Abstract
The aim of our study was to compare the richness and composition of macroinvertebrate assemblages in natural reed and artificial rip-rap habitats in a shallow lake 50+ years after shoreline modifications. Lake Balaton (Hungary) provided a unique study system as approximately half of its shoreline (c.105 km) has been modified. Littoral macroinvertebrates were collected in two habitat types (artificial rip-rap and natural reed) around the shoreline over two seasons. We found that native taxon richness of rip-rap habitat was only one-twentieth of the natural reed habitat. Rip-rap habitat harboured significantly more alien species. We found that the proportion of alien taxa was higher in rip-rap habitat (89.2%) than in reed habitat (16.7%). The composition of macroinvertebrate assemblages in the two habitats was also significantly different with limited to moderate overlap. Furthermore, all 8 indicator taxa of rip-rap habitat were alien, whilst all 28 indicator taxa were native in reed habitat. These results suggest that artificial engineering structure creates a novel ecosystem dominated by alien species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lakes and their shorelines are important to ecosystem services in several aspects. Lakeshores are important because of the ecological significance for ecosystem functioning (Vadeboncoeur et al., 2002), and because their different habitats are hotspots of biodiversity considering different organisms groups (Vadeboncoeur et al., 2011). Freshwater species and habitats are increasingly threatened worldwide (Allan & Flecker 1993; Saunders et al., 2002; Reid et al., 2019; Tickner et al., 2020), due to the importance of lakeshores for humans causing a continuous increase of shore developments worldwide (Schnaiberg et al., 2002; Schmieder, 2004).
The consequence of human shoreline development in the last half century included a reduction of littoral biodiversity and an alteration of littoral communities, shown in several studies (e.g. Radomski & Goeman 2001; Scheuerell & Schindler 2004; Brauns et al., 2007; Miler & Brauns, 2020). Human shoreline development has been recognized as a significant threat to the ecological integrity of lakes through the simplification of the littoral zone (Ostendorp et al., 2004; Brauns et al., 2007; Verdonschot et al., 2013). It has a negative impact on the composition and condition of near-shore aquatic emergent and submerged macrophyte communities (Radomski & Goeman 2001; Miler & Brauns, 2020). Moreover, human modification of shores and littoral zones reduces the abundance and changes the taxonomic composition of macroinvertebrate assemblages (Brauns et al., 2007; McGoff et al., 2013; Pätzig et al., 2015).
Shoreline development may also facilitate the establishment of invasive species (Johnson et al., 2008; Brabender et al., 2016), especially in lakes connected to navigable rivers (Bobeldyk et al., 2005). Invasive alien aquatic species have increasingly important environmental, social and economic impacts (Laverty et al., 2015). As global trade and travel increase (Dick & Platvoet 2000; Keller et al., 2011), the strength of ecosystem services is diminishing, also as invasive species reduce native biodiversity, which in turn harms the environment and thus the economy (Simberloff et al., 2013). New species may preadapt due to the similarity of their ancestral habitat, leading to relatively rapid colonization, whilst richness of native species may simultaneously decrease by anthropogenic modifications. Thus, successful invasive species may be able to exploit resources more efficiently, disrupt native food webs (Paolucci et al., 2013), or spread diseases for which native species lack immunity (Laverty et al., 2015). It is now also known that invasive aquatic macroinvertebrates not only disrupt aquatic ecosystems, but also impact terrestrial food webs via cross-ecosystem flow of resources (Gergs et al., 2014).
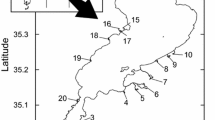
Lake Balaton is the largest shallow lake in Central Europe, and one of the region's foremost touristic destinations. Approx. 100 years ago, reeds (helophytic perennial plants) were dominant almost along the entire coast (sometimes up to 100 m wide). This habitat type has declined significantly in the last 90 years due to the spread of bathing culture and tourism. Today, the coastline is scattered, out of the 240 km shoreline, reed patches are present along approximately 112 km of shoreline, and cover a total area of 16 km2 (Stratoulias & Tóth, 2020). The remaining approx. 128 km long shoreline is almost entirely transformed to rip-rap covered concrete structures. This type of artificial shoreline was constructed between 1930 and 1965 (Balogh et al., 2008), and its structure follows a unified layout around the entire shoreline (see Fig. 1).
Several invasive species appeared in Lake Balaton during or after shoreline modification (Table 1). Spreading of aquatic invertebrate alien species is an ongoing threat in Europe, mainly due to the historical development of inland waterways (Laverty et al., 2015). Lake Balaton is connected to the Ponto-Caspian region through the Southern corridor, which is one of the major invasive corridors of Europe. Large artificial rip-rap shorelines were colonized by several Ponto-Caspian species including Dreissena polymorpha (Pallas, 1771) and D. bugensis (Andrusov, 1897) (Balogh et al., 2008). These two Dreissena mussel species are the most abundant invasive species in Lake Balaton, and their colonization has had various effects: it has changed the abundance of planktonic mussels, the mussel colonies improve water transparency by filtering out particles (both live and dead) and provide an important source of food for many fish (Balogh et al., 2008). The amphipod Chelicorophium curvispinum (G.O. Sars, 1895) utilizes algae and detritus as food and is itself an important fish food item (Muskó et al., 2007). Limnomysis benedeni Czerniavsky, 1882 is also utilized as fish food in Lake Balaton (Szalontai, 2008).
The objective of the present study was to compare macroinvertebrate assemblages in natural reed and artificial rip-rap habitats of Lake Balaton, Hungary. We examined the role of the two habitats (1) in maintaining native taxa richness and (2) in supporting alien taxa. Finally, (3) we examined whether the assemblage composition of the two habitats differed emphasizing the long-term success of the alien species. To achieve these objectives we examined macroinvertebrate assemblages of Lake Balaton. We hypothesized that although artificial rip-rap habitats are surrounded by natural habitats, which might act as sources for the colonization of native species, rip-rap habitat will host a reduced richness of native, and an increased richness of alien aquatic macroinvertebrates. We expect that our comparison of macroinvertebrate assemblages in natural reed and artificial rip-rap habitat provides information on how habitat differences influence the spread and success of alien species in the two habitats.
Materials and methods
Study area and design
Lake Balaton (N 46° 50′, E 17° 44′) is the largest shallow freshwater lake in Central Europe (surface area: 593 km2, average depth: 3.14 m, length: 77.9 km, average width: 7.2 km) (Padisák, 1992; Crossetti et al., 2013). To protect the shoreline of the lake, approximately 105 km of rip-rap, built from red sandstone, was constructed between 1930 and 1965 (Balogh et al., 2008). Consequently, half of the littoral zone of the lake is modified, and reed vegetated areas are now fragmented, with a total area of only 11 km2 (Erős et al., 2008; Tóth & Szabó, 2012) corresponding to a 110-km length of shoreline. We selected 10 rip-rap and 10 reed sites distributed evenly along the shoreline of the lake (Fig. 2, Suppl. Table 1), representing the actual proportion of natural and artificial habitats in the lake. We aimed to choose pairs of rip-rap and reed sites located close to each other.
Sampling and identification of macroinvertebrates
Two different sampling methods were used for reed and rip-rap habitats. Both sampling techniques are considered as an adequate qualitative sampling method and are generally applied in community ecology studies. In reed habitat, we collected macroinvertebrates using a hand net (250 mm wide, 500 µm mesh size) following an established sampling method (McGoff et al., 2013; Pätzig et al., 2015; Porst et al., 2019) from an area of approximately 0.5 m2. During the sampling, the net was swept intensively through the habitat (i.e. vegetation, debris, and surface sediment). This procedure was repeated five times (five subsamples) to cover the entire width of reed from the closest point to the shore to the closest point to the open water, and subsamples were aggregated and preserved in 70% ethanol. At rip-rap sites, we followed Brauns et al. (2007), Porst et al. (2019), and Schreiber & Brauns (2010): A piece of stone was randomly selected and placed on a tray. Macroinvertebrates attempting to escape were collected by hand net. All individuals from the estimated surface of the stone were collected. We repeated this procedure until we reached an approximate surface area of 0.5 m2. Macroinvertebrates were preserved in 70% ethanol and transferred to the laboratory. Macroinvertebrates were sampled in spring (May 2020) and autumn (Sept 2020). Subsequently, the study design includes 40 sampling units: 10 sites, 2 habitats (reed and rip-rap), and 2 seasons (spring and autumn).
Macroinvertebrates were identified to the lowest taxonomic level possible. We used the identification keys of Elliott & Mann (1979), Richnovszky & Pintér (1979), Savage (1989), Csabai (2000), Csabai et al. (2002), Kontschán et al. (2002), Eiseler (2005), Waringer and Graf (2011), Ambrus et al. (2018), Glöer (2019). Following Kobak et al. (2010), and Balogh et al. (2018), small Dreissena individuals were identified as Dreissena sp. (juv). Diptera were identified only to family level. We decided to use only presence/absence data to avoid errors as a result of abundance differences caused by different sampling methods (Elliott & Drake, 1981; Drake & Elliott, 1982; Blocksom & Flotemersch 2005; Cao et al., 2005).
Taxa were classified as non-native (alien) and native based on the DAISIE database (DAISIE, 2009). However, we had to adjust the categorization for two species [Planorbarius corneus (Linnaeus, 1758)] and Synurella ambulans (F. Müller, 1846), which are classified as alien in Western Europe but not in Hungary (Fehér et al., 2006; Sidorov & Palatov, 2012). As in Hungary only two Dreissena species exist (D. polymorpha and D. bugensis, both alien), we considered Dreissena sp. (juv) as an alien taxa. Protected species are listed in the corresponding 100/2012 (IX. 28.) decree of the Hungarian Ministry of Environment.
Statistical analyses
Linear models (LMs) were used to examine whether the numbers of native and alien taxa were influenced by habitat (categorical predictor with two levels: reed and rip-rap), season (categorical predictor with two levels: spring and autumn) and an interaction of these factors. We selected the best-fit models based on the Akaike Information Criterion corrected for the number of cases and parameters estimated (AICc) and Akaike weights (Garamszegi & Mundry, 2014). Delta AICc indicates the difference in the fit between a particular model considered and that of the best-fit model. Models with delta AICc < 10 are considered to be better fitting models. AIC weight was calculated among all possible pairs. Generalized linear models (GLMs) with binomial distribution were used to test whether habitat, season, and their interaction influenced the presence of protected taxa. We applied this approach because protected taxa were rare (range between 0 and 2) and the distribution was zero inflated. As in linear models, best-fit models were selected based on AICc.
Constrained Analysis of Principal Coordinates (CAP, Anderson & Willis, 2003) with Sørensen Distance (Podani, 2000) was used to test the separation of macroinvertebrate communities in reed and rip-rap habitats. We ran an ANOVA-like permutation test for the significance of the separation. Indicator Value analysis (Dufrene & Legendre, 1997; De Cáceres, 2020) was used to identify indicator taxa for reed and rip-rap habitat. Permutational tests (Dufrene & Legendre, 1997; n = 999) were run for assessing significance. Analyses were run in the R statistical environment (R Core Team, 2021) using the ‘indicspecies’ (De Cáceres, 2020), 'MASS' (Venables & Ripley, 2002), 'MuMIn' (Barton, 2020), and 'vegan' (Oksanen et al., 2020) packages.
Results
Altogether 111 taxa were found among the 62,055 individuals (Suppl. Table 2). We identified 3 protected and 11 alien taxa (Suppl. Table 1). The most abundant taxa were Dreissena sp. (juv) (alien) (24,490 individuals), Dreissena bugensis (alien) (19,524 individuals), Dikerogammarus villosus (Sowinsky, 1894) (alien) (4881 individuals), Valvata cristata O. F. Müller, 1774 (native) (2281 individuals), Chelicorophium curvispinum (alien) (2075 individuals), Theodoxus fluviatilis (Linnaeus, 1758) (alien) (1957 individuals), and Dreissena polymorpha (alien) (1692 individuals).
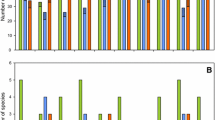
The best-supported model (with the lowest AICc) revealed that the native taxa richness of macroinvertebrates was influenced only by the habitat (Table 2). This statistical model explained the observed data well (adjusted R2 = 0.80) and suggested that rip-rap habitat had a lower number of native taxa than reed habitat (LM, estimate for rip-rap = − 19.50, s.e. = 1.545, t-value = − 12.62, P < 0.001). The average number of native taxa richness in reed habitat was 20.50, whilst in rip-rap habitat it was 1.0, representing a 95% decrease (Fig. 3).
Model selection showed that the best statistical model included only habitat in explaining the presence of protected species (Table 3, Fig. 4). However, we did not find statistical evidence for a difference between the occurrence of protected species in the reed (60%) and rip-rap (0%) habitats (GLM, estimate for rip-rap: − 19.972, s.e. = 2404.670, z-value = − 0.008, P = 0.993). The average proportion of native taxa in the reed habitat was 83.3%, whilst in the rip-rap habitat it was 10.8%
The best-supported linear model showed that the alien taxa richness of macroinvertebrates was explained by the joint effects of habitat and season, whilst the second-best model indicated the importance of habitat, season and their interaction (Table 2). The best-fit models explained the observed data well (adjusted R2 = 0.621) and suggested that the number of alien taxa was higher in rip-rap habitat compared to reed habitat (LM, estimate for rip-rap = 4.100 s.e. = 0.524, t-value = 7.820, P < 0.001, Fig. 5). This model also showed that the number of alien species was significantly different in autumn and in spring (LM, estimate for spring = − 1.100, s.e. = 0.524, t-value = − 2.098, P = 0.043). The average proportion of alien taxa in the reed habitat was 16.7%, whilst in the rip-rap habitat it was 89.2%.
Constrained Analysis of Principal Coordinates (Suppl. Table 3) showed that the natural reed and the artificial rip-rap habitat differed in the composition of macroinvertebrate communities (ANOVA-like permutations: F1,38 = 34.445, P = 0.001). The ordination plot showed that a single constrained axis (CAP1) could separate reed and rip-rap assemblages (Fig. 6). The distribution range of points along the vertical unconstrained axis (MDS1) suggested that reed communities showed considerable variability compared to communities in the rip-rap habitat.
We found 28 indicator taxa for reed and 8 indicator taxa for the rip-rap habitats (Table 4). In reed habitat all 28 indicator taxa were native, whereas the indicator taxa of the rip-rap habitat were all alien taxa.
Discussion
Our study reports drastic differences between the macroinvertebrate assemblages in natural and human-modified shorelines of Lake Balaton. We observed that only 5% of the native taxa richness of natural reed was present in artificial rip-rap habitat. We found also that artificial rip-rap habitat harboured significantly more alien taxa. Finally, we observed compositional differences between the macroinvertebrate assemblages of the two habitats. These results suggest that the transformation of natural shorelines into artificial rip-rap habitat decreases native richness and supports the spread of alien species.
We found that artificial habitat supported a lower richness of native macroinvertebrates compared to native habitats. This finding concurs with studies examining the effects of human shoreline modification on littoral macroinvertebrates in lakes (Pätzig et al., 2015, 2018; Porst et al., 2019). Our study is, however, the first that reports such a drastic difference between the richness of macroinvertebrates in natural and human-modified shorelines of a lake: we observed that only 5% of the native taxa richness of reed was present in rip-rap habitat. A possible explanation is that reed beds present a wide variety of abiotic and biotic variables and thus offer several diverse niches for macroinvertebrate taxa. Moreover, rip-rap habitat provides exclusively hard mineral substrate, a microhabitat that is not available in reed habitat. Thus, the reduction in native taxa richness can be explained by the differences in the conservation status of the two habitats (Grzybowski & Glińska-Lewczuk, 2019), as well as by the interaction between habitat quality and alien species (Strayer, 2010). Our results disagree with the results of Brauns et al. (2007), who did not find a difference in macroinvertebrate richness between the natural and artificial rip-rap shorelines of seven German lowland lakes. This comparison suggests that individual lakes might show considerable differences and thus further studies are needed to reveal the general impact of human-induced shoreline modification on biodiversity.
We observed that the richness of alien taxa was higher in the artificial habitat than in the natural reed habitat. This result concurs with other studies stating that artificial habitats are more susceptible to invasions (Strayer & Findlay, 2010) and that invasive species prefer artificial habitats (Schreiber et al., 2003). In agreement with this, some studies showed that the traits of native and invasive macroinvertebrate species differ (Stazner et al., 2008), and this might explain the different adaptions to different habitats. According to our results, the proportion of alien taxa was 16.7% in the natural habitat and 89.2% in the artificial habitat. These values suggest that alien taxa occupy a sizable proportion of the natural habitat and can have extreme dominance in the artificial habitat (Bozóki et al., 2018). However, underlying mechanisms explaining this pattern are unknown. A possible explanation is that rip-rap and reed habitats act as a source-sink relationship considering invasive species. Unfortunately, studies focussing on the effect of lakeshore modification on littoral macroinvertebrates did not provide such information (Brauns et al., 2007; Pätzig et al., 2015, 2018; Porst et al., 2019).
We found that the macroinvertebrate composition of natural and artificial shorelines differed. Indicator species analysis identified exclusively native indicator taxa for the natural reed-vegetated shoreline, and only alien indicator taxa for the artificial rip-rap habitat. The majority of these alien indicator taxa, such as Dreissena bugensis, Dreissena polymorpha, Dreissena sp. (juv), Chelicorophium curvispinum, Jaera sp., Dikerogammarus villosus, and Dikerogammarus haemobaphes (Eichwald, 1841) originate from the Ponto-Caspian region (Borza, 2012; Bódis et al., 2012), and cause serious problems world-wide by displacing native species. For instance, the invasive species Dikerogammarus haemobaphes shows strong competition with native species for resources, and thus can replace native species (Guareschi et al., 2021). It follows that low native diversity in rip-rap habitat might reflect the effect of habitat degradation, but might also reflect the effect of invasive species (Schmidlin et al., 2012; Guareschi et al., 2021). It seems that further studies are needed to disentangle the separate effects of habitat degradation and alien species.
Seasonal differences are a well-known feature of freshwater macroinvertebrate communities (Beche et al., 2006; Hill et al., 2016). Our results showed seasonal differences, mainly in the natural habitat (reed field). The species composition of the macroinvertebrate community of the rip-rap was constant and dominated by sessile Bivalvia and moderately mobile Amphipoda taxa. This finding is in line with Pätzig et al. (2015) suggesting that a single seasonal sampling event is sufficient to capture the compositional differences of macroinvertebrate communities associated with human lakeshore modifications.
From a conservation standpoint, our study showed that the natural reed-vegetated shoreline of Lake Balaton supports a rich and valuable macroinvertebrate fauna, which is threatened by habitat modification. Our study presents clear evidence that the transformation of the natural reed-vegetated shoreline to artificial rip-rap habitat alone, or together with the spread of alien taxa, caused a significant and drastic loss in native taxa richness. The loss together with compositional changes suggests that the altered environmental conditions in rip-rap habitats lead to the creation of novel ecosystems for almost completely new macroinvertebrate communities. Our documented change in native richness calls the attention to management decision plans to reverse global freshwater biodiversity loss (Tickner et al., 2020).
Conclusion
Although there is a clear human need for transforming the natural shoreline of lakes to artificial habitat, we have limited information on how this action influences biodiversity. To fill this knowledge gap, we compared macroinvertebrate assemblages in natural reed and artificial rip-rap habitats in Lake Balaton, Hungary. We found that the native taxa richness of rip-rap habitat is one eighth of that of natural reed habitat, and the proportion of alien taxa can be as high as 89%. We found also that in reed habitat all 28 indicator taxa were native, whereas in the rip-rap all 8 indicator taxa habitat were alien—all these alien indicator species are of Ponto-Caspian origin.
These findings suggest that shoreline alteration decreases native richness and supports alien species. Our results imply that shoreline management decisions should consider not only social and economic benefits, but also the environmental costs of such habitat transformation including biodiversity loss.
Our findings have several implications for decision-making. First of all, the transformation of reed-vegetated shoreline to artificial rip-rap habitat needs to be prevented, especially where it is not an absolute necessity for human activities. Once widely-distributed reed areas of Lake Balaton are now fragmented with a total area of only 11 km2 (Tóth & Szabó, 2012). Management actions have to be undertaken to support existing reed-vegetated shoreline, and action plans should be implemented to restore artificial rip-rap habitats to natural shoreline habitats where possible. Our results suggest that these actions may mitigate the spread and impact of alien species, thereby preventing further losses of native freshwater biodiversity.
Data availability
Data is available on request from the authors.
References
Allan, J. D. & A. S. Flecker, 1993. Biodiversity conservation in running waters. Bioscience 43: 32–43.
Ambrus, A., T. Danyik, T. Kovács, &, O. Olajos, 2018. Magyarország szitakötőinek kézikönyve. MondAt Kft.
Anderson, M.J., & T.J. Willis, 2003. Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology 84: 511–525.
Balogh, C., I. B. Muskó, L. G. Tóth & L. Nagy, 2008. Quantitative trends of zebra mussels in Lake Balaton (Hungary) in 2003–2005 at different water levels. Hydrobiologia 613: 57–69.
Balogh, C., A. Vláčilová, G. László & Z. Serfőző, 2018. Dreissenid colonization during the initial invasion of the quagga mussel in the largest Central European shallow lake, Lake Balaton, Hungary. Journal Great Lakes Research 44: 114–125.
Barton, K., 2020. MuMIn: multi-model inference. R Package Version 1(47): 17.
Beche, L. A., E. P. McElravy & V. H. Resh, 2006. Long-term seasonal variation in the biological traits of benthic-macroinvertebrates in two Mediterranean climate streams in California, U.S.A. Freshwater Biology 1: 56–75.
Bíró, K. & P. Gulyás, 1974. Zoological investigations in the open water Potamogeton perfoliatus stands of Lake Balaton. Annales Instituti Biologici (tihany) Hungaricae Academiae Scientiarum 41: 181–203.
Blocksom, K. A. & J. E. Flotemersch, 2005. Comparison of macroinvertebrate sampling methods for non wadeable streams. Environmental Monitoring and Assessment 102: 243–262.
Bobeldyk, A. M., J. M. Bossenbroek, M. A. Evans-White, D. M. Lodge & G. A. Lamberti, 2005. Secondary spread of zebra mussels (Dreissena polymorpha) in coupled lake–stream systems. Ecoscience 12: 339–346.
Borza, P., 2012. (Ponto-kaszpikus magasabbrendű rákok (Crustacea: Malacostraca: Mysida, Amphipoda, Isopoda) faunisztikai és taxonómiai vizsgálata a Duna vízrendszerében. Ph.D dissertation. MTA ÖK Duna-kutató Intézet.) [Faunistic and taxonomic study of higher order Ponto-Caspian crustaceans (Crustacea: Malacostraca: Mysida, Amphipoda, Isopoda) in the Danube water system. Ph.D dissertation, p.65., Eötvös Loránd Research Network Centre for Ecological Research].
Bódis, E., P. Borza, I. Potyó, A. Weiperth & G. Guti, 2012. Invasive Mollusc, Crustacean, fish and reptile species along the Hungarian stretch of the River Danube and some connected waters. Acta Zoologica Academiae Scientiarum Hungaricae 58: 29–45.
Bozóki, T., E. Á. Krasznai-Kun, A. Csercsa, G. Várbíró & P. Boda, 2018. Temporal and spatial dynamics in aquatic macroinvertebrate communities along a small urban stream. Environmental Earth Sciences 77(15): 1–10.
Brabender, M., M. Weitere, C. Anlanger & M. Brauns, 2016. Secondary production and richness of native and non-native macroinvertebrates are driven by human-altered shoreline morphology in a large river. Hydrobiologia 776: 51–65.
Brauns, M., X.-F. Garcia, N. Walz & M. T. Pusch, 2007. Effects of human shoreline development on littoral macroinvertebrates in lowland lakes. Journal of Applied Ecology 44: 1138–1144.
Cao, Y., C. P. Hawkins & A. W. Storey, 2005. A method for measuring the comparability of different sampling methods used in biological surveys: implications for data integration and synthesis. Freshwater Biology 50: 1105–1115.
Crossetti, L. O., C. Stenger-Kovács & J. Padisák, 2013. Coherence of phytoplankton and attached diatom-based ecological status assessment in Lake Balaton. Hydrobiologia 716: 87–101.
Csabai, Z., 2000. Vízibogarak határozója, I., Környezetgazdálkodási Intézet, Környezet-és Természetvédelmi Szakkönyvtár és Tájékoztatási Központ.
Csabai, Z., Z. Gidó & G. Szél, 2002. Vízibogarak kishatározója. II., Környezetgazdálkodási Intézet, Környezet-és Természetvédelmi Szakkönyvtár és Tájékoztatási Központ.
DAISIE, 2009. Handbook of Alien Species in Europe, Springer, Dordrecht:
De Cáceres, M., 2020. How to use the indicspecies package (ver. 1.7.8), Forest Sciences and Technology Center of Catalonia, Spain.
Dick, J. T. A. & D. Platvoet, 2000. Invading predatory crustacean Dikerogammarus villosus eliminates both native and exotic species. Proceedings of the Royal Society b: Biological Sciences 267: 977–983.
Drake, C. M. & M. J. Elliott, 1982. A comparative study of three air-lift samplers used for benthic macro-invertebrates in rivers. Freshwater Biology 12: 511–533.
Dufrene, M. & P. Legendre, 1997. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecological Monographs 67: 345–366.
Eiseler, B., 2005. Bildbestimmungsschlüssel für die Eintagsfliegenlarven der deutschen Mittelgebirge und des Tieflandes. Lauterbornia 53.
Elliott, J. M. & C. M. Drake, 1981. A comparative study of seven grabs used sampling macroinvertebrates in rivers. Freshwater Biology 11: 99–120.
Elliott, J. M. & K. H. Mann, 1979. A key to the British freshwater leeches with notes on their life cycles and ecology. Freshwater Biological Association Scientific Publication 40.
Erős, T., B. Tóth, A. Sevcsik & D. Schmera, 2008. Comparison of fish assemblage diversity in natural and artificial rip-rap habitats in the littoral zone of a large river (River Danube, Hungary). International Review Hydrobiology 93: 88–105.
Fehér, Z., G. Majoros & A. Varga, 2006. A scoring method for the assessment of rarity and conservation value of the Hungarian freshwater Molluscs. Heldia 6: 101–114.
Garamszegi, L. Z. & R. Mundry, 2014. Multimodel-inference in comparative analyses. In Garamszegi, L. Z. (ed), Modern phylogenetic comparative methods and their application in evolutionary biology. Concepts and practice Springer, Heidelberg: 305–331.
Gergs, R., M. Koester, R. S. Schulz & R. Schulz, 2014. Potential alteration of cross-ecosystem resource subsidies by an invasive aquatic macroinvertebrate: implications for the terrestrial food web. Freshwater Biology 59(12): 2645–2655.
Glöer, P., 2019. The freshwater Gastropods of the West-Palaearctis. Peter Glöer.
Grzybowski, M. & K. Glińska-Lewczuk, 2019. Principal threats to the conservation of freshwater habitats in the continental biogeographical region of Central Europe. Biodiversity and Conservation 28: 4065–4097.
Guareschi, S., A. Laini, J. England, T. Johns, M. Winter & P. J. Wood, 2021. Invasive species influence macroinvertebrate biomonitoring tools and functional diversity in British rivers. Journal of Applied Ecology 58: 135–147.
Hill, M. J., C. D. Sayer & P. J. Wood, 2016. When is the best time to sample aquatic macroinvertebrates in ponds for biodiversity assessment? Environmental Monitoring Assessment 188: 194–205.
Johnson, P. T. J., J. D. Olden & M. J. Vander Zanden, 2008. Dam invaders: impoundments facilitate biological invasions into freshwaters. Frontiers in Ecology and the Environment 6: 357–363.
Keller, R. P., J. Geist, J. M. Jeschke & I. Kühn, 2011. Invasive species in Europe: ecology, status, and policy. Environmental Sciences Europe 23: 23.
Kertai, E., M. Kozák & L. Kővári, 1974. Magyarország nagyobb vízépítésű műtárgyai, tavi kikötők, Az Országos Vízügyi Hivatal és a Budapesti Műszaki Egyetem Kiadványa.) [Hungary's major waterworks and lake harbours, Publication of the General Directorate of Water Management and the Budapest University of Technology and Economics.]
Kobak, J., T. Kakareko & M. Poznanska, 2010. Changes in attachment strength and aggregation of zebra mussel, Dreissena polymorpha in the presence of potential fish predators of various species and size. Hydrobiologia 644: 195–206.
Kontschán, J., B. Muskó Ilona & D. Murányi, 2002. A felszíni vizekben előforduló felemáslábú rákok (Crustacea: Amphipoda) rövid határozója és előfordulásuk Magyarországon. [Short descriptions of the crustaceans (Crustacea: Amphipoda) occurring in surface waters and their occurrence in Hungary]. Folia Historico-Naturalia Musei Matraensis 26: 151–157.
Laverty, C., W. Nentwig, J. T. Dick & F. E. Lucy, 2015. Alien aquatics in Europe: assessing the relative environmental and socioeconomic impacts of invasive aquatic macroinvertebrates and other taxa. Management of Biological Invasions 6(4): 341–350.
McGoff, E., A. G. Solimini, M. T. Pusch, T. Jurca & L. Sandin, 2013. Does lake habitat alteration and land-use pressure homogenize European littoral macroinvertebrate communities? Journal of Applied Ecology 50: 1010–1018.
Miler, O. & M. Brauns, 2020. Hierarchical response of littoral macroinvertebrates to altered hydromorphology and eutrophication. Science of the Total Environment 743: 140582–140594.
Muskó, I. B., 1992. Amphipoda species found in Lake Balaton since 1897. Miscellanea Zoologica Hungarica 7: 59–64.
Muskó, I. B., 1994. Occurrence of Amphipoda in Hungary since 1853. Crustaceana 66: 144–152.
Muskó, I. B., C. Balogh, Á. P. Tóth, É. Varga & G. Lakatos, 2007. Differential response of invasive malacostracan species to lake level fluctuations. Hydrobiologia 590: 65–74.
Müller, J. C., S. Schramm & A. Seitz, 2002. Genetic and morphological differentiation of Dikerogammarus invaders and their invasion history in Central Europe. Freshwater Biology 47: 2039–2048.
Nesemann, H., M. Pöckl & K. J. Wittmann, 1995. Distribution of epigean Malacostraca in the middle and upper Danube (Hungary, Austria, Germany). Miscellanea Zoologica Hungarica 10: 49–68.
Oksanen, J., F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O'hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, E. Szöcs & H. Wagner, 2020. vegan: Community Ecology Package R. package version 2.5–7.
Ostendorp, W., K. Schmieder & K. Jönk, 2004. Assessment of human pressures and their hydromorphological impacts on lakeshores in Europe. Ecohydrology and Hydrobiology 4: 380–395.
Padisák, J., 1992. Seasonal succession of phytoplankton in a large shallow lake (Lake Balaton, Hungary)—a dynamic approach to ecological memory, its possible role and mechanisms. Journal of Ecology 80: 217–230.
Paolucci, E. M., H. J. MacIsaac & A. Ricciardi, 2013. Origin matters: alien consumers inflict greater damage on prey populations than do native consumers. Diversity and Distributions 19: 988–995.
Pätzig, M., B. Grüneberg & M. Brauns, 2015. Water depth but not season mediates the effects of human lakeshore modification on littoral macroinvertebrates in a large lowland lake. Fundamental and Applied Limnology 186: 311–321.
Pätzig, M., Y. Vadeboncoeur & M. Brauns, 2018. Lakeshore modification reduces secondary production of macroinvertebrates in littoral but not deeper zones. Freshwater Science 37: 845–856.
Podani, J., 2000. Introduction into the Exploration of Multivariate Biological Data, Backhuys, Leiden:
Porst, G., M. Brauns, K. Irvine, A. Solimini, L. Sandin, M. Pusch & O. Miler, 2019. Effects of shoreline alteration and habitat heterogeneity on macroinvertebrate community composition across European lakes. Ecological Indicators 98: 285–296.
R Core Team, 2021. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. R version 4.1.0 (2021-05-18)
Radomski, P. & T. J. Goeman, 2001. Consequences of human lakeshore development on emergent and floating-leaf vegetation abundance. North American Journal of Fisheries Management 21: 46–61.
Reid, A. J., A. K. Carlson, I. F. Creed, E. J. Eliason, P. A. Gell, P. T. J. Johnson, K. A. Kidd, T. J. MacCormack, J. D. Olden, S. J. Ormerod, J. P. Smol, W. W. Taylor, K. Tockner, J. C. Vermaire, D. Dudgeon & S. J. Cooke, 2019. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biological Reviews 94: 849–873.
Richnovszky, A. & L. Pintér, 1979. Vizicsigák és kagylók (Mollusca) kishatározója. Vízügyi Hidrobiologia 6., Vízügyi Dokumentációs és Továbbképző Intézet, Budapest.
Saunders, D. L., J. J. Meeuwig & A. C. J. Vincent, 2002. Freshwater protected areas: strategies for conservation. Conservation Biology 16: 30–41.
Savage, A. A., 1989. Adults of the British aquatic Hemiptera Heteroptera: a key with ecological notes. Freshwater biological association.
Scheuerell, M. D. & D. E. Schindler, 2004. Changes in the spatial distribution of fishes in lakes along a residential development gradient. Ecosystems 7: 98–106.
Schmieder, K., 2004. European lakeshores in danger—concepts for a sustainable development. Limnologica 34: 3–14.
Schmidlin, S., D. Schmera & B. Baur, 2012. Alien molluscs affect the composition and diversity of native macroinvertebrates in a sandy flat of Lake Neuchatel, Switzerland. Hydrobiologia 679: 233–249.
Schnaiberg, J., J. Riera, M. G. Turner & P. R. Voss, 2002. Explaining human settlement patterns in a recreational lake district: Vilas County, Wisconsin, USA. Environmental Management 30: 24–34.
Schreiber, J. & M. Brauns, 2010. How much is enough? Adequate sample size for littorar macroinvertebrates in lowland lakes. Hydrobiologia 649: 365–373.
Schreiber, E. S. G., G. P. Quinn & P. S. Lake, 2003. Distribution of an alien aquatic snail in relation to flow variability, human activities and water quality. Freshwater Biology 48: 951–961.
Sebestyén, O., 1938. Colonization of two new fauna-elements of Pontus-origin (Dreissensia polymorpha Pall. and Corophium curvispinum G. O. Sars forma devium Wundsch) in Lake Balaton. Verhandlungen Internationale Vereinigung Theoretische Und Angewandte Limnologie 8: 169–181.
Sidorov, D. & D. Palatov, 2012. Taxonomy of the spring dwelling amphipod Synurella ambulans (Crustacea: Crangonyctidae) in West Russia: with notes on its distribution and ecology. European Journal of Taxonomy 23: 1–19.
Simberloff, D., J.-L. Martin, P. Genovesi, V. Maris, D. A. Wardle, J. Aronson, F. Courchamp, B. Galil, E. García-Berthou, M. Pascal, P. Pyšek, R. Sousa, E. Tabacchi & M. Vilà, 2013. Impacts of biological invasions: what’s what and the way forward. Trends in Ecology and Evolution 28: 58–66.
Stazner, B., N. Bonada & S. Dolédec, 2008. Biological attributes discriminating invasive from native European stream macroinvertebrates. Biological Invasions 10: 517–530.
Straskraba, M., 1962. Amphipoden der Tschekoslowakei nach den Sammlungen von Prof. Hrabe. i. Acta Societatis Zoologicae Bohemoslovenicae XXV I(2): 117–145.
Stratoulias, D. & V. R. Tóth, 2020. Photophysiology and spectroscopy of sun and shade leaves of Phragmites australis and the effect on patches of different densitiesal. Remote Sensing 12(1): 200–218.
Stratoulias, D., H. Balzter, A. Zlinszky & V. R. Tóth, 2018. A comparison of airborne hyperspectral-based classifications of emergent wetland vegetation at Lake Balaton, Hungary. International Journal of Remote Sensing 39: 5689–5715.
Strayer, D. L., 2010. Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshwater Biology 55: 152–174.
Strayer, D. L. & S. E. G. Findlay, 2010. Ecology of freshwater shore zones. Aquatic Sciences 72: 127–163.
Szalontai, K., 2008. A pontusi tanúrák (Limnomysis benedeni Czerniavsky, 1882) állományfelmérése, populáció-dinamikája és produkciója a Balatonban [Stock assessment, population dynamics and production of Limnomysis benedeni (Czerniavsky, 1882) in Lake Balaton]. University of Debrecen, Debrecen: 104. (PhD Thesis)
Takács, P., A. Ács, B. Bánó, I. Czeglédi, J. Csaba, T. Erős, M. Fésűs-Móré, B. Preiszner, Á. Staszny, Z. Vitál, A. Weiperth & Á. Ferincz, 2019. “Invasion in progress”: first occurrence and spread of river nerite (Theodoxus fluviatilis L., 1758) in the largest Central European shallow lake, Lake Balaton, Hungary. BioInvasions Records 8(2): 273–280.
Tickner, D., J. J. Opperman, R. Abell, M. Acreman, A. H. Arthington, S. E. Bunn, S. J. Cooke, J. Dalton, W. Darwall, G. Edwards, I. Harrison, K. Hughes, T. Jones, D. Leclère, A. J. Lynch, P. Leonard, M. E. McClain, D. Muruven, J. D. Olden, S. J. Ormerod, J. Robinson, R. E. Tharme, M. Thieme, K. Tockner, M. Wright & L. Young, 2020. Bending the curve of global freshwater biodiversity loss: an emergency recovery plan. BioScience 70: 330–342.
Tóth, V. R. & K. Szabó, 2012. Morphometric structural analysis of Phragmites australis stands in Lake Balaton. Annales De Limnologie-International Journal of Limnology 48: 241–251.
Vadeboncoeur, Y., M. J. Vander Zanden & D. M. Lodge, 2002. Putting the lake back together: reintegrating benthic pathways into lake food web models. Bioscience 52: 44–54.
Vadeboncoeur, Y., P. B. McIntyre & M. J. Vander Zanden, 2011. Borders of biodiversity: life at the edge of the world’s large lakes. Bioscience 61: 526–537.
Venables, W. N. & B. D. Ripley, 2002. Modern applied statistics with S, 4th ed. Springer, New York:
Verdonschot, P. F. M., B. M. Spears, C. K. Feld, S. Brucet, H. E. Keizer-Vlek, A. Borja, M. Elliott, M. Kernan & R. K. Johnson, 2013. A comparative review of recovery processes in rivers, lakes, estuarine and coastal waters. Hydrobiologia 704: 453–474.
Waringer, J. & W. Graf, 2011. Atlas of Central European Trichoptera larvae. Erik Mauch Verlag.
Woynárovich, E., 1955. Vorkommen der Limnomysis benedeni Czern. im ungarischen Donauabschnitt. Acta Zoologica Academiae Scientiarum Hungaricae 1: 177–185.
Acknowledgements
This research was supported by the NKFIH K140352, NKFIH FK 135136, NP2022-II-3/2022 and RRF-2.3.1-21-2022-00014 projects. We thank László Kovács and Borbála Király for their support in the field work, and Andrew Hamer for his comments on the manuscript. PB is financially supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences BO-00106-21 and by the ÚNKP-21-5 New National Excellence Program of the Ministry of Innovation and Technology from the source of the National Research, Development and Innovation Fund.
Funding
Open access funding provided by Balaton Limnological Research Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Handling editor: Sally A. Entrekin
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karádi-Kovács, K., Boda, P., Csabai, Z. et al. Negligible native and significant alien colonization of artificial shoreline by macroinvertebrates in a large shallow lake (Lake Balaton, Hungary). Hydrobiologia 850, 1837–1848 (2023). https://doi.org/10.1007/s10750-023-05186-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05186-7