Abstract
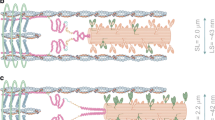
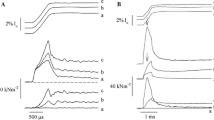
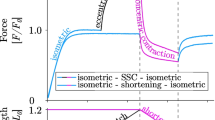
Titin is a filamentous protein spanning the half-sarcomere, with spring-like properties in the I-band region. Various structural, signaling, and mechanical functions have been associated with titin, but not all of these are fully elucidated and accepted in the scientific community. Here, I discuss the primary mechanical functions of titin, including its accepted role in passive force production, stabilization of half-sarcomeres and sarcomeres, and its controversial contribution to residual force enhancement, passive force enhancement, energetics, and work production in shortening muscle. Finally, I provide evidence that titin is a molecular spring whose stiffness changes with muscle activation and actin–myosin-based force production, suggesting a novel model of force production that, aside from actin and myosin, includes titin as a “third contractile” filament. Using this three-filament model of sarcomeres, the stability of (half-) sarcomeres, passive force enhancement, residual force enhancement, and the decrease in metabolic energy during and following eccentric contractions can be explained readily.









Similar content being viewed by others
References
Abbott BC, Aubert XM (1952) The force exerted by active striated muscle during and after change of length. J Physiol 117:77–86
Allinger TL, Epstein M, Herzog W (1996) Stability of muscle fibers on the descending limb of the force- length relation. A theoretical consideration. J Biomech 29:627–633
Anderson BR, Bogomolovas J, Labeit S, Granzier HLM (2010) The effects of PKCalpha phosphorylation on the extensibility of titin’s PEVK element. J Struct Biol 170:270–277
Astier C, Raynaud F, Lebart MC, Roustan C, Benyamin Y (1998) Binding of a native titin fragment to actin is regulated by PIP2. FEBS Lett 429:95–98
Bartoo ML, Linke WA, Pollack GH (1997) Basis of passive tension and stiffness in isolated rabbit myofibrils. Am J Phys 273:C266–C276
Bianco P, Nagy A, Kengyel A et al (2007) Interaction forces between F-actin and titin PEVK domain measured with optical tweezers. Biophys J 93:2102–2109
Borbely A, Falcao-Pires I, van Heerebeek L et al (2009) Hypophosphorylation of the stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res 104(6):780–786
Bullimore SR, Leonard TR, Rassier DE, Herzog W (2007) History-dependence of isometric muscle force: effect of prior stretch or shortening amplitude. J Biomech 40:1518–1524
Campbell KS (2009) Interactions between connected half-sarcomeres produce emergent mechanical behavior in a mathematical model of muscle. PLoS Comput Biol 5:e1000560
Cazorla O, Freiburg A, Helmes M et al (2000) Differential expression of cardiac tintin isoforms and modulation of cellular stiffness. Circ Res 86:59–67
Chung CS, Bogomolovas J, Gasch A et al (2011) Titin-actin interaction: PEVK-actin-based viscosity in a large animal. J Biomed Biotechnol 2011:310791
Colomo F, Piroddi N, Poggesi C, Te KG, Tesi C (1997) Active and passive forces of isolated myofibrils from cardiac and fast skeletal muscle of the frog. J Physiol 500(Pt 2):535–548
de Brito Fontana H, Herzog W (2016) Vastus lateralis maximum force-generating potential occurs at optimal fascicle length regardless of activation level. Eur J Appl Physiol 116:1267–1277
DuVall MM, Gifford JL, Amrein M, Herzog W (2013) Altered mechanical properties of titin immunoglobulin domain 27 in the presence of calcium. Eur Biophys J 42:301–307
DuVall M, Jinha A, Schappacher-Tilp G, Leonard T, Herzog W (2017) Differences in Titin segmental elongation between passive and active stretch in skeletal muscle. J Exp Biol 220(Pt 23):4418–4425
Edman KAP, Tsuchiya T (1996) Strain of passive elements during force enhancement by stretch in frog muscle fibres. J Physiol 490(1):191–205
Edman KAP, Elzinga G, Noble MIM (1982) Residual force enhancement after stretch of contracting frog single muscle fibers. J Gen Physiol 80:769–784
Epstein M, Herzog W (1998) Theoretical models of skeletal muscle:biological and mathematical considerations. John Wiley & Sons Ltd., New York
Forcinito M, Epstein M, Herzog W (1998) Can a rheological muscle model predict force depression/enhancement? J Biomech 31:1093–1099
Fortuna R, Power GA, Mende E, Seiberl W, Herzog W (2016) Residual force enhancement following shortening is speed-dependent. Sci Rep 5:21513
Freiburg A, Trombitas K, Hell W et al (2000) Series of exon-skipping events in the elastic spring region of titin as the structural basis for myofibrillar elastic diversity. Circ Res 86:1114–1121
Fukunaga T, Ichinose Y, Ito M, Kawakami Y, Fukashiro S (1997) Determination of fascicle length and pennation in a contracting human muscle in vivo. J Appl Physiol 82:354–358
Funatsu T, Higuchi H, Ishiwata S (1990) Elastic filaments in skeletal muscle revealed by selective removal of thin filaments with plasma gelsolin. J Cell Biol 110:53–62
Fusi L, Brunello E, Yan Z, Irving M (2016) Thick filament mechano-sensing is a calcium-independent regulatory mechanism in skeletal muscle. Nat Commun 7:13281
Gordon AM, Huxley AF, Julian FJ (1966) The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol 184:170–192
Granzier HLM (2010) Activation and stretch-induced passive force enhancement—are you pulling my chain? Focus on “regulation of muscle force in the absence of actin-myosin-based cross-bridge interaction”. Am J Physiol Cell Physiol 299:C11–C13
Granzier HLM, Irving TC (1995) Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J 68:1027–1044
Granzier HLM, Labeit S (2002) Cardiac titin: an adjustable multi-functional spring. J Physiol 541(2):335–342
Granzier HLM, Labeit S (2007) Structure-function relations of the giant elastic protein titin in striated and smooth muscle cells. Muscle Nerve 36:740–755
Granzier HLM, Trombitas K, Kellermayer MSZ, Helmes M, Stockman B (1996) Titin: a bi-directional spring and modulator of filament sliding. Proc Can Soc Biomech 9:10–11
Granzier HLM, Labeit D, Wu Y, Labeit S (2002) Titin as a modular spring: emerging mechanisms for elasticity control by titin in cardiac physiology and pathophysiology. J Muscle Res Cell Motil 23:457–471
Granzier HLM, Hutchinson KR, Tonino P et al (2014) Deleting titin’s I-band/A-band junction reveals critical roles for titin in biomechanical sensing and cardiac function. Proc Natl Acad Sci USA 111:14589–14594
Hahn D, Seiberl W, Schmidt S, Schweizer K, Schwirtz A (2010) Evidence of residual force enhancement for multi-joint leg extension. J Biomech 43:1503–1508
Herzog W (2014a) Mechanisms of enhanced force production in lengthening (eccentric) muscle contractions. J Appl Physiol 116:1407–1417
Herzog W (2014b) The role of titin in eccentric muscle contraction. J Exp Biol 217:2825–2833
Herzog W, Leonard TR (2002) Force enhancement following stretching of skeletal muscle: a new mechanism. J Exp Biol 205:1275–1283
Herzog W, Leonard TR (2005) The role of passive structures in force enhancement of skeletal muscles following active stretch. J Biomech 38:409–415
Herzog W, Schachar R, Leonard TR (2003) Characterization of the passive component of force enhancement following active stretching of skeletal muscle. J Exp Biol 206:3634–3643
Herzog W, Lee EJ, Rassier DE (2006) Residual force enhancement in skeletal muscle. J Physiol Lond 574:635–642
Herzog JA, Leonard TR, Jinha A, Herzog W (2012) Are titin properties reflected in single myofibrils? J Biomech 45:1893–1899
Herzog JA, Leonard TR, Jinha A, Herzog W (2014) Titin (visco-) elasticity in skeletal muscle myofibrils. MCB 11:1–17
Herzog W, Powers K, Johnston K, DuVall M (2015) A new paradigm for muscle contraction: review. Front Physiol 6:174–185
Herzog W, Schappacher G, DuVall M, Leonard TR, Herzog JA (2016) Residual force enhancement following eccentric contractions: a new mechanism involving Titin. Physiology 31:300–312
Hill AV (1953) The mechanics of active muscle. Proc R Soc Lond 141:104–117
Hisey B, Leonard TR, Herzog W (2009) Does residual force enhancement increase with increasing stretch magnitudes? J Biomech 42:1488–1492
Horowits R (1992) Passive force generation and titin isoforms in mammalian skeletal muscle. Biophys J 61:392–398
Horowits R, Podolsky RJ (1987) The positional stability of thick filaments in activated skeletal muscle depends on sarcomere length: evidence for the role of titin filaments. J Cell Biol 105:2217–2223
Horowits R, Podolsky RJ (1988) Thick filament movement and isometric tension in activated skeletal muscle. Biophys J 54:165–171
Horowits R, Maruyama K, Podolsky RJ (1989) Elastic behaviour of connecting filaments during thick filament movement in activated skeletal muscle. J Cell Biol 109:2169–2176
Hudson BD, Hidalgo CG, Gotthardt M, Granzier HLM (2010) Excision of titin’s cardiac PEVK spring element abolishes PKCalpha-induced increases in myocardial stiffness. J Mol Cell Cardiol 48:972–978
Huxley HE (1953) Electron microscope studies of the organization of the filaments in striated muscle. Biochim Biophys Acta 12:387–394
Huxley AF (1957a) Muscle structure and theories of contraction. Prog Biophys Biophys Chem 7:255–318
Huxley HE (1957b) The double array of filaments in cross-striated muscle. Biochem Biophys Acta 12:387–394
Huxley HE (1969) The mechanism of muscular contraction. Science 164:1356–1366
Huxley AF (1980) Reflections on muscle. Liverpool University Press, Liverpool
Huxley HE, Hanson J (1954) Changes in cross-striations of muscle during contraction and stretch and their structural implications. Nature 173:973–976
Huxley AF, Niedergerke R (1954) Structural changes in muscle during contraction. Interference microscopy of living muscle fibres. Nature 173:971–973
Huxley AF, Peachey LD (1961) The maximum length for contraction in vertebrate striated muscle. J Physiol Lond 156:150–165
Huxley AF, Simmons RM (1971) Proposed mechanism of force generation in striated muscle. Nature 233:533–538
Ichinose Y, Kawakami Y, Ito M, Fukunaga T (1997) Estimation of active force-length characteristics of human vastus lateralis muscle. Acta Anat (Basel) 159:78–83
Iwazumi T (1979) In: Sugi H, Pollack GH (eds) Crossbridge mechanism in muscle contraction. University of Tokyo Press, Tokyo, pp 611–632
Iwazumi T, Noble M (1989) An electrostatic mechanism of muscular contraction. Int J Cardiol 24:267–275
Johnston K, Jinha A, Herzog W (2016) The role of sarcomere length non-uniformities in residual force enhancement of skeletal muscle myofibrils. Royal Soc Open Sci 3:150657
Joumaa V, Herzog W (2013) Energy cost of force production is reduced after active stretch in skinned muscle fibres. J Biomech 46:1135–1139
Joumaa V, Rassier DE, Leonard TR, Herzog W (2007) Passive force enhancement in single myofibrils. Pflügers Arch 455:367–371
Joumaa V, Leonard TR, Herzog W (2008a) Residual force enhancement in myofibrils and sarcomeres. Proc R Soc B 275:1411–1419
Joumaa V, Rassier DE, Leonard TR, Herzog W (2008b) The origin of passive force enhancement in skeletal muscle. Am J Physiol Cell Physiol 294:C74–C78
Julian FJ, Morgan DL (1979) The effects of tension on non-uniform distribution of length changes applied to frog muscle fibres. J Physiol 293:379–392
Julian FJ, Sollins MR, Moss RL (1978) Sarcomere length non-uniformity in relation to tetanic response of stretched skeletal muscle fibres. Proc R Soc Lond B 200:109–116
Kellermayer MSZ, Smith SB, Granzier HLM, Bustamante C (1997) Folding-unfolding transitions in single titin molecules characterized with laser tweezers. Science 276:1112–1116
Kruger M, Linke WA (2009) Titin-based mechanical signaling in normal and failing myocardium. J Mol Cell Cardiol 46(4):490–498
Kulke M, Fuijita-Becker S, Rostkova E et al (2001) Interaction between PEVK-titin and actin filaments: origin of a viscous force component in cardiac myofibrils. Circ Res 89:874–881
Labeit D, Watanabe K, Witt C et al (2003) Calcium-dependent molecular spring elements in the giant protein titin. Proc Natl Acad Sci USA 100:13716–13721
Lee HD, Herzog W (2002) Force enhancement following muscle stretch of electrically and voluntarily activated human adductor pollicis. J Physiol 545:321–330
Lee EJ, Herzog W (2008) Residual force enhancement exceeds the isometric force at optimal sarcomere length for optimized stretch conditions. J Appl Physiol 105:457–462
Leonard TR, Herzog W (2010) Regulation of muscle force in the absence of actin-myosin based cross-bridge interaction. Am J Physiol Cell Physiol 299:C14–C20
Leonard TR, Duvall M, Herzog W (2010) Force enhancement following stretch in a single sarcomere. Am J Physiol Cell Physiol 299(6):C1398–C1401
LeWinter MM, Granzier HLM (2010) Cardiac titin: a multifunctional giant. Circulation 121:2137–2145
Li Q, Jin J-P, Granzier HLM (1995) The effect of genetically expressed cardiac titin fragments on in vitro actin motility. Biophys J 69:1508–1518
Linke WA, Fernandez JM (2002) Cardiac titin: molecular basis of elasticity and cellular contribution to elastic and viscous stiffness components in myocardium. J Muscle Res Cell Motil 23:483–497
Linke WA, Kruger M (2010) The giant protein titin as an integrator of myocyte signaling pathways. Physiology (Bethesda) 25:186–198
Linke WA, Popov VI, Pollack GH (1994) Passive and active tension in single cardiac myofibrils. Biophys J 67:782–792
Linke WA, Ivemeyer M, Olivieri N et al (1996) Towards a molecular understanding of the elasticity of titin. J Mol Biol 261:62–71
Linke WA, Ivemeyer M, Labeit S et al (1997) Actin-titin interaction in cardiac myofibrils: probing a physiological role. Biophys J 73:905–919
Linke WA, Ivemeyer M, Mundel P, Stockmeier MR, Kolmerer B (1998) Nature of PEVK-titin elasticity in skeletal muscle. Proc Natl Acad Sci U S A 95:8052–8057
Linke WA, Kulke M, Li H et al (2002) PEVK domain of titin: an entropic spring with actin-binding properties. J Struct Biol 137:194–205
Liversage AD, Holmes D, Knight PJ, Tskhovrebova L, Trinick J (2001) Titin and the sarcomere symmetry paradox1. J Mol Biol 305:401–409
Llewellyn ME, Barretto RPJ, Delp SL, Schnitzer MJ (2008) Minimally invasive high-speed imaging of sarcomere contractile dynamics in mice and humans. Nature 454:784–788
Maruyama K (1976) Connectin, an elastic protein from myofibrils. J Biochem 80:405–407
Maruyama K, Kimura S, Kuroda M, Handa S (1977) Connectin, an elastic protein of muscle. J Biochem 82:347–350
Minajeva A, Kulke M, Fernandez JM, Linke WA (2001) Unfolding of Titin domains explains the Viscoelastic behavior of skeletal myofibrils. Biophys J 80(3):1442–1451
Moo EK, Fortuna R, Sibole SC, Abusara Z, Herzog W (2016) In vivo sarcomere lengths and sarcomere elongations are not uniform across an intact muscle. Front Physiol 7:1–9
Morgan DL (1990) New insights into the behavior of muscle during active lengthening. Biophys J 57:209–221
Morgan DL (1994) An explanation for residual increased tension in striated muscle after stretch during contraction. Exp Physiol 79:831–838
Morgan DL, Proske U (2004) Popping sarcomere hypothesis explains stretch-induced muscle damage. Clin Exp Pharmacol Physiol 31:541–545
Morgan DL, Proske U (2006) Can all residual force enhancement be explained by sarcomere non-uniformities? J Physiol 578(2):613–615
Morgan DL, Whitehead NP, Wise AK, Gregory JE, Proske U (2000) Tension changes in the cat soleus muscle following slow stretch or shortening of the contracting muscle. J Physiol 522(3):503–513
Nagy A, Cacciafesta P, Grama L et al (2004) Differential actin binding along the PEVK domain of skeletal muscle titin. J Cell Sci 117:5781–5789
Noble MIM (1992) Enhancement of mechanical performance of striated muscle by stretch during contraction. Exp Physiol 77:539–552
Novak I, Truskinovsky L (2014) Nonaffine response of skeletal muscles on the ‘descending limb’. Math Mech Solids 20:1–24
Oskouei AE, Herzog W (2005) Observations on force enhancement in sub-maximal voluntary contractions of human adductor pollicis muscle. J Appl Physiol 98:2087–2095
Perkin J, Slater R, Del Favero G et al (2015) Phosphorylating Titin’s cardiac N2B element by ERK2 or CaMKII delta lowers the single molecule and cardiac muscle force. Biophys J 109:2592–2601
Peterson D, Rassier DE, Herzog W (2004) Force enhancement in single skeletal muscle fibres on the ascending limb of the force-length relationship. J Exp Biol 207:2787–2791
Powers K, Schappacher-Tilp G, Jinha A et al (2014) Titin force is enhanced in actively stretched skeletal muscle. J Exp Biol 217:3629–3636
Prado LG, Makarenko I, Andresen C et al (2005) Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. J Gen Physiol 126:461–480
Pun C, Syed A, Rassier DE (2010) History-dependent properties of skeletal muscle myofibrils contracting along the ascending limb of the force–length relationship. Proc Biol Sci 277:475–484
Rassier DE, Pavlov I (2012) Force produced by isolated sarcomeres and half-sarcomeres after an imposed stretch. Am J Physiol Cell Physiol 302:C240–C248
Rassier DE, Herzog W, Pollack GH (2003a) Stretch-induced force enhancement and stability of skeletal muscle myofibrils. Adv Exp Med Biol 538:501–515
Rassier DE, Herzog W, Pollack GH (2003b) Dynamics of individual sarcomeres during and after stretch in activated single myofibrils. Proc R Soc Lond B 270:1735–1740
Rassier DE, Herzog W, Wakeling JM, Syme D (2003c) Stretch-induced, steady-state force enhancement in single skeletal muscle fibers exceeds the isometric force at optimal fibre length. J Biomech 36:1309–1316
Rayment I, Holden HM, Whittaker M et al (1993) Structure of the actin-myosin complex and its implications for muscle contraction. Science 261:58–65
Rivas-Pardo JA, Eckels EC, Popa I et al (2016) Work done by Titin protein folding assists muscle contraction. Cell Rep 14:1–9
Schappacher-Tilp G, Leonard T, Desch G, Herzog W (2015) A novel three-filament model of force generation in eccentric contraction of skeletal muscles. PLoS One 10:e0117634
Schwarz ML, Witt SH, Schneider-Wald B et al (2008) Titin expression in human articular cartilage and cultured chondrocytes: a novel component in articular cartilage biomechanical sensing? Biomed Pharmacother 62:339–347
Scott KA, Steward A, Fowler SB, Clarke J (2002) Titin: a multidomain protein that behaves as the sum of its parts. J Mol Biol 315:819–829
Seiberl W, Hahn D, Herzog W, Schwirtz A (2012) Feedback controlled force enhancement and activation reduction of voluntarily activated quadriceps femoris during sub-maximal muscle action. J Electromyogr Kinesiol 22:117–123
Stoecker U, Telley IA, Stüssi E, Denoth J (2009) A multisegmental cross-bridge kinetics model of the myofibril. J Theor Biol 259:714–726
Sugi H, Tsuchiya T (1988) Stiffness changes during enhancement and deficit of isometric force by slow length changes in frog skeletal muscle fibres. J Physiol Lond 407:215–229
Ter Keurs HE, Rijnsburger WH, van Heuningen R, Nagelsmit MJ (1980) Tension development and sarcomere length in rat cardiac trabeculae: evidence of length-dependent activation. Circ Res 46:703–714
Trombitas K, Granzier HLM (1997) Actin removal from cardiac myocytes shows that near Z line titin attaches to actin while under tension. Am J Phys 273:C662–C670
Trombitas K, Pollack GH (1993) Elastic properties of the titin filament in the z-line region of vertebrate striated-muscle. J Muscle Res Cell Motil 14:416–422
Trombitas K, Redkar A, Centner T et al (2000) Extensibility of isoforms of cardiac titin: variation in contour length of molecular subsegments provides a basis for cellular passive stiffness diversity. Biophys J 79:3226–3234
Vaz MA, de la Rocha FC, Leonard T, Herzog W (2012) The force-length relationship of the cat soleus muscle. Muscles Ligaments Tendons J 2:79–84
Walcott S, Herzog W (2008) Modeling residual force enhancement with generic cross-bridge models. Math Biosci 216:172–186
Wang K, Mcclure J, Tu A (1979) Titin: major myofibrillar components of striated muscle. Proc Natl Acad Sci USA 76:3698–3702
Yamasaki R, Berri M, Wu Y et al (2001) Titin-Actin interaction in mouse myocardium: passive tension modulation and its regulation by calcium/S100A1. Biophys J 81:2297–2313
Yamasaki R, Wu Y, McNabb M et al (2002) Protein kinase a phosphorylates titin’s cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ Res 90:1181–1188
Yasuda K, Shindo Y, Ishwata S (1996) Synchronous behavior of spontaneous oscillations of sarcomeres in skeletal myofibrils under isotonic conditions. Biophys J 70:1823–1829
Zahalak GI (1997) Can muscle fibers be stable on the descending limbs of their sarcomere length–tension relations? J Biomech 30:1179–1182
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Walter Herzog declares that he has no conflict of interest.
Ethical approval
Ethics approvals for all experiments described in this study were obtained by the Life Sciences and Animal Research Ethics Commitee of the University of Calgary.
Rights and permissions
About this article
Cite this article
Herzog, W. The multiple roles of titin in muscle contraction and force production. Biophys Rev 10, 1187–1199 (2018). https://doi.org/10.1007/s12551-017-0395-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-017-0395-y