Abstract
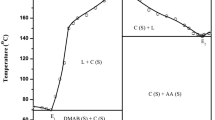
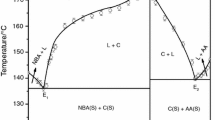
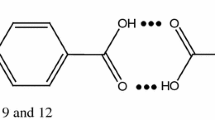
Phase diagrams of o-phenylenediamine (OPDA)–N,N-dimethylaminobenzaldehyde (DMAB) and salicylamide (SAM)–m-nitro benzoic acid (NBA) systems, determined by the thaw-melt method, show the formation of two eutectics and one inter-molecular compound (IMC) in each case. These inter-molecular compounds were synthesized by the solvent-free method involving thermally initiated molten state reactions. Their characterization by the IR and the 1H NMR techniques suggest the formation of a new compound. Absorption spectrum of IMC of OPDA–DMAB system shows band at 245 nm due to the π → π* transition and another broad band observed from 280 to 440 nm is attributed to the n → π* transition. Their IMC shows fluorescence at 388 nm with a Stokes shift (93 nm) and quantum efficiency (0.0032) upon excitation at 350 nm in ethyl alcohol. Emission and absorption were observed in case of SAM–NBA system too. While the values of enthalpy and entropy of fusion of inter-molecular compound in case of OPDA–DMAB are 23.58 kJ mol−1 and 57.20 J mol−1, respectively, in the case of SAM–NBA system the corresponding values are 69.50 kJ mol−1 and 170.00 J mol−1. The values of interfacial energy in case of OPDA–DMAB and SAM–NBA inter-molecular compounds are 37.84 × 10−3 and 40.39 × 10−3 J m−2, respectively.











Similar content being viewed by others
References
Chen M, Ma P. Solid–liquid equilibria of several systems containing acetic acid. J Chem Eng Data. 2004;49:756–9.
Boettinger WJ, Coriell SR, Greer AL, Kerma A, Kurz W, Rappaz M, Trivedi R. Solidification microstructures: recent developments, future directions. Acta Mater. 2000;48:43–70.
Rudrakshi GB, Pathak JP, Ojha SN. Processing characteristics of liquid immiscible alloys based on Al-Pb system. Indian Foundry J. 2002;48:17–29.
Elliott R. Eutectic solidification processing. London: Butterworths; 1983.
Herlach DM, Cochrane RF, Egry I, Fecht HJ, Greer AL. Containerless processing in the study of metallic melts and their solidification. Int Mater Rev. 1993;38:273–347.
Majumdar B, Chattopadhyay K. Aligned monotectic growth in unidirectionally solidified Zn-Bi alloys. Metall Mater Trans. 2000;31(7):1833–42.
Predel B. Constitution and thermodynamics of monotectic alloys—a survey. J Phase Equilib. 1997;18:327–37.
Rai US, Singh M. An overview of the progress in solidification of binary monotectics. J Mater Chem Eng. 2013;1:75–84.
Rai RN, Reddi RSB, Rai US, Developments and future directions of phase diagram, physicochemical and optical studies of binary organic complexes. 2013;59:73–111.
Rice JW, Fu J, Suuberg EM. Anthracene + Pyrene solid mixtures: eutectic and azeotropic character. J Chem Eng Data. 2010;55(9):3598–605.
Asta M, Beckermann C, Karma A, Kurz W, Napolitano R, Plapp M, Purdy G, Rappaz M, Trivedi R. Solidification microstructures and solid-state parallels: recent developments, future directions. Acta Mater. 2009;57:941–71.
Singh J, Singh NB. Solidification and thermodynamic parameters of binary faceted organic alloys: picricacid-resorcinol system. J Cryst Growth. 2015;424:14–23.
Parimaladevi P, Kavitha C, Srinivasan K. Investigation of the effect of liquid–liquid phase separation (LLPS) on nucleation and different growth stages of vanillin and bulk growth of defect-free single crystals from aqueous solution—a new approach. CrystEngComm. 2014;16:2565–9.
Rai RN, Ramasamy P, Lan CW. Synthesis and crystal growth of binary organic NLO material UNBA. J Cryst Growth. 2002;235:499–504.
Rai RN, Lan CW. Crystal structure and properties of new organic nonlinear optical material. J Mater Res. 2002;17(7):1587–91.
Dean JA. Lange’s handbook of chemistry. New York: McGraw-Hill; 1985.
Singh M, Pandey P, Rai RN, Rai US. Solid–liquid equilibrium, thermal, and physicochemical studies on salicylamide–4-nitrophenol and 2-cyanoacetamide–4-aminoacetophenone organic eutectic systems. J Therm Anal Calorim. 2013;113:977–83.
Rai RN, Varma KBR. Phase diagram and dielectric studies of binary organic materials. Mater Lett. 2000;44:284–93.
Rajkumar M, Synthesis A. growth, structural, optical, thermal, electrical and mechanical properties of hydrogen bonded organic salt crystal: triethylammonium-3,5-dinitrosalicylate. J Mol Struct. 2017;1134:762–9.
Singh M, Rai RN, Rai US. Synthesis, crystal growth and physicochemical studies on a novel organic inter-molecular compound; 3,5-dinitrobenzoic acid and salicylamide system. J Crystal Growth. 2015;419:114–22.
Sharma KP, Rai RN. Synthesis and characterization of novel binary organic monotectic and eutectic alloys. Thermochim Acta. 2012;535:66–70.
Singh NB, Das SS, Singh NP, Agrawal Tanvi. Computer simulation, thermodynamic and microstructural studies of benzamide–benzoic acid eutectic system. J Cryst Growth. 2008;310:2878–84.
Singh M, Rai RN, Rai US. Some physicochemical and thermal studies on organic analog of a nonmetal-nonmetal monotectic alloy; 2-cyanoacetamide–4-chloronitrobenzene system. Am J Anal Chem. 2011;2:953–61.
Singh J, Singh NB. Solidification and computational analysis of o-nitroaniline–a-naphthol eutectic system. Fluid Phase Equilib. 2015;386:168–79.
Dwivedi Y, Kant S, Rai SB, Rai RN. Synthesis, physicochemical and optical characterization of novel fluorescing complex: o-phenylenediamine—Benzoin. J Flour. 2011;21:1255–63.
Li X-G, Wang H-Y, Huang M-R. Synthesis, film-forming, and electronic properties of o-phenylenediamine copolymers displaying an uncommon tricolor. Macromolecules. 2007;40(5):1489–96.
Bani-Yaseen AD, Hammad F, Ghanem BS, Mohammad EG. On the photophysicochemical properties of selected fluoroquinolones: solvatochromic and fluorescence spectroscopy study. J Fluoresc. 2013;23:93–101.
Acknowledgements
We wish to express our thanks to the Head, Chemistry Department, Institute of Science, Banaras Hindu University, Varanasi, for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rai, U.S., Singh, M. & Rai, R.N. Some physicochemical studies on organic eutectics and inter-molecular compounds. J Therm Anal Calorim 130, 967–974 (2017). https://doi.org/10.1007/s10973-017-6429-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6429-7