Abstract
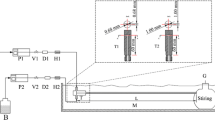
To get the controlled size and monodispersed silica nanoparticle is a popular topic since its broad applications are in a lot of fields such as biological medicine, military project, catalyst, and chip industry. The Stöber process was often used to prepare silica nanoparticle because of its low cost and easy operation. Here, the combined method of microchannel and microwave was proposed to accelerate the rate of both nucleation and growth reactions, which is important to the dimension and uniformity of the monodispersed silica nanoparticle. It was found that the particle size ranging from 15 to 400 nm was achieved by varying the concentration of TEOS and NH4OH with the combined method of microchannel-mixed (MC) and microchannel-microwave (MM). X-ray powder diffraction, Fourier transform infrared spectroscopy, dynamic light scattering, and scanning electron microscopy were used to characterize all the particles. Compared with the MC way, it showed a larger particle size and narrower distribution under the same operating condition experiment with the MM method due to the higher reaction rate of the nucleation and growth. When the concentration of NH4OH is 0.2 M, the size range of produced particles for the MC method is 17.7–28 nm comparing to the MM method is 151–213 nm. Besides, the higher yield of the silica nanoparticles was obtained by the MM method than the MC way because of the better mass and heat transfer. When the concentration of TEOS is 0.25 M, the yield of silica produced by the MC method is 70–77% comparing to the data of 87–92% by the MM combined method. The results of this paper may have important implications for developing the productivity of the industry.

Highlights
-
Fast and continuous synthesis of silica nanoparticles by microchannel-microwave combined method for the first time.
-
Two methods (the microchannel-mixed method and microchannel-microwave method) were investigated and compared in different concentrations of TEOS and NH4OH.
-
The particle size was larger and better distributed by using the microchannel-microwave method than that of the microchannel-mixed method under the same operating condition.
-
The higher yield of the silica nanoparticles was obtained by the microchannel-microwave combined method.









Similar content being viewed by others
References
Brinker CJ (1988) Hydrolysis and condensation of silicates—effects on structure. J Non-Cryst Solids 100(1–3):31–50. https://doi.org/10.1016/0022-3093(88)90005-1
Yang CM, Cho AT, Pan FM, Tsai TG, Chao KJ (2001) Spin-on mesoporous silica films with ultralow dielectric constants, ordered pore structures, and hydrophobic surfaces. Adv Mater 13(14):1099–1102. https://doi.org/10.1002/1521-4095(200107)13:14<1099::Aid-Adma1099>3.0.Co;2-0
Abe HY, Abe I, Sato K, Naito M (2005) Dry powder processing of fibrous fumed silica compacts for thermal insulation. J Am Ceram Soc 88(5):1359–1361. https://doi.org/10.1111/j.1551-2916.2005.00317.x
Arif AF, Taniguchi S, Izawa T, Kamikubo K, Iwasaki H, Ogi T (2018) Microwave-assisted synthesis of C/SiO2 composite with controllable silica nanoparticle size. ACS Omega 3(4):4063–4069. https://doi.org/10.1021/acsomega.8b00340
Guo Q, Yang GQ, Huang DC, Cao WB, Ge L, Li L (2018) Synthesis and characterization of spherical silica nanoparticles by modified Stober process assisted by slow-hydrolysis catalyst. Colloid Polym Sci 296(2):379–384. https://doi.org/10.1007/s00396-017-4260-0
Stober W, Fink A, Bohn E (1968) Controlled growth of monodisperse silica spheres in micron size range. J Colloid Inter Sci 26(1):62–69. https://doi.org/10.1016/0021-9797(68)90272-5
Drummond C, McCann R, Patwardhan SV (2014) A feasibility study of the biologically inspired green manufacturing of precipitated silica. Chem Eng J 244:483–492. https://doi.org/10.1016/j.cej.2014.01.071
Guerrero-Martinez A, Perez-Juste J, Liz-Marzan LM (2010) Recent progress on silica coating of nanoparticles and related nanomaterials. Adv Mater 22(11):1182–1195
Bogush GH, Tracy MA, Zukoski CF (1988) Preparation of monodisperse silica particles—control of size and mass fraction. J Non-Cryst Solids 104(1):95–106. https://doi.org/10.1016/0022-3093(88)90187-1
Brinker CJ (1990) Better ceramics through chemistry—an overview of sol-gel technology. Abstr Pap Am Chem S 200:200–217
Klemperer WG, Ramamurthi SD (1990) A Flory-Stockmayer analysis of silica sol-gel polymerization. J Non-Cryst Solids 121(1–3):16–20. https://doi.org/10.1016/0022-3093(90)90096-5
Matsoukas T, Gulari E (1988) Dynamics of growth of silica particles from ammonia-catalyzed hydrolysis of tetra-ethyl-orthosilicate. J Colloid Inter Sci 124(1):252–261. https://doi.org/10.1016/0021-9797(88)90346-3
Vanblaaderen A, Kentgens APM (1992) Particle morphology and chemical microstructure of colloidal silica spheres made from alkoxysilanes. J Non-Cryst Solids 149(3):161–178. https://doi.org/10.1016/0022-3093(92)90064-Q
Bazula PA, Arnal PM, Galeano C, Zibrowius B, Schmidt W, Schuth F (2014) Highly microporous monodisperse silica spheres synthesized by the Stober process. Micropor Mesopor Mat 200:317–325. https://doi.org/10.1016/j.micromeso.2014.07.051
Vanhelden AK, Jansen JW, Vrij A (1981) Preparation and characterization of spherical monodisperse silica dispersions in non-aqueous solvents. J Colloid Inter Sci 81(2):354–368. https://doi.org/10.1016/0021-9797(81)90417-3
Plumere N, Ruff A, Speiser B, Feldmann V, Mayer HA (2012) Stober silica particles as basis for redox modifications: particle shape, size, polydispersity, and porosity. J Colloid Inter Sci 368:208–219. https://doi.org/10.1016/j.jcis.2011.10.070
Wang XD, Shen ZX, Sang T, Cheng XB, Li MF, Chen LY, Wang ZS (2010) Preparation of spherical silica particles by Stober process with high concentration of tetra-ethyl-orthosilicate. J Colloid Inter Sci 341(1):23–29. https://doi.org/10.1016/j.jcis.2009.09.018
Guo Q, Huang DC, Kou XL, Cao WB, Li L, Ge L, Li JG (2017) Synthesis of disperse amorphous SiO2 nanoparticles via sol-gel process. Ceram Int 43(1):192–196. https://doi.org/10.1016/j.ceramint.2016.09.133
Yokoi T, Sakamoto Y, Terasaki O, Kubota Y, Okubo T, Tatsumi T (2006) Periodic arrangement of silica nanospheres assisted by amino acids. J Am Chem Soc 128(42):13664–13665. https://doi.org/10.1021/ja065071y
Davis TM, Snyder MA, Krohn JE, Tsapatsis M (2006) Nanoparticles in lysine-silica sols. Chem Mater 18(25):5814–5816. https://doi.org/10.1021/cm061982v
Matsoukas J, Cordopatis P, Belte U, Goghari MH, Ganter RC, Franklin KJ, Moore GJ (1988) Importance of the N-terminal domain of the type-II angiotensin antagonist sarmesin for receptor blockade J Med Chem 31(7):1418–1421. https://doi.org/10.1021/jm00402a028
Matsoukas KA, Demertzis MA (1988) Fluorimetric determination of calcium in serum with calcein blue. Analyst 113(2):251–253. https://doi.org/10.1039/an9881300251
Chernysh AM, Bogushevich MS, Tsydenov MM (1991) The mechanism of the occurrence of segmental stress nonuniformities in the left ventricle during disordered excitation processes (Mekhanizm vozniknoveniia segmentarnykh neodnorodnostei napriazhenii v levom zheludochke pri narusheniiakh protsessov vozbuzhdeniia.). Fiziologicheskii Zh SSSR Im I M Sechenova 77(7):35–42
Karpukhin AV, Salimov AG, Veiko NN, Nemtsova MV, Bogush AI, Spitkovskii DM (1991) Nonradioactive detection of DNA probes during in situ hybridization using a new photoactivated reagent for biotinylation of nucleic acids (Neradioaktivnaia detektsiia DNK-zondov pri gibridizatsii in situ s pomoshch’iu novogo fotoaktiviruemogo reagenta dlia biotinilirovaniia nukleinovykh kislot.). Molekuliarnaia genetika, mikrobiologiia i virusologiia 11:13–14
Vylegzhanina TA, Bogush NA (1991) Reaction of the adrenal glands to laser puncture during combined effects of ionizing radiation (Reaktsiia nadpochechnikov na lazeropunkturu v usloviiakh sochetannogo vozdeistviia ioniziruiushchei radiatsii.). Radiobiologiia 31(4):515–520
Vanblaaderen A, Vangeest J, Vrij A (1992) Monodisperse colloidal silica spheres from tetraalkoxysilanes—particle formation and growth-mechanism. J Colloid Inter Sci 154(2):481–501. https://doi.org/10.1016/0021-9797(92)90163-g
Boukari H, Lin JS, Harris MT (1997) Probing the dynamics of the silica nanostructure formation and growth by SAXS. Chem Mater 9(11):2376–2384. https://doi.org/10.1021/cm9702878
LaMer VK, Dinegar RH (1950) Theory, production and mechanism of formation of monodispersed hydrosols. J Am Chem Soc 72(11):4847–4854. https://doi.org/10.1021/ja01167a001
Gutierrez L, Gomez L, Irusta S, Arruebo M, Santamaria J (2011) Comparative study of the synthesis of silica nanoparticles in micromixer-microreactor and batch reactor systems. Chem Eng J 171(2):674–683. https://doi.org/10.1016/j.cej.2011.05.019
Chang CH, Paul BK, Remcho VT, Atre S, Hutchison JE (2008) Synthesis and post-processing of nanomaterials using microreaction technology. J Nanopart Res 10(6):965–980. https://doi.org/10.1007/s11051-007-9355-y
Su M, Su HJ, Du BL, Li XT, Ren GY, Wang SD (2014) The properties of silica nanoparticles with high monodispersity synthesized in the microreactor system. J Sol-Gel Sci Techn 72(2):375–384. https://doi.org/10.1007/s10971-014-3445-y
Tsuji M, Hashimoto M, Nishizawa Y, Kubokawa M, Tsuji T (2005) Microwave-assisted synthesis of metallic nanostructures in solution. Chem-Eur J 11(2):440–452. https://doi.org/10.1002/chem.200400417
Kappe CO (2004) Controlled microwave heating in modern organic synthesis. Angew Chem Int Ed 43(46):6250–6284. https://doi.org/10.1002/anie.200400655
Mahltig B, Gutmann E, Reibold M, Meyer DC, Bottcher H (2009) Synthesis of Ag and Ag/SiO2 sols by solvothermal method and their bactericidal activity. J Sol-Gel Sci Techn 51(2):204–214. https://doi.org/10.1007/s10971-009-1972-8
Conner WC, Tompsett GA (2008) How could and do microwaves influence chemistry at interfaces? J Phys Chem B 112(7):2110–2118. https://doi.org/10.1021/jp0775247
Stenzel C, Brinkmann M, Muller J, Schertlen R, Wiesbeck YVW (2001) A Novel Microwave Applicator for Tailoring the Energy Input for Hydrothermal Synthesis of Zeolites. J Microw Power Ee 36(3):155–168
Abbaspour A, Ghaffarinejad A (2009) Method for Preparation of a Sol-Gel-Derived Carbon Ceramic Electrode Using Microwave Irradiation. Anal Chem 81(9):3660–3664. https://doi.org/10.1021/ac802690s
Chen XX, Yan WF, Cao XJ, Yu JH, Xu RR (2009) Fabrication of silicalite-1 crystals with tunable aspect ratios by microwave-assisted solvothermal synthesis. Micropor Mesopor Mat 119(1-3):217–222. https://doi.org/10.1016/j.micromeso.2008.10.015
Chen XX, Yan WF, Shen WL, Yu JH, Cao XJ, Xu RR (2007) Morphology control of self-stacked silicalite-1 crystals using microwave-assisted solvothemial synthesis. Micropor Mesopor Mat 104(1-3):296–304. https://doi.org/10.1016/j.micromeso.2007.05.015
Zhou HF, Yi R, Li JH, Su Y, Liu XH (2010) Microwave-assisted synthesis and characterization of hexagonal Fe3O4 nanoplates. Solid State Sci 12(1):99–104. https://doi.org/10.1016/j.solidstatesciences.2009.10.012
Washington AL, Strouse GF (2008) Microwave synthesis of CdSe and CdTe nanocrystals in nonabsorbing alkanes. J Am Chem Soc 130(28):8916–8922. https://doi.org/10.1021/ja711115r
Satapathy LN, Ramesh PD, Agrawala D, Roy R (2005) Microwave synthesis of phase-pure, fine silicon carbide powder. Mater Res Bull 40(10):1871–1882. https://doi.org/10.1016/j.materresbull.2005.04.034
Agrawal DK (1998) Microwave processing of ceramics. Curr Opin Solid St M 3(5):480–485. https://doi.org/10.1016/S1359-0286(98)80011-9
Louzguine-Luzgin DV, Xie GQ, Li S, Inoue A, Yoshikawa N, Mashiko K, Taniguchi S, Sato M (2009) Microwave-induced heating and sintering of metallic glasses. J Alloy Compd 483(1-2):78–81. https://doi.org/10.1016/j.jallcom.2008.07.158
Peng JH, Binner J, Bradshaw S (2002) Microwave initiated self-propagating high temperature synthesis of materials. Mater Sci Tech-Lond 18(12):1419–1427. https://doi.org/10.1179/026708302225006142
Slangen PM, Jansen JC, vanBekkum H (1997) The effect of ageing on the microwave synthesis of zeolite NaA. Microporous Mater 9(5-6):259–265. https://doi.org/10.1016/S0927-6513(96)00119-8
Su M, Su HJ, Du BL, Li XT, Ren GY, Wang SD (2015) Mesoporous silica with monodispersed pores synthesized from the controlled self-assembly of silica nanoparticles. Korean J Chem Eng 32(5):852–859. https://doi.org/10.1007/s11814-014-0270-5
Dubey RS, YBRD Rajesh, More MA (2015) Synthesis and Characterization of SiO2 Nanoparticles via Sol-gel Method for Industrial Applications. Mater Today-Proc 2(4-5):3575–3579. https://doi.org/10.1016/j.matpr.2015.07.098
Gholami T, Salavati-Niasari M, Bazarganipour M, Noori E (2013) Synthesis and characterization of spherical silica nanoparticles by modified Stober process assisted by organic ligand. Superlattice Microst 61:33–41. https://doi.org/10.1016/j.spmi.2013.06.004
Vanblaaderen A, Vrij A (1992) Synthesis and Characterization of Colloidal Dispersions of Fluorescent, Monodisperse Silica Spheres. Langmuir 8(12):2921–2931. https://doi.org/10.1021/la00048a013
Su M (2017) Synthesis of highly monodisperse silica nanoparticles in the microreactor system. Korean J Chem Eng 34(2):484–494. https://doi.org/10.1007/s11814-016-0297-x
Vasconcelos DCL, Campos WR, Vasconcelos V, Vasconcelos WL (2002) Influence of process parameters on the morphological evolution and fractal dimension of sol-gel colloidal silica particles. Mat Sci Eng a-Struct 334(1-2):53–58. https://doi.org/10.1016/S0921-5093(01)01762-2. Pii S0921-5093(01)01762-2
Kim KD, Kim HT (2002) New process for the preparation of monodispersed, spherical silica particles. J Am Ceram Soc 85(5):1107–1113
Huang Y, Pemberton JE (2010) Synthesis of uniform, spherical sub-100 nm silica particles using a conceptual modification of the classic LaMer model. Colloid Surf A 360(1-3):175–183. https://doi.org/10.1016/j.colsurfa.2010.02.031
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ren, G., Su, H. & Wang, S. The combined method to synthesis silica nanoparticle by Stöber process. J Sol-Gel Sci Technol 96, 108–120 (2020). https://doi.org/10.1007/s10971-020-05322-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05322-y