Abstract
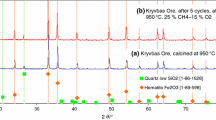
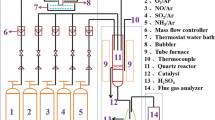
Sintered iron ore fines were selected as oxygen carriers in chemical looping combustion. The reactivity of the sintered iron ore was investigated in redox cycles using thermogravimetric methods under isothermal or non-isothermal conditions. The used sintered iron ore samples from the redox cycles in isothermal tests, which were chosen to evaluate their structural changes, were characterized using the N2 adsorption–desorption, SEM–EDS, XRD and Raman spectroscopy. Results revealed that multiple redox cycle was an activation process of the selected sintered iron ore to promote its redox kinetics, as well as formations of the new crystalline phase. This was attributed to variations of the sintered iron ore in both their physical and chemical structural changes during redox cycles. The N2 adsorption–desorption analysis indicated an increase of its surface areas of sintered iron ore during redox cycles. The SEM–EDS results revealed the appearance of tiny cracks on the tested ore sample surfaces. Both XRD and Raman results presented appearance of a new crystalline phase, such as the lepidocrocite (γ-FeOOH), which was apparently generated in the reduction reaction of chemical looping cycles. The formation of the new lepidocrocite phase seemed correlated with the decrease of the oxygen transport capacity of the sintered iron ore. The sintered iron ore performed properly in the carbon-laden atmospheres, and there was no carbon deposition on its surface.










Similar content being viewed by others
References
Stewart C, Hessami M-A. A study of methods of carbon dioxide capture and sequestration—the sustainability of a photosynthetic bioreactor approach. Energy Convers Manag. 2005;46(3):403–20.
Yang Z-Z, Zhao Y-N, He L-N. CO2 chemistry: task-specific ionic liquids for CO2 capture/activation and subsequent conversion. RSC Adv. 2011;1(4):545–67.
Kenarsari SD, Yang D, Jiang G, Zhang S, Wang J, Russell AG, et al. Review of recent advances in carbon dioxide separation and capture. RSC Adv. 2013;3(45):22739–73.
Liu L, Cao Y, Liu Q. Kinetics studies and structure characteristics of coal char under pressurized CO2 gasification conditions. Fuel. 2015;146:103–10.
Cao Y, Casenas B, Pan W-P. Investigation of chemical looping combustion by solid fuels. 2. Redox reaction kinetics and product characterization with coal, biomass, and solid waste as solid fuels and CuO as an oxygen carrier. Energy Fuels. 2006;20(5):1845–54.
Zhang H, Xiao R, Song M, Shen D, Liu J. Hydrogen production from bio-oil by chemical looping reforming. J Therm Anal Calorim. 2014;115(2):1921–7.
Hossain MM, de Lasa HI. Chemical-looping combustion (CLC) for inherent CO2 separations—a review. Chem Eng Sci. 2008;63(18):4433–51.
Lyngfelt A, Leckner B, Mattisson T. A fluidized-bed combustion process with inherent CO2 separation; application of chemical-looping combustion. Chem Eng Sci. 2001;56(10):3101–13.
Wang K, Yu Q, Qin Q. The thermodynamic method for selecting oxygen carriers used for chemical looping air separation. J Therm Anal Calorim. 2013;112(2):747–53.
Wang K, Yu QB, Qin Q, Li JC. Feasibility study for copper/zirconium oxides as oxygen carrier in chemical looping air separation (CLAS). Adv Mater Res. 2013;683:479–83.
Cao Y, Zhao H-Y, Sit SP, Pan W-P. Lanthanum-promoted copper-based oxygen carriers for chemical looping combustion process. J Therm Anal Calorim. 2014;116(3):1257–66.
Zhao H, Cao Y, Orndorff W, Pan W. Study on modification of Cu-based oxygen carrier for chemical looping combustion. J Therm Anal Calorim. 2013;113(3):1123–8.
Wang B, Xiao G, Song X, Zhao H, Zheng C. Chemical looping combustion of high-sulfur coal with NiFe2O4-combined oxygen carrier. J Therm Anal Calorim. 2014;118(3):1593–602.
Cui Y, Cao Y, Pan WP. Preparation of copper-based oxygen carrier supported by titanium dioxide. J Therm Anal Calorim. 2013;114(3):1089–97.
Sarshar Z, Kleitz F, Kaliaguine S. Novel oxygen carriers for chemical looping combustion: La1−xCexBO3 (B = Co, Mn) perovskites synthesized by reactive grinding and nanocasting. Energy Environ Sci. 2011;4(10):4258–69.
Penthor S, Mayer K, Kern S, Kitzler H, Wöss D, Pröll T, et al. Chemical-looping combustion of raw syngas from biomass steam gasification—coupled operation of two dual fluidized bed pilot plants. Fuel. 2014;127:178–85. doi:10.1016/j.fuel.2014.01.062.
Corbella BM, Palacios JM. Titania-supported iron oxide as oxygen carrier for chemical-looping combustion of methane. Fuel. 2007;86(1):113–22.
Huang Z, He F, Zhao K, Feng Y, Zheng A, Chang S, et al. Natural iron ore as an oxygen carrier for biomass chemical looping gasification in a fluidized bed reactor. J Therm Anal Calorim. 2014;116(3):1315–24.
Abad A, Adánez J, Cuadrat A, García-Labiano F, Gayán P, De Diego LF. Kinetics of redox reactions of ilmenite for chemical-looping combustion. Chem Eng Sci. 2011;66(4):689–702.
Leion H, Mattisson T, Lyngfelt A. Use of ores and industrial products as oxygen carriers in chemical-looping combustion. Energy Fuels. 2009;23(4):2307–15.
Liu X-L, Yin X-J, Zhang H. Reaction characteristics of CO and sintering ore used as an oxygen carrier in chemical looping combustion. Energy Fuels. 2014;28(9):6066–76. doi:10.1021/ef5009677.
Sohn H, Szekely J. A structural model for gas-solid reactions with a moving boundary—III: A general dimensionless representation of the irreversible reaction between a porous solid and a reactant gas. Chem Eng Sci. 1972;27(4):763–78.
Navrotsky A, Mazeina L, Majzlan J. Size-driven structural and thermodynamic complexity in iron oxides. Science. 2008;319(5870):1635–8.
Cornell RM, Schwertmann U. The iron oxides: structure, properties, reactions, occurrences and uses. London: Wiley; 2006.
Adánez J, Cuadrat A, Abad A, Gayán P, de Diego LF, García-Labiano F. Ilmenite activation during consecutive redox cycles in chemical-looping combustion. Energy Fuels. 2010;24(2):1402–13. doi:10.1021/ef900856d.
Navarro C, Díaz M, Villa-García MA. Physico-chemical characterization of steel slag. Study of its behavior under simulated environmental conditions. Environ Sci Technol. 2010;44(14):5383–8.
Frost R, Kloprogge J, Russell S, Szetu J. Dehydroxylation and structure of alumina gels prepared from trisecbutoxyaluminium. Thermochim Acta. 1999;329(1):47–56.
Namduri H, Nasrazadani S. Quantitative analysis of iron oxides using Fourier transform infrared spectrophotometry. Corros Sci. 2008;50(9):2493–7.
Li Y-S, Church JS, Woodhead AL. Infrared and Raman spectroscopic studies on iron oxide magnetic nano-particles and their surface modifications. J Magn Magn Mater. 2012;324(8):1543–50.
Chen C-T, Chen Y-C. Fe3O4/TiO2 core/shell nanoparticles as affinity probes for the analysis of phosphopeptides using TiO2 surface-assisted laser desorption/ionization mass spectrometry. Anal Chem. 2005;77(18):5912–9.
Pinna N, Grancharov S, Beato P, Bonville P, Antonietti M, Niederberger M. Magnetite nanocrystals: nonaqueous synthesis, characterization, and solubility. Chem Mater. 2005;17(11):3044–9.
Xi G, Wang C, Wang X. The oriented self-assembly of magnetic Fe3O4 nanoparticles into monodisperse microspheres and their use as substrates in the formation of Fe3O4 nanorods. Eur J Inorg Chem. 2008;2008(3):425–31.
Shebanova ON, Lazor P. Raman spectroscopic study of magnetite (FeFe2O4): a new assignment for the vibrational spectrum. J Solid State Chem. 2003;174(2):424–30.
Thibeau RJ, Brown CW, Heidersbach RH. Raman spectra of possible corrosion products of iron. Appl Spectrosc. 1978;32(6):532–5.
De Faria D, Venâncio Silva S, De Oliveira M. Raman microspectroscopy of some iron oxides and oxyhydroxides. J Raman Spectrosc. 1997;28(11):873–8.
Shebanova ON, Lazor P. Raman study of magnetite (Fe3O4): laser-induced thermal effects and oxidation. J Raman Spectrosc. 2003;34(11):845–52. doi:10.1002/jrs.1056.
Pimenta HP, Seshadri V. Characterisation of structure of iron ore sinter and its behaviour during reduction at low temperatures. Ironmak Steelmak. 2002;29(3):169–74. doi:10.1179/030192302225002009.
Cui X, Antonietti M, Yu SH. Structural effects of iron oxide nanoparticles and iron ions on the hydrothermal carbonization of starch and rice carbohydrates. Small. 2006;2(6):756–9.
Tay H-L, Li C-Z. Changes in char reactivity and structure during the gasification of a Victorian brown coal: comparison between gasification in O2 and CO2. Fuel Process Technol. 2010;91(8):800–4.
Acknowledgements
This work was financially supported by Overseas Academic Leader Fund of Anhui University (J10117700073), NSF-CHE-MRI under the Award ID of 1338072, NSF-MRI under the Award ID of 1429563 and the National Natural Science Funds of China (51204220). The authors also thank China Scholarship Council for the financial support. The material is based upon the work supported by the National Science Foundation under Cooperative Agreement No. 1355438.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, L., Liu, Q., Cao, Y. et al. Investigation of sintered iron ore fines as an oxygen carrier in chemical looping combustion. J Therm Anal Calorim 125, 459–469 (2016). https://doi.org/10.1007/s10973-016-5320-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5320-2