Abstract
The Abraham linear free energy relationship model has been used to characterize a hydrophilic interaction liquid chromatography (HILIC) silica column with acetonitrile/water and methanol/water mobile phases. Analysis by the model for acetonitrile/water mobile phases points to solute volume and hydrogen bond basicity as the main properties affecting retention, whereas solute hydrogen bond acidity, dipolarity and polarizability practically do not affect it. Formation of a cavity is easier in acetonitrile-rich mobile phases than in the aqueous stationary phase, and hence increase of solute volume decreases retention. Conversely, hydrogen bond acidity is stronger in the aqueous stationary phase than in the acetonitrile-rich mobile phase and thus an increase of solute hydrogen bond basicity increases retention. Results are similar for methanol/water mobile phases with the difference that solute hydrogen bond acidity is significant too. Increase in hydrogen bond acidity of the solute decreases retention showing that methanol mobile phases must be better hydrogen bond acceptors than acetonitrile ones, and even than water-rich stationary phases. The results are like the ones obtained in zwitterionic HILIC columns bonded to silica or polymer supports for acetonitrile/water mobile phases, but different for solute hydrogen bond acidity for a polymer bonded zwitterionic column with methanol/water mobile phases, indicating that bonding support plays an important role in HILIC retention. Comparison to RPLC characterized systems confirms the complementarity of HILIC systems to RPLC ones because the main properties affecting retention are the same but with reversed coefficients. The least retained solutes in RPLC are the most retained in HILIC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hydrophilic interaction liquid chromatography (HILIC) is a modern mode of liquid chromatography complementary to reversed-phase liquid chromatography (RPLC). Despite that the retention time is very short, HILIC is able to separate polar compounds, that cannot be separated by RPLC. In contrast to RPLC, HILIC uses a polar stationary phase with a much less polar mobile phase. The difference to normal-phase liquid chromatography (NPLC), which uses pure or mixed non-aqueous solvents as mobile phases, is that HILIC mobile phases are similar to the RPLC ones (i.e., they are aqueous organic solvent mixtures, such as acetonitrile–water or methanol–water), with the difference that water contents are low enough (5–40%) to keep a polarity much lower than that of the stationary phase. Presence of water in the mobile phase facilitates the solubility of very polar and ionic solutes which would not be solubilized in NPLC and that would be almost not retained in RPLC [1,2,3,4,5].
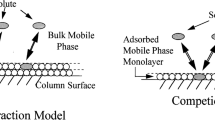
The retention mechanism in HILIC is more complex than in RPLC or NPLC. The polar stationary phase adsorbs water from the mobile phase and several water-rich layers of graduated variable composition and mobility are formed between the column stationary phase surface and the bulk mobile phase [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17]. Polar solutes partition between the mobile phase and these adsorbed or semi-adsorbed layers and, since the transitional mobility of these layers are reduced in reference to the mobile phase, the solutes are delayed and eluted later, i.e., they are retained. In fact, the different solute–solvent interactions that contribute to solute partitioning between the bulk hydroorganic mobile phase and the water-rich layers partially immobilized on the stationary phase are considered to be the main retention mechanism in HILIC [1]. However, other interactions with the column components can also be involved in the retention depending on the solute characteristics, the functional groups of the bonded phase and support, and the solvent composition of the mobile phase [2,3,4,5,6, 8, 11, 13, 14].
The stationary phase in HILIC must be polar enough to interact with the water contained in the mobile phase and adsorb part of it. Bare silica, the classical stationary phase for NPLC, was one of the first supports used in HILIC, but many other polar phases, such as amino, amide, diol, cyano and especially zwitterionic phases, bonded to different supports have been developed [2, 4,5,6, 8]. Although all these phases may appear to be very different, they have in common that they are the support for the adsorbed water-rich layers acting as main stationary phase, and thus analytes are expected to have similar partition properties in them. However, some differences may be also expected because water adsorption is different depending on the column functionalization [16, 17] and distinct additional interactions between the solute and the different bonded phases and supports are expected too.
Regarding mobile phases, acetonitrile–water mixtures are by far the most used HILIC eluents, as in RPLC but with a much lower proportion of water (3–40%) to keep a low polarity. Other organic modifiers of different eluotropic strength commonly used in RPLC, such as methanol, isopropanol or tetrahydrofuran, have also been investigated as HILIC mobile phases [4]. Variation of the nature (organic solvent) and composition (%) of the mobile phase produces changes in the amount of adsorbed water [16, 17] and of course variation of the additional interactions with the bonded phase and support.
The main purpose of this paper is the characterization of the different solute–solvent interactions that led to HILIC retention in a silica column with acetonitrile/water and methanol/water mobile phases by means of the general linear free energy relationship (LFER) developed by Abraham [18]. The Abraham model has been applied to characterize many RPLC systems [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62] and some NPLC ones [63,64,65,66,67,68], but as far as we know only three HILIC columns, all them with a sulfobetaine phase bonded to a silica [69] or a polymeric [70] support, have previously been characterized for neutral solutes retention, although some modifications of the LFER approach have been proposed for the retention of ionic or partially ionized solutes in HILIC columns [69, 71, 72].
Characterization of a pure silica column (with no bonded phase) will provide information on the different types of solute–solvent interactions (dipolarity, polarizability, hydrogen bonding, creation of cavities in the solvents) that contribute to HILIC partition and retention, interactions that are commonly described by the unspecific term of “polarity”. The provided information can be contrasted with the one already known for RPLC and NPLC, and since some functionalized HILIC columns have been already characterized, an additional goal will be comparison of the effect of the sulfobetaine bonded phase and support on the characterized solute–solvent interactions.
2 Theory
2.1 Abraham LFER Model
Abraham developed several models based in linear free energy relationships to relate many physicochemical and biological properties to solute–solvent interactions [18]. In these models a free energy related parameter (such as the logarithm of an equilibrium constant) is linearly related to the combination of solute and solvent descriptors, describing each descriptor a particular type of interaction. The model for the partition between two condensed phases applied to liquid chromatography (also named solvation parameter model) usually takes the form of Eq. 1.
Since the equilibrium constant for the partition between stationary and mobile phases is difficult to measure in liquid chromatography, the retention factor (k), which is directly related to the partition constant by the phase ratio (ratio between the volumes of stationary and mobile phases) is used instead of the constant.
In the Abraham model of Eq. 1, the v·V term accounts for the difference in free energy for cavity formation in the two solvents (stationary and mobile phases) together with residual solute–solvent dispersion interactions. The e·E term models the difference in polarizability contributions from n- and π-electron pairs, s·S the dipole-type interactions (orientation and induction) differences, a·A the hydrogen bond donation from the solute to solvent phases, and b·B the hydrogen bond donation from solvents to solute. c is the system constant, which includes the phase ratio, normalization of descriptors and other factors independent of the probe solutes terms.
E, S, A, B, and V are solute descriptors, either experimentally determined or calculated. V is the McGowan molar volume. E is the solute excess molar refractivity. S is the solute dipolarity/polarizability, A and B are the overall hydrogen bond acidity and basicity, respectively [18]. The descriptors are known for about 9000 compounds [73, 74], and free [73] and commercial [74] software is available for the calculation, if necessary. More recently, Poole has developed an alternative database of descriptors from chromatographic data [75].
e, s, a, b, and v are the system coefficients obtained by multilinear regression of the free energy related property (log10 k in this case) against the solute descriptors. They reflect the difference in solute interaction between the stationary and mobile phases. The sign (positive or negative) and magnitude of these coefficients lead to the characterization of chromatographic systems, finding the key features responsible for retention and allowing the comparison between different retention modes, columns, and mobile phases. This information can be easily obtained by measuring the chromatographic retention of an adequate series of solutes with well-known descriptors and regressing its retention (log10 k) against their descriptors. A tutorial for the practical application of the method to partition processes, and in particular to liquid chromatography, has recently been presented by Poole [76].
The Abraham LFER model has been successfully applied to a large number of physicochemical and biological processes [18], and in particular many liquid chromatography ones [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68], to obtain chemical and biological information about the intermolecular interactions governing the processes being studied [39].
Application of the model to liquid chromatography requires the accurate determination of the retention factor (k). k is calculated from the measured retention volume (VR) or time (tR) of the solute and the hold-up volume (VM) or time (tM) according to Eq. 2.
Please notice that extracolumn volumes (or times) were subtracted from gross retention values obtained from chromatographic peaks, in order to refer retention only to the column and thus be independent of the particular instrument used.
2.2 Determination of the Hold-Up Volume by the Abraham LFER Approach
Determination of the chromatographic hold-up volume (a measure of the volume of the effective mobile phase) is not an easy task, especially in HILIC where a significant part of the mobile phase is adsorbed and acts as stationary phase. The Abraham LFER model offers a simple way to determine the hold-up volume by measuring the retention of different members of a homologous series [12].
As Table 1 shows, solute members of a particular homologous series have almost identical Abraham descriptors except for their McGowan’s volume (V). Therefore, all terms of Eq. 1, with the exception of the volume, can be considered constant, and combination of Eqs. 1 and 2 leads to Eq. 3.
being r0 a constant defined in Eq. 4:
The hold-up volume can easily be obtained, together with r0, by non-linear regression of the retention volumes of the homologous series members against their LFER descriptor volumes (V).
Different homologous series may be used for the extrapolation of VM which may led to slightly different hold-up volumes. The different series can be combined in a unique equation to obtaining an unique VM value, as well as an unique v parameter, by using flag descriptors as described by Eq. 5 [16, 17]:
where n is the number of homologous series included in the model, and fi are binary flag descriptors (1 or 0) used as independent variables in the fitting (i.e., for homologues of i-th series, fi = 1 and fj ≠ i = 0). Hold-up volume (VM) and v are the same for each and every considered homologous series, and their values are fitted together from all retention data. On the contrary, the r0 value is series dependent, and it is calculated only from the retention data of the corresponding series setting f = 1 for this series and f = 0 for the other series, avoiding in this way the influence of the data from other series to each particular series r0 calculation.
3 Experimental
3.1 Chemicals and Solvents
Injected analytes were from Acros Organics, Alfa Aesar, Baker, Fluka, J.T. Baker, Merck, and Sigma-Aldrich; all of high purity grade (≥ 99%). Water was obtained from a Milli-Q plus system (Millipore, Billerica, USA) with a resistivity of 18.2 MΩ cm. Acetonitrile and methanol were HPLC gradient grade and purchased from Fisher (Loughborough, UK).
3.2 Instrumentation and Chromatographic Conditions
HPLC measurements were performed on a Shimadzu (Kyoto, Japan) HPLC system consisting of two LC-10ADvp pumps, a SIL-10ADvp auto-injector, an SPD-M10AVvp diode array detector and a CTO-10ASvp oven at 25 °C and a SCL-10Avp controller. A 150 × 4.6 mm Kinetex 5 μm HILIC 100 Å (unbonded silica) column (Phenomenex, Torrance, CA, USA) was employed. Injection volume and flow rate were 1 μL and 1 mL⋅min−1, respectively.
3.3 Procedure
Extracolumn volume was determined injecting 1 μL of 0.5 mg⋅mL−1 aqueous solution of potassium bromide (Merck, > 99%) in absence of column and using water as eluent at the flow rate of 0.50 mL⋅min−1, in triplicate. The overall extracolumn volume in the particular chromatograph employed was 0.12 mL. This volume was subtracted from gross retention volumes obtained from all chromatograms.
Stock solutions of injected analytes were prepared in methanol at concentrations of 5–10 mg⋅mL−1, and diluted to 0.5–1 mg⋅mL−1 before injection. The column was equilibrated with the new eluent for at least 20 min when changing the mobile phase.
4 Results and Discussion
4.1 Hold-Up Volume Determination by the LFER Method
The measurement of the retention factors (k) of the considered solutes (Eq. 2) requires a previous determination of the hold-up volume (VM) of the studied HILIC systems. This determination has been done by the Abraham LFER approach described in Sect. 2.2.
Three homologous series were selected: n-alkyl benzenes, n-alkyl phenones, and n-alkyl ketones. The Abraham descriptors of the homologues are presented in Table 1 together with the descriptors of the rest of solutes studied in the characterization.
Conjoint hold-up volumes were determined from the results of the three series simultaneously analyzed by Eq. 5. The obtained results for the studied systems are presented in Table 2 together with the statistics of the fitting. Figure 1 presents the plots obtained for all studied series and mobile phase compositions. The fitted VM value in Eq. 5 represents a non-linear extrapolation of the molecular volume of the homologues to infinite, and this might involve a significant degree of uncertainty. However, differences between the fitted VM and the VR of the closest homologue (dodecylbenzene) is, in most of the chromatographic systems studied in this work, less than 1%, never higher than 3%, and thus extrapolations were performed from a relative short distance in retention. The good precision of the extrapolation is reflected in the small standard deviations of the extrapolated VM values presented in Table 2 (about 0.01 mL).
Hold-up volume fittings (Eq. 5) from retention data of homologous series showing a typical HILIC behavior
Acetonitrile/water and methanol/water mobile phases representative of HILIC retention were selected for the study. HILIC mobile phases must contain some water to be adsorbed as stationary phase, but not so much for the polarity of the mobile phase to be higher than or even like that of the stationary phase. For the studied silica column and mobile phases this range becomes approximately 50–90% of acetonitrile or 70–90% of methanol. In these composition ranges the retention vs. solute volume plots for homologous series show a clear HILIC behavior (see the downwards plots in Fig. 1), being for a particular series the largest solutes less retained than the smallest ones [12, 16, 17]. When the water contents of the mobile phase increase, the largest solutes of the series become more retained than expected (in relation to the expected trend from smaller series members) because of partial RPLC retention, giving rise to a dual HILIC-RPLC retention mechanism also observed in other columns [13, 15,16,17]. For mobile phases very rich in water only the RPLC retention mechanism is observed in HILIC columns, obtaining typical retention vs. volume upwards plots [16, 17].
Table 2 shows that the hold-up volumes for acetonitrile/water mobile phases slightly decrease in the range 90–70% acetonitrile, i.e., the volume of mobile phase decreases when its water content increases because more water is adsorbed as stationary phase. However, a further increase of water content in the mobile phase (range 70–50% acetonitrile) produces a slight increase of VM (i.e., less adsorption of water) probably because of the increase of mobile phase polarity.
For methanol/water mobile phases VM values are similar for all compositions and larger than for acetonitrile/water, which implies a smaller and more constant water adsorption. This behavior has been observed in other columns too [17].
4.2 LFER Characterization of the HILIC Silica Column
A set of 75 solutes was selected for the column characterization. The selected set was similar to those used previously [70], complemented with the members of the homologous series used for VM determination.
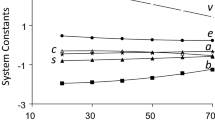
Retention data was measured for the whole set of solutes in the eight HILIC mobile phases studied, and the k value of each compound was calculated from the solute retention volume and the hold-up volume at the particular mobile phase composition (Table 2). Later, retention factors for each mobile phase were correlated against the solute descriptors according to Eq. 1. The solutes with residuals higher than 2.5 times the standard deviation of the linear regression in any of the studied chromatographic systems were marked as outliers. The number of outliers was low, 2–6 for acetonitrile/water mobile phases and 8–9 for methanol/water ones, depending on the mobile phase composition. The results obtained in the correlations are presented in Table 3 with the statistics of the fits. The variation of coefficients obtained in the fits with the mobile phase composition are presented in Fig. 2.
Characterization (Eq. 1) of the Kinetex HILIC (unbound silica) column in acetonitrile/water and methanol/water mobile phases showing HILIC behavior
The main solute properties affecting the retention in mobile phases containing acetonitrile are the molecular volume (V) and the hydrogen bond acceptor capability (B). Since intermolecular interactions in acetonitrile-rich mobile phases are weaker than in the water-rich adsorbed stationary phase, the formation of a cavity in the mobile phase requires a lower amount of energy and therefore the higher the molecular volume, the lower the retention (v < 0). Regarding hydrogen bonding, the b coefficient is positive and large showing that the water-rich stationary phase can interact stronger by hydrogen bond donation to the hydrogen bond acceptor solute than the acetonitrile-rich mobile phase. In contrast, the a coefficient is negative, but not significant (by Student’s t-test at 95% confidence level) demonstrating that the mobile and stationary phases must be similar in terms of hydrogen bond acceptor abilities. We think that these results are reasonable, because water (mostly in the stationary phase) is a good donor and acceptor of hydrogen bonds, whereas acetonitrile (mostly in the mobile phase) is also a good acceptor (although a bad donor). Solvent hydrogen bond basicity must be similar in both phases and thus hydrogen bond donation from solute to the solvent cancels out retention effects (a·A ≈ 0). However, hydrogen bond acceptor solutes favour retention by a strong hydrogen bond donation from the water-rich stationary phase to the solute, which is not possible in the acetonitrile-rich mobile phase (b·B > 0). Water and acetonitrile are both quite dipolar and poorly polarizable, and this type of interactions must be comparable in both phases leading to non-significant s and e coefficients.
Slightly different results are obtained for methanol/water mobile phases. Like in acetonitrile/water, volume and hydrogen bond basicity are the most significant solute properties affecting retention. The volume coefficient (v) is large and negative and b large and positive, as in acetonitrile/water. However, the a coefficient is significant and negative showing that an increase of the hydrogen bond acidity of the solute decreases retention, i.e., the methanol-rich mobile phase must be more hydrogen bond basic than the water-rich stationary phase. Dipolarity is somewhat significant too, and positive. Hence, an increase in the dipolarity of the solute increases retention, implying that the stationary phase must be slightly more dipolar than the mobile phase. The e coefficient is not significant at all by Student’s t-test at 95% confidence level, as in acetonitrile/water mobile phases.
As an example, Table 4 shows the products of the chromatographic system coefficients and the molecular descriptors for chlorobenzene and 4-chlorophenol in two mobile phases containing 80% of organic solvent, one with acetonitrile and the other with methanol. Each product accounts for the contribution of the considered specific interaction to the chromatographic retention. The structural difference of these solutes is the hydroxy group, and thus the phenol is just slightly larger (ΔV = 0.06) and with somewhat improved hydrogen bond basicity (ΔB = 0.13) and polarizability from n- and π-electron pairs (ΔE = 0.20) interactions. The main differences lie in the dipole-type interactions (ΔS = 0.43) and particularly hydrogen bond acidity capabilities (ΔA = 0.67). In acetonitrile the contribution of A is not relevant, since the hydrogen bond basicity of the system (a) is practically zero (Table 3), and thus the product a·A is insignificant. Differences in dipolarity (ΔS) are also poorly relevant due to the low value of the s coefficient. The hydrogen bond acidity of the system is relevant (b), but since ΔB is small the differences in the product b·B are not substantial. Consequently, similar chromatographic retentions are expected (and experimentally confirmed) for chlorobenzene and 4-chlorophenol at 80% acetonitrile. However, when methanol is used in the mobile phase the solute hydrogen bond acidity gains importance (a = − 0.42), and thus the retention of the phenol is reduced (a·A = − 0.28) in relation to the benzene, which lacks hydrogen bond donor capacities. Therefore, 4-chlorophenol is less retained than chlorobenzene in the studied underivatized silica column at 80% methanol.
4.3 Comparison to Other HPLC Systems
HILIC was developed as complementary to RPLC for separation of polar compounds that cannot be well separated in RPLC because they are weakly retained. Then, it is expected that solute–solvent interactions are completely different and even mainly reversed compared to those of RPLC. It is well known [19,20,21,22,23,24,25, 28,29,30,31,32,33,34, 36, 37, 39,40,41, 43,44,45,46,47, 49, 53,54,55,56,57,58,59, 61] that the main interactions affecting RPLC retention are solute volume (v·V) and hydrogen bond basicity (b·B), as in our HILIC-acetonitrile system but with reversed signs of the coefficients. Conversely to HILIC, in RPLC large solutes with poor hydrogen bonding basicity are well retained. Complementarity of HILIC to RPLC can be demonstrated by comparison of the coefficients of typical RPLC and HILIC systems.
In RPLC, retention is very dependent of the composition of the mobile phase, although the proportions of the predominant interactions are rather constant [70]. Then, it is convenient to normalize the coefficients for comparison of the different compositions and columns [77]. Normalization is done by dividing each coefficient by the length (l) of the correlation vector, which can be easily obtained from Eq. 6
Thus, the normalized coefficients, which correspond to the unitary correlation vector, eu, su, au, bu, and vu are obtained. Normalized coefficients are relative weights of the respective solute–solvent interactions in the solute retention.
The normalized coefficients for the two most representative HILIC systems studied in this work (90% acetonitrile and 90% methanol) are presented in Table 5, together with representative normalized coefficients of RPLC systems. RPLC normalized coefficients are the means of different C8 and C18 with different acetonitrile/water (20–90% acetonitrile) and methanol/water (10–80% methanol) mobile phases and were taken from a previous work [70]. Standard deviations of the coefficients are very low (< 0.1, see the original reference) and prove the representativity of the coefficient’s values. Data for a typical NPLC system with a silica column and a non-aqueous organic solvent mixture (n-hexane with 5% propan-2-ol as modifier) is given too [66, 70], which is expected to be more similar to HILIC systems. In fact, HILIC is sometimes considered a type of NPLC.
Similarities and differences between the compared systems can be easily observed by a radial plot such as the one presented in Fig. 3A. It can be observed that the HILIC system are similar to the NPLC representative system than to the RPLC systems. The acetonitrile-HILIC system is the closest to the NPLC ones, whereas the methanol-HILIC system is quite close too with the exception of the hydrogen bond donation interaction from solute to solvent (a coefficient). Acetonitrile- and methanol-RPLC systems are very different from HILIC and NPLC systems (except for the polarizability e·E interaction), especially for the volume v·V and hydrogen bond donation from solvent to solute b·B interactions. The RPLC systems are all very similar in all relative interactions regardless of the organic modifier of the mobile phase (acetonitrile or methanol) and their compositions and columns.
Radial plot of normalized system coefficients for (A) typical reversed-phase systems (C8-C18, 20–90% acetonitrile and 10–80% methanol), normal-phase [Nucleosil Silica n-hexane/propan-2-ol (95/5)] and Kinetex HILIC (unbound silica, 90% acetonitrile and 90% methanol) chromatographic systems; and B different HILIC columns (Kinetex HILIC, ZIC-HILIC, ZIC-pHILIC, and Nucleodur HILIC) in hydroorganic mobile phases containing 80% acetonitrile or 80% methanol)
The few HILIC systems characterized in literature by the Abraham model for neutral solutes have been compared to the HILIC systems studied here. We have literature data for 80% acetonitrile in a ZIC-HILIC and a Nucleodur HILIC columns [69], which have a zwitterionic sulfobetaine bonded to a silica support, and 80–95% acetonitrile and 80–95% methanol for a ZIC-pHILIC column [70] with a zwitterionic sulfobetaine bonded to a polymeric support. We have selected 80% acetonitrile (available in all columns) and 80% methanol mobile phases for comparison with our results in the Kinetex silica column (with no bonding) for the same mobile phases. Normalized coefficients are presented in Table 4 and the comparison of the normalized coefficients can be observed in Fig. 3B.
Figure 3B shows that the most different HILIC system is the one of the ZIC-pHILIC column with methanol, especially for b coefficient which is negative (like in RPLC) whereas in the other HILIC columns it is positive. We suppose that the reason may lay in the polymeric support, which is different from the silica support of the other columns. However, the same column with acetonitrile/water is more similar to the other HILIC columns with acetonitrile/water too. Our pure silica column with acetonitrile/water is similar to the two sulfobetaine functionalized silica columns ZIC-HILIC and Nucleodur HILIC, suggesting that functionalization of the column does not have a big effect in its HILIC character. Our pure silica column with methanol/water mobile phase is different in the a coefficient. It is negative (like in RPLC) whereas in the other HILIC systems is zero or positive. Comparison confirms that HILIC columns with acetonitrile/water mobile phases have a HILIC character more marked than the same columns with methanol/water mobile phases. Surprisingly, the support (silica or polymer) seems to be more determinant of the HILIC behavior (at least for methanol/water mobile phases) than the presence of the bonded phase (sulfobetaine), although characterization of more HILIC systems with different bonding would be needed to confirm it.
5 Conclusions
Application of the Abraham LFER model for neutral solutes to a HILIC silica column with acetonitrile/water and methanol/water mobile phases allows to characterize de main solute–solvent interactions responsible of HILIC retention. Creation of a cavity in the phases and hydrogen bond donation from the phases are the main interactions. Increase of solute volume favors elution, whereas hydrogen bond acceptor ability (basicity) of the solute increases retention. For mobile phase with methanol/water, hydrogen bond donation from solute to phases is also important, and a decrease in hydrogen bond donor ability (acidity) of the solute favors retention. Solute dipolarity and polarizability, as well as hydrogen bond acidity for acetonitrile/water mixtures, do not seem to have a significant effect in the retention of compounds.
Comparison to reversed-phase and normal-phase shows that hydrophilic interaction liquid chromatography systems are closer to normal-phase systems than to reversed-phase ones, especially using acetonitrile/water mobile phases. In general, acetonitrile/water eluents in HILIC columns show stronger HILIC interactions than methanol/water eluents.
Comparison of diverse HILIC systems indicate that functionalization of the column may not have a big effect on the HILIC solute–solvent interactions, whereas the support of the functionalization (silica or polymer) and the lack of it (pure silica columns) may be more important.
References
Alpert, A.J.: Hydrophilic-interaction chromatography for the separation of peptides, nucleic acids and other polar compounds. J. Chromatogr. A 499, 177–196 (1990). https://doi.org/10.1016/S0021-9673(00)96972-3
Buszewski, B., Noga, S.: Hydrophilic interaction liquid chromatography (HILIC)—a powerful separation technique. Anal. Bioanal. Chem. 402, 231–247 (2012). https://doi.org/10.1007/s00216-011-5308-5
Kumar, A., Heaton, J.C., McCalley, D.V.: Practical investigation of the factors that affect the selectivity in hydrophilic interaction chromatography. J. Chromatogr. A 1276, 33–46 (2013). https://doi.org/10.1016/j.chroma.2012.12.037
McCalley, D.V.: Understanding and manipulating the separation in hydrophilic interaction liquid chromatography-a review. J. Chromatogr. A 1523, 49–71 (2017). https://doi.org/10.1016/j.chroma.2017.06.026
Jandera, P., Janás, P.: Recent advances in stationary phases and understanding of retention in hydrophilic interaction chromatography. A review. Anal. Chim. Acta 967, 12–32 (2017). https://doi.org/10.1016/j.aca.2017.01.060
McCalley, D.V.: Study of the selectivity, retention mechanisms and performance of alternative silica-based stationary phases for separation of ionised solutes in hydrophilic interaction chromatography. J. Chromatogr. A 1217, 3408–3417 (2010). https://doi.org/10.1016/j.chroma.2010.03.011
Dinh, N.P., Jonsson, T., Irgum, K.: Probing the interaction mode in hydrophilic interaction chromatography. J. Chromatogr. A 1218, 5880–5891 (2011). https://doi.org/10.1016/j.chroma.2011.06.037
Jandera, P.: Stationary and mobile phases in hydrophilic interaction chromatography: a review. Anal. Chim. Acta 692, 1–25 (2011). https://doi.org/10.1016/j.aca.2011.02.047
Dinh, N.P., Jonsson, T., Irgum, K.: Water uptake on polar stationary phases under conditions for hydrophilic interaction chromatography and its relation to solute retention. J. Chromatogr. A 1320, 33–47 (2013). https://doi.org/10.1016/j.chroma.2013.09.061
Soukup, J., Jandera, P.: Adsorption of water from aqueous acetonitrile on silica-based stationary phases in aqueous normal-phase liquid chromatography. J. Chromatogr. A 1374, 102–111 (2014). https://doi.org/10.1016/j.chroma.2014.11.028
Guo, Y.: Recent progress in the fundamental understanding of hydrophilic interaction chromatography (HILIC). Analyst 140, 6452–6466 (2015). https://doi.org/10.1039/C5AN00670H
Subirats, X., Justicia, A., Rosés, M.: Chasing the elusive hold-up time from an LFER approach. J. Chromatogr. A 1571, 176–184 (2018). https://doi.org/10.1016/j.chroma.2018.08.017
Jandera, P., Hájek, T.: Mobile phase effects on the retention on polar columns with special attention to the dual hydrophilic interaction-reversed-phase liquid chromatography mechanism, a review. J. Sep. Sci. 41, 145–162 (2018). https://doi.org/10.1002/jssc.201701010
Shamshir, A., Dinh, N.P., Jonsson, T., Sparrman, T., Irgum, K.: Probing the retention mechanism of small hydrophilic molecules in hydrophilic interaction chromatography using saturation transfer difference nuclear magnetic resonance spectroscopy. J. Chromatogr. A 1623, 461130 (2020). https://doi.org/10.1016/j.chroma.2020.461130
Jandera, P., Hájek, T.: Dual-mode hydrophilic interaction normal phase and reversed phase liquid chromatography of polar compounds on a single column. J. Sep. Sci. 43, 70–86 (2020). https://doi.org/10.1002/jssc.201900920
Redón, L., Subirats, X., Rosés, M.: HILIC characterization: estimation of phase volumes and composition for a zwitterionic column. Anal. Chim. Acta 1130, 39–48 (2020). https://doi.org/10.1016/j.aca.2020.06.035
Redón, L., Subirats, X., Rosés, M.: Volume and composition of semi-adsorbed stationary phases in hydrophilic interaction liquid chromatography. Comparison of water adsorption in common stationary phases and eluents. J. Chromatogr. A 1656, 462543 (2021). https://doi.org/10.1016/j.chroma.2021.462543
Abraham, M.H.: Scales of solute hydrogen-bonding: their construction and application to physicochemical and biochemical processes. Chem. Soc. Rev. 22, 73 (1993). https://doi.org/10.1039/cs9932200073
Abraham, M.H., Rosés, M.: Hydrogen bonding. 38. Effect of solute structure and mobile phase composition on reversed-phase high-performance liquid chromatographic capacity factors. J. Phys. Org. Chem. 7, 672–684 (1994). https://doi.org/10.1002/poc.610071205
Tan, L.C., Carr, P.W., Abraham, M.H.: Study of retention in reversed-phase liquid chromatography using linear solvation energy relationships I. The stationary phase. J. Chromatogr. A 752, 1–18 (1996). https://doi.org/10.1016/S0021-9673(96)00459-1
Li, J., Carr, P.W.: Characterization of polybutadiene-coated zirconia and comparison to conventional bonded phases by use of linear solvation energy relationships. Anal. Chim. Acta 334, 239–250 (1996). https://doi.org/10.1016/S0003-2670(96)00302-9
Abraham, M.H., Rosés, M., Poole, C.F., Poole, S.K.: Hydrogen bonding. 42. Characterization of reversed-phase high-performance liquid chromatographic C18 stationary phases. J. Phys. Org. Chem. 10, 358–368 (1997)
Tan, L.C., Carr, P.W.: Study of retention in reversed-phase liquid chromatography using linear solvation energy relationships. J. Chromatogr. A 799, 1–19 (1998). https://doi.org/10.1016/S0021-9673(97)01054-6
Blackwell, J.A., Carr, P.W.: Study of the effect of mobile phase additives on retention in reversed phase HPLC using linear solvation energy relationships. J. High Resolut. Chromatogr. 21, 427–434 (1998). https://doi.org/10.1002/(SICI)1521-4168(19980801)21:8%3c427::AID-JHRC427%3e3.0.CO;2-3
Zhao, J., Carr, P.W.: Comparison of the retention characteristics of aromatic and aliphatic reversed phases for HPLC using linear solvation energy relationships. Anal. Chem. 70, 3619–3628 (1998). https://doi.org/10.1021/ac980173v
Rosés, M., Bolliet, D., Poole, C.F.: Comparison of solute descriptors for predicting retention of ionic compounds (phenols) in reversed-phase liquid chromatography using the solvation parameter model. J. Chromatogr. A 829, 29–40 (1998). https://doi.org/10.1016/S0021-9673(98)00746-8
Bolliet, D., Poole, C.F., Rosés, M.: Conjoint prediction of the retention of neutral and ionic compounds (phenols) in reversed-phase liquid chromatography using the solvation parameter model. Anal. Chim. Acta 368, 129–140 (1998). https://doi.org/10.1016/S0003-2670(98)00190-1
Wang, A., Tan, L.C., Carr, P.W.: Global linear solvation energy relationships for retention prediction in reversed-phase liquid chromatography. J. Chromatogr. A. 848, 21–37 (1999). https://doi.org/10.1016/S0021-9673(99)00464-1
Reta, M., Carr, P.W., Sadek, P.C., Rutan, S.C.: Comparative study of hydrocarbon, fluorocarbon, and aromatic bonded RP-hplc stationary phases by linear solvation energy relationships. Anal. Chem. 71, 3484–3496 (1999). https://doi.org/10.1021/ac990081l
Li, L., Carr, P.W., Evans, J.F.: Studies of retention and stability of a horizontally polymerized bonded phase for reversed-phase liquid chromatography. J. Chromatogr. A 868, 153–167 (2000). https://doi.org/10.1016/S0021-9673(99)01194-2
Du, C.M., Valko, K., Bevan, C., Reynolds, D., Abraham, M.H.: Characterizing the selectivity of stationary phases and organic modifiers in reversed-phase high-performance liquid chromatographic systems by a general solvation equation using gradient elution. J. Chromatogr. Sci. 38, 503–511 (2000). https://doi.org/10.1093/chromsci/38.11.503
Valkó, K., Espinosa, S., Du, C., Bosch, E., Rosés, M., Bevan, C., Abraham, M.: Unique selectivity of perfluorinated stationary phases with 2,2,2-trifluoroethanol as organic mobile phase modifier. J. Chromatogr. A 933, 73–81 (2001). https://doi.org/10.1016/S0021-9673(01)01254-7
Zissimos, A.M., Abraham, M.H., Du, C.M., Valko, K., Bevan, C., Reynolds, D., Wood, J., Tam, K.Y.: Calculation of Abraham descriptors from experimental data from seven HPLC systems; evaluation of five different methods of calculation. J. Chem. Soc. Perkin Trans. 2, 2001–2010 (2002). https://doi.org/10.1039/b206927j
Lepont, C., Poole, C.F.: Retention characteristics of an immobilized artificial membrane column in reversed-phase liquid chromatography. J. Chromatogr. A 946, 107–124 (2002). https://doi.org/10.1016/S0021-9673(01)01579-5
Espinosa, S., Bosch, E., Rosés, M.: Retention of ionizable compounds on high-performance liquid chromatography. J. Chromatogr. A 945, 83–96 (2002). https://doi.org/10.1016/S0021-9673(01)01486-8
Kiridena, W., Poole, C.F., Koziol, W.W.: Reversed-phase chromatography on a polar endcapped octadecylsiloxane-bonded stationary phase with water as the mobile phase. Chromatographia 57, 703–707 (2003). https://doi.org/10.1007/BF02491754
Ali, Z., Poole, C.F.: Insights into the retention mechanism of neutral organic compounds on polar chemically bonded stationary phases in reversed-phase liquid chromatography. J. Chromatogr. A 1052, 199–204 (2004). https://doi.org/10.1016/j.chroma.2004.08.109
Sun, Y., Cabovska, B., Evans, C.E., Ridgway, T.H., Stalcup, A.M.: Retention characteristics of a new butylimidazolium-based stationary phase. Anal. Bioanal. Chem. 382, 728–734 (2005). https://doi.org/10.1007/s00216-004-3051-x
Vitha, M., Carr, P.W.: The chemical interpretation and practice of linear solvation energy relationships in chromatography. J. Chromatogr. A 1126, 143–194 (2006). https://doi.org/10.1016/j.chroma.2006.06.074
Poole, C.F., Kiridena, W., DeKay, C., Koziol, W.W., Rosencrans, R.D.: Insights into the retention mechanism on an octadecylsiloxane-bonded silica stationary phase (HyPURITY C18) in reversed-phase liquid chromatography. J. Chromatogr. A 1115, 133–141 (2006). https://doi.org/10.1016/j.chroma.2006.02.089
Gil-Agustí, M., Esteve-Romero, J., Abraham, M.H.: Solute–solvent interactions in micellar liquid chromatography. J. Chromatogr. A 1117, 47–55 (2006). https://doi.org/10.1016/j.chroma.2006.03.046
Sun, Y., Stalcup, A.M.: Mobile phase effects on retention on a new butylimidazolium-based high-performance liquid chromatographic stationary phase. J. Chromatogr. A 1126, 276–282 (2006). https://doi.org/10.1016/j.chroma.2006.06.092
Shearer, J.W., Ding, L., Olesik, S.V.: Solvation parameter models for retention on perfluorinated and fluorinated low temperature glassy carbon stationary phases in reversed-phase liquid chromatography. J. Chromatogr. A 1141, 73–80 (2007). https://doi.org/10.1016/j.chroma.2006.12.003
Liu, J., Sun, J., Wang, Y., Liu, X., Sun, Y., Xu, H., He, Z.: Characterization of microemulsion liquid chromatography systems by solvation parameter model and comparison with other physicochemical and biological processes. J. Chromatogr. A 1164, 129–138 (2007). https://doi.org/10.1016/j.chroma.2007.06.066
Kiridena, W., Atapattu, S.N., Poole, C.F., Koziol, W.W.: Comparison of the separation characteristics of the organic-inorganic hybrid stationary phases XBridge C8 and phenyl and XTerra phenyl in RP-LC. Chromatographia 68, 491–500 (2008). https://doi.org/10.1365/s10337-008-0778-0
Torres-Lapasió, J.R., Ruiz-Ángel, M.J., García-Álvarez-Coque, M.C., Abraham, M.H.: Micellar versus hydro-organic reversed-phase liquid chromatography: a solvation parameter-based perspective. J. Chromatogr. A 1182, 176–196 (2008). https://doi.org/10.1016/j.chroma.2008.01.010
Tian, M., Row, K.H.: Study of retention in micellar liquid chromatography on a C18 column on the basis of linear solvation energy relationships. Bull. Korean Chem. Soc. 29, 979–984 (2008). https://doi.org/10.5012/bkcs.2008.29.5.979
Van Meter, D.S., Sun, Y., Parker, K.M., Stalcup, A.M.: Retention characteristics of a new butylimidazolium-based stationary phase. Part II: anion exchange and partitioning. Anal. Bioanal. Chem. 390, 897–905 (2008). https://doi.org/10.1007/s00216-007-1739-4
Ruiz-Ángel, M.J., Carda-Broch, S., Torres-Lapasió, J.R., García-Álvarez-Coque, M.C.: Retention mechanisms in micellar liquid chromatography. J. Chromatogr. A 1216, 1798–1814 (2009). https://doi.org/10.1016/j.chroma.2008.09.053
Van Meter, D.S., Oliver, N.J., Carle, A.B., Dehm, S., Ridgway, T.H., Stalcup, A.M.: Characterization of surface-confined ionic liquid stationary phases: impact of cation and anion identity on retention. Anal. Bioanal. Chem. 393, 283–294 (2009). https://doi.org/10.1007/s00216-008-2482-1
Fields, P.R., Sun, Y., Stalcup, A.M.: Application of a modified linear solvation energy relationship (LSER) model to retention on a butylimidazolium-based column for high performance liquid chromatography. J. Chromatogr. A 1218, 467–475 (2011). https://doi.org/10.1016/j.chroma.2010.11.058
VanMiddlesworth, B.J., Stalcup, A.M.: Characterization of surface confined ionic liquid stationary phases: impact of cation revisited. J. Chromatogr. A 1364, 171–182 (2014). https://doi.org/10.1016/j.chroma.2014.08.079
Poole, C.F., Lenca, N.: Applications of the solvation parameter model in reversed-phase liquid chromatography. J. Chromatogr. A 1486, 2–19 (2017). https://doi.org/10.1016/j.chroma.2016.05.099
Subirats, X., Muñoz-Pascual, L., Abraham, M.H., Rosés, M.: Revisiting blood-brain barrier: a chromatographic approach. J. Pharm. Biomed. Anal. 145, 98–109 (2017). https://doi.org/10.1016/j.jpba.2017.06.027
Atapattu, S.N., Poole, C.F., Praseuth, M.B.: Insights into the retention mechanism for small neutral compounds on silica-based phenyl phases in reversed-phase liquid chromatography. Chromatographia 81, 225–238 (2018). https://doi.org/10.1007/s10337-017-3451-7
Atapattu, S.N., Poole, C.F., Praseuth, M.B.: Insights into the retention mechanism of small neutral compounds on octylsiloxane-bonded and diisobutyloctadecylsiloxane-bonded silica stationary phases in reversed-phase liquid chromatography. Chromatographia 81, 373–385 (2018). https://doi.org/10.1007/s10337-017-3454-4
Poole, C.F.: Chromatographic test methods for characterizing alkylsiloxane-bonded silica columns for reversed-phase liquid chromatography. J. Chromatogr. B 1092, 207–219 (2018). https://doi.org/10.1016/j.jchromb.2018.06.011
Poole, C.F.: Influence of solvent effects on retention of small molecules in reversed-phase liquid chromatography. Chromatographia 82, 49–64 (2019). https://doi.org/10.1007/s10337-018-3531-3
Riering, H., Bilmann, N.: Characterisation of RP sorbents by linear solvation energy relationships (LSER). Labmate. 2019, 8–12 (2019)
Žuvela, P., Skoczylas, M., Jay Liu, J., Ba̧czek, T., Kaliszan, R., Wong, M.W., Buszewski, B.: Column characterization and selection systems in reversed-phase high-performance liquid chromatography. Chem. Rev. 119, 3674–3729 (2019). https://doi.org/10.1021/acs.chemrev.8b00246
Amézqueta, S., Fernández-Pumarega, A., Farré, S., Luna, D., Fuguet, E., Rosés, M.: Lecithin liposomes and microemulsions as new chromatographic phases. J. Chromatogr. A 1611, 460596 (2020). https://doi.org/10.1016/j.chroma.2019.460596
Soriano-Meseguer, S., Fuguet, E., Abraham, M.H., Port, A., Rosés, M.: Linear free energy relationship models for the retention of partially ionized acid-base compounds in reversed-phase liquid chromatography. J. Chromatogr. A 1635, 461720 (2021). https://doi.org/10.1016/j.chroma.2020.461720
Cheong, W.J., Choi, J.D.: Linear solvation energy relationships in normal phase liquid chromatography based on retention data on silica in 2-propanol/hexane eluents. Anal. Chim. Acta 342, 51–57 (1997). https://doi.org/10.1016/S0003-2670(96)00511-9
Li, J., Whitman, D.A.: Characterization and selectivity optimization on diol, amino, and cyano normal phase columns based on linear solvation energy relationships. Anal. Chim. Acta 368, 141–154 (1998). https://doi.org/10.1016/S0003-2670(98)00193-7
Park, J.H., Yoon, M.H., Ryu, Y.K., Kim, B.E., Ryu, J.W., Jang, M.D.: Characterization of some normal-phase liquid chromatographic stationary phases based on linear solvation energy relationships. J. Chromatogr. A 796, 249–258 (1998). https://doi.org/10.1016/S0021-9673(97)01022-4
Oumada, F.Z., Rosés, M., Bosch, E., Abraham, M.H.: Solute–solvent interactions in normal-phase liquid chromatography: a linear free-energy relationships study. Anal. Chim. Acta 382, 301–308 (1999). https://doi.org/10.1016/S0003-2670(98)00787-9
Li, J., Robison, T.: Application of linear solvation energy relationships to guide selection of polar modifiers in normal-phase liquid chromatographic separations. Anal. Chim. Acta 395, 85–99 (1999). https://doi.org/10.1016/S0003-2670(99)00268-8
Van Meter, D.S., Stuart, O.D., Carle, A.B., Stalcup, A.M.: Characterization of a novel pyridinium bromide surface confined ionic liquid stationary phase for high-performance liquid chromatography under normal phase conditions via linear solvation energy relationships. J. Chromatogr. A 1191, 67–71 (2008). https://doi.org/10.1016/j.chroma.2008.02.048
Chirita, R.-I., West, C., Zubrzycki, S., Finaru, A.-L., Elfakir, C.: Investigations on the chromatographic behaviour of zwitterionic stationary phases used in hydrophilic interaction chromatography. J. Chromatogr. A 1218, 5939–5963 (2011). https://doi.org/10.1016/j.chroma.2011.04.002
Subirats, X., Abraham, M.H., Rosés, M.: Characterization of hydrophilic interaction liquid chromatography retention by a linear free energy relationship. Comparison to reversed- and normal-phase retentions. Anal. Chim. Acta 1092, 132–143 (2019). https://doi.org/10.1016/j.aca.2019.09.010
Schuster, G., Lindner, W.: Comparative characterization of hydrophilic interaction liquid chromatography columns by linear solvation energy relationships. J. Chromatogr. A 1273, 73–94 (2013). https://doi.org/10.1016/j.chroma.2012.11.075
Schuster, G., Lindner, W.: Additional investigations into the retention mechanism of hydrophilic interaction liquid chromatography by linear solvation energy relationships. J. Chromatogr. A 1301, 98–110 (2013). https://doi.org/10.1016/j.chroma.2013.05.065
Ulrich S., Brown, T.N., Watanabe, N., Bronner, G., Abraham, M.H., Goss, K.-U.: UFZ-LSER database v 3.2 [Internet], http://www.ufz.de/lserd, (2017)
ACD/Percepta, build 2726: Advanced Chemistry Development, Inc., Toronto, ON, Canada (5 May 2014). www.acdlabs.com
Poole, C.F.: Wayne State University experimental descriptor database for use with the solvation parameter model. J. Chromatogr. A 1617, 460841 (2020). https://doi.org/10.1016/j.chroma.2019.460841
Poole, C.F.: Solvation parameter model: tutorial on its application to separation systems for neutral compounds. J. Chromatogr. A 1645, 462108 (2021). https://doi.org/10.1016/j.chroma.2021.462108
Lázaro, E., Ràfols, C., Abraham, M.H., Rosés, M.: Chromatographic estimation of drug disposition properties by means of immobilized artificial membranes (IAM) and C18 columns. J. Med. Chem. 49, 4861–4870 (2006). https://doi.org/10.1021/jm0602108
Acknowledgements
This paper is dedicated to the memory of Prof. Michael H. Abraham, whose outstanding collaboration to our research during the last 30 years is gratefully acknowledged. The project was supported by the Ministry of Science, Innovation and Universities of Spain (Project CTQ2017-88179-P AEI/FEDER, UE).
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflict of interest to declare.
Additional information
In memory of Professor Michael H. Abraham (1931–2021).
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cortés, S., Subirats, X. & Rosés, M. Solute–Solvent Interactions in Hydrophilic Interaction Liquid Chromatography: Characterization of the Retention in a Silica Column by the Abraham Linear Free Energy Relationship Model. J Solution Chem 51, 1081–1100 (2022). https://doi.org/10.1007/s10953-022-01161-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-022-01161-3