retina of choroideremia mice and human cells ex vivo. J Mol Med (Berl). https://doi.org/10.1007/s00109-013-1006-4
Vasireddy V, Mills JA, Gaddameedi R, Basner-Tschakarjan E, Kohnke M, Black AD, Alexandrov K, Zhou S, Maguire AM, Chung DC, Mac H, Sullivan L, Gadue P, Bennicelli JL, French DL, Bennett J (2013) Aav-mediated gene therapy for choroideremia: preclinical studies in personalized models. PLoS ONE. https://doi.org/10.1371/journal.pone.0061396
MacLaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, Clark KR, During MJ, Cremers FP, Black GC, Lotery AJ, Downes SM, Webster AR, Seabra MC (2014) Retinal gene therapy in patients with choroideremia: ınitial findings from a phase 1/2 clinical trial. Lancet. https://doi.org/10.1016/S0140-6736(13)62117-0
Tolmachova T, Tolmachov OE, Wavre-Shapton ST, Tracey-White D, Futter CE, Seabra MC (2012) Chm/rep1 cdna delivery by lentiviral vectors provides functional expression of the transgene in the retinal pigment epithelium of choroideremia mice. J Gene Med. https://doi.org/10.1002/jgm.1652
Soheili T, Durand A, Sepulveda FE, Riviere J, Lagresle-Peyrou C, Sadek H, de Saint BG, Martin S, Mavilio F, Cavazzana M, Andre-Schmutz I (2017) Gene transfer into hematopoietic stem cells reduces hlh manifestations in a murine model of munc13–4 deficiency. Blood Adv. https://doi.org/10.1182/bloodadvances.2017012088
Cooray SS, Hacohen Y, Worth A, Eleftheriou D, Hemingway C (2022) Treatment strategies for central nervous system effects in primary and secondary haemophagocytic lymphohistiocytosis in children. Curr Treat Opt Neurol. https://doi.org/10.1007/s11940-022-00705-8
Sahenk Z, Galloway G, Clark KR, Malik V, Rodino-Klapac LR, Kaspar BK, Chen L, Braganza C, Montgomery C, Mendell JR (2014) AAV1.Nt-3 gene therapy for Charcot-Marie-Tooth neuropathy. Mol Ther. https://doi.org/10.1038/mt.2013.250
Ikawa Y, Hess R, Dorward H, Cullinane AR, Huizing M, Gochuico BR, Gahl WA, Candotti F (2015) In vitro functional correction of hermansky-pudlak syndrome type-1 by lentiviral-mediated gene transfer. Mol Genet Metab. https://doi.org/10.1016/j.ymgme.2014.11.006
Nieto-Alamilla G, Behan M, Hossain M, Gochuico BR, Malicdan MCV (2022) Hermansky-pudlak syndrome: gene therapy for pulmonary fibrosis. Mol Genet Metab. https://doi.org/10.1016/j.ymgme.2022.08.008
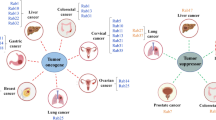
Gopal Krishnan PD, Golden E, Woodward EA, Pavlos NJ, Blancafort P (2020) Rab gtpases: emerging oncogenes and tumor suppressive regulators for the editing of survival pathways in cancer. Cancers. https://doi.org/10.3390/cancers12020259
Xu BH, Li XX, Yang Y, Zhang MY, Rao HL, Wang HY, Zheng XF (2015) Aberrant amino acid signaling promotes growth and metastasis of hepatocellular carcinomas through rab1a-dependent activation of mtorc1 by rab1a. Oncotarget. https://doi.org/10.18632/oncotarget.5175
Su H, Li T, Li C, Liu X, Ling H, Li X (2020) Expression of rab1a in bladder cancer and its clinical implications. Exp Ther Med. https://doi.org/10.3892/etm.2020.9174
Kajiho H, Kajiho Y, Frittoli E, Confalonieri S, Bertalot G, Viale G, Di Fiore PP, Oldani A, Garre M, Beznoussenko GV, Palamidessi A, Vecchi M, Chavrier P, Perez F, Scita G (2016) Rab2a controls mt1-mmp endocytic and e-cadherin polarized golgi trafficking to promote invasive breast cancer programs. EMBO Rep. https://doi.org/10.1552/embr.201642032
Kim JK, Lee SY, Park CW, Park SH, Yin J, Kim J, Park JB, Lee JY, Kim H, Kim SC (2014) Rab3a promotes brain tumor initiation and progression. Mol Biol Rep. https://doi.org/10.1007/s11033-014-3465-2
Luo Q, Liu Y, Yuan Z, Huang L, Diao B (2021) Expression of rab3b in human glioma: Influence on cell proliferation and apoptosis. Curr Pharm Des. https://doi.org/10.2174/1381612826666200917145228
Chang YC, Su CY, Chen MH, Chen WS, Chen CL, Hsiao M (2017) Secretory rab gtpase 3c modulates il6-stat3 pathway to promote colon cancer metastasis and is associated with poor prognosis. Mol Cancer. https://doi.org/10.1186/s12943-017-0687-7
Yang J, Liu W, Lu X, Fu Y, Li L, Luo Y (2015) High expression of small gtpase rab3d promotes cancer progression and metastasis. Oncotarget. https://doi.org/10.1832/oncotarget.3575
Luo Y, Ye GY, Qin SL, Mu YF, Zhang L, Qi Y, Qiu YE, Yu MH, Zhong M (2016) High expression of rab3d predicts poor prognosis and associates with tumor progression in colorectal cancer. Int J Biochem Cell Biol. https://doi.org/10.1016/j.biocel.2016.03.017
Frittoli E, Palamidessi A, Marighetti P, Confalonieri S, Bianchi F, Malinverno C, Mazzarol G, Viale G, Martin-Padura I, Garré M, Parazzoli D, Mattei V, Cortellino S, Bertalot G, Di Fiore PP, Scita G (2014) A rab5/rab4 recycling circuitry induces a proteolytic invasive program and promotes tumor dissemination. J Cell Biol. https://doi.org/10.1083/jcb.201403127
Silva P, Mendoza P, Rivas S, Díaz J, Moraga C, Quest AF, Torres VA (2016) Hypoxia promotes rab5 activation, leading to tumor cell migration, invasion and metastasis. Oncotarget. https://doi.org/10.18632/oncotarget.8794
Liu Q, Bai Y, Shi X, Guo D, Wang Y, Wang Y, Guo WZ, Zhang S (2022) High ras-related protein rab-7a (rab7a) expression is a poor prognostic factor in pancreatic adenocarcinoma. Sci Rep. https://doi.org/10.1038/s41598-022-22355-1
Liu Y, Wang X, Zhang Z, Xiao B, An B, Zhang J (2019) The overexpression of rab9 promotes tumor progression regulated by xbp1 in breast cancer. OncoTargets ther. https://doi.org/10.2147/ott.S183748
Boulay PL, Mitchell L, Turpin J, Huot-Marchand J, Lavoie C, Sanguin-Gendreau V, Jones L, Mitra S, Livingstone JM, Campbell S, Hallett M, Mills GB, Park M, Chodosh L, Strathdee D, Norman JC, Muller WJ (2016) Rab11-fip1c is a critical negative regulator in erbb2-mediated mammary tumor progression. Cancer Res. https://doi.org/10.1158/0008-5472.Can-15-2782
Chen P, Chen G, Wang C, Mao C (2019) Rab13 as a novel prognosis marker promotes proliferation and chemotherapeutic resistance in gastric cancer. Biochem Biophys Res Commun. https://doi.org/10.1016/j.bbrc.2019.08.141
Ioannou MS, Bell ES, Girard M, Chaineau M, Hamlin JN, Daubaras M, Monast A, Park M, Hodgson L, McPherson PS (2015) Dennd2b activates rab13 at the leading edge of migrating cells and promotes metastatic behavior. J Cell Biol. https://doi.org/10.1083/jcb.201407068
Chen TW, Yin FF, Yuan YM, Guan DX, Zhang E, Zhang FK, Jiang H, Ma N, Wang JJ, Ni QZ, Qiu L, Feng J, Zhang XL, Bao Y, Wang K, Cheng SQ, Wang XF, Wang X, Li JJ, Xie D (2019) Chml promotes liver cancer metastasis by facilitating rab14 recycle. Nat Commun. https://doi.org/10.1038/s41467-019-10364-0
Wang K, Mao Z, Liu L, Zhang R, Liang Q, Xiong Y, Yuan W, Wei L (2015) Rab17 inhibits the tumourigenic properties of hepatocellular carcinomas via the erk pathway. Tumour Biol: Journal Int Soc Oncodev Biol Med. https://doi.org/10.1007/s13277-015-3251-3
Jiang X, Yang L, Gao Q, Liu Y, Feng X, Ye S, Yang Z (2022) The role of rab gtpases and its potential in predicting immunotherapy response and prognosis in colorectal cancer. Front genet. https://doi.org/10.3389/fgene.2022.828373
Ge J, Chen Q, Liu B, Wang L, Zhang S, Ji B (2017) Knockdown of rab21 inhibits proliferation and induces apoptosis in human glioma cells. Cell Mol Biol Lett. https://doi.org/10.1186/s11658-017-0062-0
Liu Y, Zeng C, Bao N, Zhao J, Hu Y, Li C, Chi S (2015) Effect of rab23 on the proliferation and apoptosis in breast cancer. Oncol Rep. https://doi.org/10.3892/or.2015.4152
Jiang Y, Han Y, Sun C, Han C, Han N, Zhi W, Qiao Q (2016) Rab23 is overexpressed in human bladder cancer and promotes cancer cell proliferation and invasion. Tumour Biol. https://doi.org/10.1007/s13277-015-4590-9
Cheng KW, Lahad JP, Gray JW, Mills GB (2005) Emerging role of rab gtpases in cancer and human disease. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-05-0573
Cheng KW, Lahad JP, Kuo WL, Lapuk A, Yamada K, Auersperg N, Liu J, Smith-McCune K, Lu KH, Fishman D, Gray JW, Mills GB (2004) The rab25 small gtpase determines aggressiveness of ovarian and breast cancers. Nat Med. https://doi.org/10.1038/nm1125
Jeong BY, Cho KH, Jeong KJ, Park YY, Kim JM, Rha SY, Park CG, Mills GB, Cheong JH, Lee HY (2018) Rab25 augments cancer cell invasiveness through a beta1 integrin/egfr/vegf-a/snail signaling axis and expression of fascin. Exp Mol Med. https://doi.org/10.1038/emm.2017.248
Jeong H, Lim KM, Kim KH, Cho Y, Lee B, Knowles BC, Roland JT, Zwerner JP, Goldenring JR, Nam KT (2019) Loss of rab25 promotes the development of skin squamous cell carcinoma through the dysregulation of integrin trafficking. J Pathol. https://doi.org/10.1002/path.5311
Dong WW, Mou Q, Chen J, Cui JT, Li WM, Xiao WH (2012) Differential expression of rab27a/b correlates with clinical outcome in hepatocellular carcinoma. World J Gastroenterol. https://doi.org/10.3748/wjg.v18.i15.1806
Tan PY, Chang CW, Chng KR, Wansa KD, Sung WK, Cheung E (2012) Integration of regulatory networks by nkx3–1 promotes androgen-dependent prostate cancer survival. Mol Cell Biol. https://doi.org/10.1128/mcb.05958-11
Worst TS, Meyer Y, Gottschalt M, Weis CA, von Hardenberg J, Frank C, Steidler A, Michel MS, Erben P (2017) Rab27a, rab27b and vps36 are downregulated in advanced prostate cancer and show functional relevance in prostate cancer cells. Int J Oncol. https://doi.org/10.3892/ijo.2017.3872
Hendrix A, Maynard D, Pauwels P, Braems G, Denys H, Van den Broecke R, Lambert J, Van Belle S, Cocquyt V, Gespach C, Bracke M, Seabra MC, Gahl WA, De Wever O, Westbroek W (2010) Effect of the secretory small gtpase rab27b on breast cancer growth, invasion, and metastasis. Je Natl Cancer Inst. https://doi.org/10.1093/jnci/djq153
Grismayer B, Solch S, Seubert B, Kirchner T, Schafer S, Baretton G, Schmitt M, Luther T, Kruger A, Kotzsch M, Magdolen V (2012) Rab31 expression levels modulate tumor-relevant characteristics of breast cancer cells. Mol Cancer. https://doi.org/10.1186/1476-4598-11-62
Yang T, Zhiheng H, Zhanhuai W, Qian X, Yue L, Xiaoxu G, Jingsun W, Shu Z, Kefeng D (2020) Increased rab31 expression in cancer-associated fibroblasts promotes colon cancer progression through hgf-met signaling. Front Oncol. https://doi.org/10.3389/fonc.2020.01747
Yang L, Tian X, Chen X, Lin X, Tang C, Gao Y, Chen S, Ge Z (2020) Upregulation of rab31 is associated with poor prognosis and promotes colorectal carcinoma proliferation via the mtor/p70s6k/cyclin d1 signalling pathway. Life Sci. https://doi.org/10.1016/j.lfs.2020.118126
Tsai CH, Cheng HC, Wang YS, Lin P, Jen J, Kuo IY, Chang YH, Liao PC, Chen RH, Yuan WC, Hsu HS, Yang MH, Hsu MT, Wu CY, Wang YC (2014) Small gtpase rab37 targets tissue inhibitor of metalloproteinase 1 for exocytosis and thus suppresses tumour metastasis. Nat Commun. https://doi.org/10.1038/ncomms5804
Sun L, Yan T, Yang B (2022) The progression related gene rab42 affects the prognosis of glioblastoma patients. Brain Sci. https://doi.org/10.3390/brainsci12060767
Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Fearon K, Hütterer E, Isenring E, Kaasa S, Krznaric Z, Laird B, Larsson M, Laviano A, Mühlebach S, Muscaritoli M, Oldervoll L, Ravasco P, Solheim T, Strasser F, de van der Schueren M, Preiser JC (2017) Espen guidelines on nutrition in cancer patients. Clin Nutr. https://doi.org/10.1016/j.clnu.2016.07.015
Chua CE, Tang BL (2015) The role of the small gtpase rab31 in cancer. J Cell Mol Med. https://doi.org/10.1111/jcmm.12403
Anand S, Khan MA, Khushman M, Dasgupta S, Singh S, Singh AP (2020) Comprehensive analysis of expression, clinicopathological association and potential prognostic significance of Rabs in pancreatic cancer. Int J Mol Sci. https://doi.org/10.3390/ijms21155580
Zhao H, Wang Q, Wang X, Zhu H, Zhang S, Wang W, Wang Z, Huang J (2016) Correlation between rab27b and p53 expression and overall survival in pancreatic cancer. Pancreas. https://doi.org/10.1097/MPA.0000000000000453
Wang Q, Ni Q, Wang X, Zhu H, Wang Z, Huang J (2015) High expression of rab27a and tp53 in pancreatic cancer predicts poor survival. Med Oncol. https://doi.org/10.1007/s12032-014-0372-2
Li J, Jin Q, Huang F, Tang Z, Huang J (2017) Effects of rab27a and rab27b on invasion, proliferation, apoptosis, and chemoresistance in human pancreatic cancer cells. Pancreas. https://doi.org/10.1097/mpa.0000000000000910
Li BY, He LJ, Zhang XL, Liu H, Liu B (2019) High expression of rab38 promotes malignant progression of pancreatic cancer. Mol Med Rep. https://doi.org/10.3892/mmr.2018.9732
Liu J, Gong X, Zhu X, Xue D, Liu Y, Wang P (2017) Rab27a overexpression promotes bladder cancer proliferation and chemoresistance through regulation of nf-κb signaling. Oncotarget. https://doi.org/10.18632/oncotarget.20775
Yoon SO, Shin S, Mercurio AM (2005) Hypoxia stimulates carcinoma invasion by stabilizing microtubules and promoting the rab11 trafficking of the alpha6beta4 integrin. Cancer Res. https://doi.org/10.1158/0008-5472.Can-04-4122
Cheng JM, Volk L, Janaki DK, Vyakaranam S, Ran S, Rao KA (2010) Tumor suppressor function of rab25 in triple-negative breast cancer. Int J Cancer. https://doi.org/10.1002/ijc.24900
Ren P, Yang XQ, Zhai XL, Zhang YQ, Huang JF (2016) Overexpression of rab27b is correlated with distant metastasis and poor prognosis in ovarian cancer. Oncol Lett. https://doi.org/10.3892/ol.2016.4801
Xu C, Liang T, Liu J, Fu Y (2022) Rab39b as a chemosensitivity-related biomarker for diffuse large b-cell lymphoma. Front Pharmacol. https://doi.org/10.3389/fphar.2022.931501
Jia LS, Michael T (2018) The function of rab35 in development and disease. In: Shihori T (ed) Peripheral membrane proteins. IntechOpen, Rijeka, p 4
Acknowledgements
This work was supported by grants from the Hacettepe University, Scientific Research Coordination Unit project nr. TUK-2019-17760 and THD-2022-19940 and the Scientific and Technological Research Council of Turkey (TÜBİTAK) project nr. 219S675.
Author information
Authors and Affiliations
Contributions
ODE and SS: drafted the first version of the article; FA-K: designed the manuscript, reviewed the contents and wrote the final version of the article to be published; all authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Consent to participations
Not applicable.
Consent to publications
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Erol, Ö.D., Şenocak, Ş. & Aerts-Kaya, F. The Role of Rab GTPases in the development of genetic and malignant diseases. Mol Cell Biochem 479, 255–281 (2024). https://doi.org/10.1007/s11010-023-04727-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-023-04727-x