Abstract
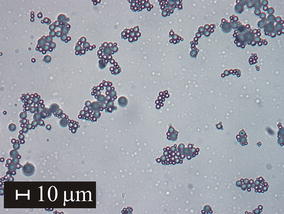
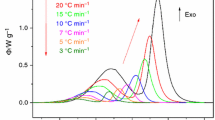
The glass of gehlenite composition was prepared by flame synthesis in the form of microspheres. The powder precursor was synthesised by standard solid-state reaction method using SiO2, Al2O3 and CaCO3. The prepared glasses were characterized from the point of view of surface morphology, phase composition and thermal properties by optical microscopy, scanning electron microscopy (SEM), X-ray diffraction (XRD) and differential scanning calorimetry (DSC), respectively. The prepared samples contained only completely re-melted spherical particles. SEM did not reveal any features indicating the presence of crystalline phases. However, traces of crystalline gehlenite were detected by XRD. The high-temperature XRD measurements (HT XRD) were carried out to identify the phase evolution during glass crystallization. In the studied temperature range, gehlenite phase was identified as the main crystalline phase. Non-isothermal DSC analysis of prepared glass microspheres was carried out from room temperature up to 1200 °C at five different heating rates: 2, 4, 6, 8 and 10 °C/min to determine the thermal properties of microspheres. In order to study the crystallization kinetics, the DSC curves were transformed into dependence of fractional extent of crystallization (α) on temperature. The Johnson–Mehl–Avrami–Kolmogorov model was found to be suitable for description of crystallization kinetics. Frequency factor A = 5.56 × 1029 ± 1.73 × 1029 min−1, apparent activation energy Eapp = 722 ± 3 kJ mol−1 and the Avrami coefficient m = 2 were determined. In the studied system, the linear temperature dependence of nucleation rate, diffusion controlled crystal growth interface and a 2D crystal growth were confirmed.
Similar content being viewed by others
References
Wu H, Hu Y, Ju G, Chen L, Wang X, Yang Z. Photoluminescence and thermoluminescence of Ce3+ and Eu2+ in Ca2Al2SiO7 matrix. J Lumin. 2011;131:2441–5. https://doi.org/10.1016/j.jlumin.2011.06.024.
Proverbio M, Dapiaggi M, Artioli G. Thermal expansion and excess properties of akermanite-gehlenite synthetic solid solution series. Mat Sci Forum. 2004;443–444:401–6. https://doi.org/10.4028/www.scientific.net/MSF.443-444.401.
Yang P, et al. Ca2Al2SiO7:Bi3+, Eu3+, Tb3+: A potential single-phased tunable-color-emitting phosphor. J Lumin. 2013;135:206–10. https://doi.org/10.1016/j.jlumin.2012.10.015.
Lejus AM, Pelletier-Allard N, Pelletier R, Vivien D. Site selective spectroscopy of Nd ions in gehlenite (Ca2Al2SiO7), a new laser material. Opt Mater. 1996;6:129–37. https://doi.org/10.1016/0925-3467(96)00041-9
Ptáček P, Opravil T, Šoukal F, Havlica Holešinský R. Kinetics and mechanism of formation of gehlenite, Al-Si spinel and anorthite from mixture of kaolinite and calcite. Solid State Sci. 2013;26:53–8. https://doi.org/10.1016/j.solidstatessciences.2013.09.014.
Viana B, Lejus AM, Saber D, Duxin N, Vivien D. Optical properties and energy transfer among Nd3+ in Nd:Ca2Al2SiO7 crystals for diode puped lasers. Opt Mater. 1994;3:307–16. https://doi.org/10.1016/0925-3467(94)90043-4.
Kodama N, Tanii Y, Yamaga M. Optical properties of long-lasting phosphorescent crystals Ce3 + -doped Ca2Al2SiO7 and CaYAl3O7. J Lumin. 2000;87–89:1076–8. https://doi.org/10.1016/S0022-2313(99)00543-8.
Yang P, Yu X, Yu H, Jiang T, Zhou D, Qiu J. Effects of crystal field on photoluminescence properties of Ca2Al2SiO7:Eu2+ phosphors. J Rare Earths. 2012;30:1208–12. https://doi.org/10.1016/S1002-0721(12)60207-5.
Majerova M, Klement R, Prnova A, Kraxner J, Bruneel E, Galusek D. Crystallization and VIS-NIR luminescence of Bi-doped gehlenite glass. R Soc Open Sci. 2018;5:181667. https://doi.org/10.1098/rsos.181667.
Moesgaard M, Yue Y. Compositional dependence of fragility and glass forming ability of calcium aluminosilicate melts. J Non-Cryst Solids. 2009;355:867–73. https://doi.org/10.1016/j.jnoncrysol.2009.04.004.
Bernardo E, Fiocco L, Prnová A, Klement R, Galusek D. Gehlenite: Eu3+ phosphors from a silicone resin and nano-sized fillers. Opt Mater. 2014;36:1243–9. https://doi.org/10.1016/j.optmat.2014.03.007.
Shih SJ, et al. Preparation and characterization of Eu-doped gehlenite glassy particles using spray pyrolysis. Ceram Int. 2016;42:11324–9. https://doi.org/10.1016/j.ceramint.2016.04.053.
Rosenflanz A, Frey M, Endres B, Anderson T, Richards E, Schardt C. Bulk glasses and ultrahard nanoceramics based on alumina and rare-earth oxides. Nature. 2004;430:761–4. https://doi.org/10.1038/nature02729.
Yigiter AO, Atakol MK, Aksu ML, Atakol O. Thermal characterization and theoretical and experimental comparison of poryl chloride derivatives of heterocyclic energetic compounds. J Therm Anal Calorim. 2017;127:2199–213. https://doi.org/10.1007/s10973-016-5766-2.
Pawlikowska M, Piatkowska M, Tomaszewicz E. Synthesis and thermal stability of rare-earths molybdates and tungstates with fluorite- and scheelite-type structure. J Therm Anal Calorim. 2017;130:69–76. https://doi.org/10.1007/s10973-017-6127-5.
Rahvard MM, Tamizifar M, Boutorabi SM. Non-isothermal crystallization kinetics and fragility of Zr56Co28Al16 and Zr56Co22Cu6Al16 bulk metallic glasses. J Therm Anal Calorim. 2018;134:903–14. https://doi.org/10.1007/s10973-018-7367-8.
Kalenda P, Koudelka L, Mošner P, Beneš L, Drobná H. Thermoanalytical study and crystallization of Ba(PO3)2–WO3 glasses. J Therm Anal Calorim. 2019;137:1911–8. https://doi.org/10.1007/s10973-019-08115-w.
Koga N, Kikuchi S. Thermal behavior of perlote concrete used in a sodium-cooled fast reactor. J Therm Anal Calorim. 2019;138:983–96. https://doi.org/10.1007/s10973-019-08351-0.
Laboureur D, Glabeke G, Gouriet JB. Aluminum nanoparticles oxidation by TGA/DSC. J Therm Anal Calorim. 2019;137:1199–210. https://doi.org/10.1007/s10973-019-08058-2.
Dande A, et al. DSC analysis of human synovial fluid samples in the diagnostics of non-septic and septic arthritis. J Therm Anal Calorim. 2017;130:1249–52. https://doi.org/10.1007/s10973-017-6179-6.
Chen J, He F, Xiao Y, Xie M, Xie J, Zhang W, Shi J. Effect of Al/Si ratio on the crystallization properties and structure of mold flux. Con Build Mat. 2019;216:19–28. https://doi.org/10.1016/j.combuildmat.2019.04.261.
Mukherjee DP, Das SK. SiO2–Al2O3–CaO glass-ceramics: effects of CaF2 on crystallization, microstructure and properties. Ceram Int. 2012;39:571–8. https://doi.org/10.1016/j.ceramint.2012.06.066.
Niculescu M, et al. Thermal and spectroscopic analysis of Co(II)-Fe(III) polyglyoxylate obtained through reaction of etylene glykol with metal nitrates. J Therm Anal Calorim. 2018;131:127–36. https://doi.org/10.1007/s10973-016-6079-1.
Lu J, Li Y, Zou Ch, Liu Z, Wang C. Effect of heating rate on the sinterability, crystallization, and mechanical properties of sintered glass-ceramics from granite waste. J Therm Anal Calorim. 2019;135:1977–85. https://doi.org/10.1007/s10973-018-7346-0.
Prnova A, et al. Thermal behavior of yttrium aluminate glasses studied by DSC, high-temperature X-ray diffraction, SEM and SEM-EDS. J Therm Anal Calorim. 2017;128(3):1407–15. https://doi.org/10.1007/s10973-016-6078-2.
Hou JG, Kumar RV, Qu YF, Krsmanovic D. Crystallization kinetics and densification of YAG nanoparticles from various chelating agents. Mater Res Bull. 2009;44:1786–91. https://doi.org/10.1016/j.materresbull.2009.03.001.
Zhang W, He F, Xie J, Liu X, Fang D, Yang H, Luo Z. Crystallization mechanism and properties of glass ceramics from modified molten blast furnace slag. J Non-Cryst Solids. 2018;502:164–71. https://doi.org/10.1016/j.jnoncrysol.2018.08.024.
Reddy AA, et al. Study of melilite based glasses and glass-ceramics nucleated by Bi2O3 for functional applications. RSC Adv. 2012;2(29):10955–67. https://doi.org/10.1039/C2RA22001F.
Malecki A, Gajerski R, Labus S, Prochovska-Klisch B, Oblakowski J. Kinetics and mechanism of crystallization of gehlenite glass pure and doped with Co2+, Eu3+, Cr3+ and Th4+. J Non-Cryst Solids. 1997;212:55–8. https://doi.org/10.1016/S0022-3093(96)00537-6.
Prnova A, Plsko A, Valuchova J, Svancarek P, Klement R, Michalkova M, Galusek D. Crystallization kinetics of yttrium aluminate glasses. J Therm Anal Calorim. 2018;133(1):227–36. https://doi.org/10.1007/s10973-017-6948-2.
Prnova A, et al. Crystallization kinetics of binary La2O3–Al2O3 glass. J Non-Cryst Solids. 2018;501:55–61. https://doi.org/10.1016/j.jnoncrysol.2018.03.001.
Vyazovkin S, et al. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19. https://doi.org/10.1016/j.tca.2011.03.034.
Johnson WA, Mehl RF, Mehl RF. Reaction kinetics in processes of nucleation and growth. Trans Am Inst Min Metall Pet Eng. 1939;135:416–42.
Šesták J, Šimon P. Thermal analysis of micro, nano- and noncrystalline materials: transformation, crystallization, kinetics and thermodynamics. Netherlands: Springer; 2013.
Tanaka H. Thermal analysis and kinetics of solid state reaction. Thermochim Acta. 1995;267:29–44. https://doi.org/10.1016/0040-6031(95)02464-6.
Johnson JB, Omland KS. Model selection in ecology and evolution. Trends Ecol Evol. 2004;19:101–8. https://doi.org/10.1016/j.tree.2003.10.013.
Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. 1974;19:716–23. https://doi.org/10.1109/TAC.1974.1100705.
Málek J. The applicability of Johnson-Mehl-Avrami model in the thermal analysis of the crystallization kinetics of glasses. Thermochim Acta. 1995;267:61–73. https://doi.org/10.1016/0040-6031(95)02466-2.
Cavanaugh JE. Criteria for linear model selection based on Kullback‘s symmetric divergence. Aust N Y Stat. 2004;46:257–74. https://doi.org/10.1111/j.1467-842X.2004.00328.x.
Kim HJ, Cavanaugh JE. Model selection criteria based on Kullback information measures for nonlinear regression. J Stat Plan Inference. 2005;134:332–49. https://doi.org/10.1016/j.jspi.2004.05.002.
Haladejova K, Prnova A, Klement R, Tuanb WH, Shihc SJ, Galusek D. Aluminate glass based phosphors for LED applications. J Eur Ceram Soc. 2016;36:2969–73. https://doi.org/10.1016/j.jeurceramsoc.2015.11.027.
Klement R, Hruska B, Hronsky V, Olcak D. Preparation and characterization of basic and Er3+ doped glasses in the system Y2O3–Al2O3–ZrO2. Acta Phys Pol A. 2014;126:302–3. https://doi.org/10.12693/APhysPolA.126.302.
Marotta A, Buri A, Valenti GL. Crystallization kinetics of gehlenite glasses. J Mat Sci. 1978;13:2483–6.
Malecki A, Lejus AM, Viana B, Vivien D, Collongues R. Spectroscopic studies of the kinetics of devitrification of Nd3+-doped glasses in the akermanite-gehlenite system. J Non-Cryst Solids. 1994;170:161–6. https://doi.org/10.1016/0022-3093(94)90042-6.
Šesták J, Šatava V, Wendlandt WW. The study of heterogeneous processes by thermal analysis. Thermochim Acta. 1973;7:333–556. https://doi.org/10.1016/0040-6031(73)87019-4.
Acknowledgements
This paper is a part of dissemination activities of project FunGlass. This project has received funding from the European Union´s Horizon 2020, research and innovation programme under Grant Agreement No 739566. The financial support of this work by the projects VEGA 1/0527/18, APVV-17-0049, VEGA 2/0026/17, VEGA 2/0164/17 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Majerová, M., Prnová, A., Plško, A. et al. Crystallization kinetics of gehlenite glass microspheres. J Therm Anal Calorim 142, 1003–1010 (2020). https://doi.org/10.1007/s10973-020-09305-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09305-7