Abstract
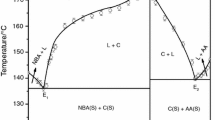
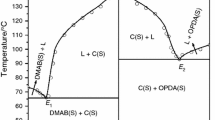
Phase diagrams of N, N-dimethylaminobenzaldehyde (DMAB)–anthranilic acid (AA) and 2-(4-(dimethylamino)benzylideneamino)benzoic acid (DMABAB)–p-nitroaniline (PNA) systems, determined by the thaw-melt method, give two eutectics and a 1:1 intermolecular compound (IMC) in each case. Appropriate amounts (10 g) of each of the eutectics and the IMCs were prepared by a green synthetic method involving a solid state reaction without any solvent. These materials were characterized by X-ray diffraction, thermal and spectral methods and the optical properties of the pure components and the IMCs were studied. While negative values of heat of mixing in the case of a DMAB–AA system suggest clustering of molecules in both eutectic melts, those of positive value in \(\hbox {E}_{1}\) and negative value in \(\hbox {E}_{2}\) of the DMABAB–PNA system indicate the formation of a quasi-eutectic structure in \(\hbox {E}_{1}\) melt and clustering of molecules in \(\hbox {E}_{2}\) melt. The IMC of DMAB–AA system shows strong dual emission with two \(\lambda _{\mathrm{max}} \) one at 380 nm and the second at 450 nm with a total quantum efficiency of 0.49. The IMC of DMABAB–PNA system also shows a similar observation with two \(\lambda _{\mathrm{max}} \) one at 390 nm and second at 435 nm with a total quantum efficiency of 0.31.








Similar content being viewed by others
References
Rudrakshi G B, Pathak J P and Ojha S N 2002 Indian Foundry J. 48 17
Ellott R 1983 Eutectic solidification processing (London: Butterworths)
Majumdar B and Chattopdhyay K 2000 Metall. Mater. Trans 31A 1
Facchetti A, Yoon M H and Marks T J 2005 Adv. Mater. 17 1705
Forest S R 2004 Nature 428 911
Loo Y L and McCulloch I 2008 Mater. Res. Bull. 33 353
Horiuchi S and Tokura Y 2008 Nat. Mater. 7 357
Horiuchi S, Kumari R and Tokura Y 2007 Angew. Chem. Int. Ed. 46 3497
Desiraju G R 1995 Angew. Chem. Int. Ed. Engl. 34 2311
Rai R N, Reddi R S B and Rai U S 2013 Prog. Cryst. Growth Character. Mater. 59 73
Muthuraman R, Masse R, Nicoud J F and Desiraju G R 2001 Chem. Mater. 13 1473
Costa M C, Rolemberg M P, Boros L A D, Kralhenbu1hl M A, de Oliveira M G and Meirelles A J A 2007 J. Chem. Eng. Data 52 30
Rice J W and Suuberg E M 2010 J. Chem. Thermodyn. 42 1356
Dwivedi Y, Kant S, Rai R N and Rai S B 2010 Appl. Phys. B 101 639
Rai R N, Mudunuri S R, Reddi R S B, Ganeshmurthi V S A K and Gupta S K 2011 J. Cryst. Growth 321 72
Dean J A 1985 Lange’s handbook of chemistry (New York: McGraw-Hill)
Singh M, Pandey P, Rai R N and Rai U S 2013 J. Therm. Anal. Calorim. 113 977
Dwivedi Y, Kant S, Rai S B and Rai R N 2011 J. Fluoresc. 21 1255
Abbott A P, Capper G, Davies D L, Rasheed R K and Tambyrajah V 2003 Chem. Commun. 1 70
Abbott A P, Boothby D, Capper G, Davies D L and Rasheed R K 2004 J. Am. Chem. Soc. 126 9142
Rai R N and Varma K B R 2000 Mater. Lett. 44 284
Stahly G P 2009 Cryst. Growth Des. 9 4212
Rai R N 2004 J. Mater. Res. 19 1348
Singh M, Rai R N and Rai U S 2011 Am. J. Anal. Chem. 2 953
Rai U S and Rai R N 1998 J. Cryst. Growth 191 234
Christian J W 1965 The theory of phase transformation in metals and alloys (Oxford: Pergamon Press) p. 992
Singh N, Singh N B, Rai U S and Singh O P 1985 Thermochim. Acta 95 291
Rai R N and Rai U S 2000 Thermochim. Acta 363 23
Kalsi P S 2005 Spectroscopy of organic compounds (India: New Age Publication) 6th edition p 1
Acknowledgements
One of the authors (Dr Manjeet Singh) would like to thank UGC for providing a research fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rai, U.S., Singh, M. & Rai, R.N. Green synthesis, characterization and optical properties of eutectics and 1:1 intermolecular compounds: N,N-dimethylaminobenzaldehyde–anthranilic acid and 2-(4-(dimethylamino)benzylideneamino)benzoic acid–p-nitroaniline systems. Bull Mater Sci 42, 67 (2019). https://doi.org/10.1007/s12034-018-1728-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-018-1728-6