Abstract
A novel nanocomposite was prepared by hybridizing polyacrylic acid/maleic acid with nano copper oxide (PAACMA/CuO) for the sorption of 60Co (II) and 152+154Eu (III) radionuclides from an aqueous solution. Nano-CuO was biochemically produced by hydrolysing its salt in the presence of the Aspergillus terreus fungus. The PAACMA/CuO nanocomposite was characterized using a variety of analytical techniques. The optimum sorption conditions (pH 4.5 for 60Co and pH 3.53 for 152+154Eu, 24 h of equilibrium time at 20 oC) were applied. The kinetic mechanism of the sorption reaction was controlled by pseudo second order based on residual charts, coefficient of determination (R2), and corrected Akaike information Criterion (AICc). The sorption reaction mechanism was controlled by Langmuir model for linear regression using the coefficient of determination and the Dubinin-Radushkevich D-R model for the AICc and residual plots error functions. The reaction mechanism throughout non-linear regression was controlled by the D-R model due to the coefficient of determination, AICc, and residual charts. The PAACMA/CuO nanocomposite had a mono-layer adsorption capacity of 11.04 mg g− 1 for Co (II) and 21.54 mg g− 1 for Eu (III). According to desorption studies, Co (II) and Eu (III) could be recovered by 0.1 mol L− 1 EDTA with efficiencies 55.46% and 95.044%, respectively. According to thermodynamic studies, the sorption of Co (II) and Eu (III) on the prepared composite was endothermic and spontaneous.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global expansion of nuclear energy applications in fields such as agriculture, industry, nuclear medicine, nuclear weapons, nuclear research, and others has disrupted the environmental balance as radioactive residues for these applications have increased. Scientists [1,2,3,4] are working hard to find a solution to this growing problem. Radioactive isotopes like 60Co and 152+154Eu are hazardous to humans due to their prolonged half-lives of 5.3 and 8.8 years, respectively. They are polluters, and they cause significant harm to the environment and to human health [5]. 60Co can cause skin burns, acute radiation sickness, and even death at extremely high doses.152+154Eu has been associated to cancers of the liver and/or bones [6]. A number of techniques for removing radionuclides from waste water have been developed such as coagulation, precipitation, sorption, ion exchange, membrane technique [7], and solvent extraction [8]. The most effective method determined by the waste treatment cost and methodology. Metal oxides, including iron oxide [9,10,11], activated aluminium oxide [12, 13], titanium oxide [14, 15], manganese oxide [16, 17], and zirconium-magnesium binary oxide [18, 19], have attracted a lot of attention as sorbents.
Yılmaz D et al. [20] found that nanomaterials offered a wide range of possibilities for producing the newest radioactive wastewater decontamination technology [21]. However, the toxicity features and expansive analysis requirements in addition to the need of skilled human resources hinder the actual/specific application of these nanomaterials [22]. The use of natural resource which named as green chemistry can solve most of these limitations [22].
Aspergillus terreus is a fungus that can produce extracellular or intracellular nanoparticles [23] through metabolic activity that bio-reduces or aggregates complex metal ions that do not dissolve into colloidal particles.
Dispersing nanoparticles in polymer matrices can enhance a variety of chemical properties, including thermal stability, electric conductivity, hydrophilicity, and the transition of chemical properties from “bulk” matrix-controlled criteria to interphase or “surface” dominated characteristics [24]. Maleic acid combined with polyacrylic acid (PAACMA) has superior adsorption capabilities for copper ions from wastewater. Its thermal stability could be improved from 40.3% to 99.84% after copper ions adsorption [25].
To the author’s knowledge, the adsorption studies of 60Co (II) and 152+154Eu (III) radionuclide onto highly and low-cost adsorbent of dispersed nano copper oxide in polyacrylic/maleic acid have not yet been published. Accordingly, authors decided to study the effective conditions and mechanism of the sorption process.
Over the last few decades, the mechanism of the sorption reaction has been designed using linear regression [26]. Current findings, on the other hand, have revealed an increasing disparity (between predictions and experimental data) and model disability, resulting in model failure [27]. Recently, expanding nonlinear has been identified as a potentially powerful and efficient tool that could lead to further advances in adsorption science [28]. The use of error functions to generate a minimal distribution between experimental equilibrium data and predicted isotherms is common. Depending on how the applied error function is defined, the error distribution between the experimental equilibrium data and the predicted isotherms will be reduced either by minimising the error function or by obtaining maximum error functions. The most popular error function used to acquire the ideal distribution between experimental equilibrium data and isotherms is the coefficient of determination, or R2 [29]. Recent studies have looked into additional error functions, such as average relative error (ARE), sum of absolute errors (EABS), and corrected Akaike information (AICc), to estimate the optimum isotherm [26, 30]. To find the best isotherm fitting, it is advised to use more than one error function.
The purpose of this research was to prepare a novel nano copper oxide composite for the sorption of radionuclides 60Co (II) and 152+154Eu (III). Nano copper oxide biosynthesised with the assistance of the metabolic fungus Aspergillus terreus. The monomers of acrylic acid, maleic acid and biosynthesized nano copper oxide were then copolymerized with radiation from a 60Co cell at a dose of 25 kGy. Calcined biosynthesized CuO nanoparticles (Nps) and PAACMA/CuO were characterized. Batch sorption experiments were performed on the radionuclides 152+154Eu (III) and 60Co (II). To optimise the sorption process, effective sorption factors such as pH, time, temperature, ionic strength, and concentration were investigated. Kinetic and equilibrium isotherms are used for determining the reaction mechanism. Fitting errors were minimised by using linear and nonlinear fitting techniques. Additionally, residual error plots, coefficient of determination (R2), and corrected Akaike information Criterion (AIKc) were used to examine the qualitative and quantitative aspects of the fitting results. To show the potential of using PAACMA/CuO nanocomposite as a radioactive waste sorbent, thermodynamic studies and the desorption process of 60Co (II) and 152+154Eu (III) were conducted.
Experimental
Materials and Procedure
All precursors used during the study were of analytical grade and used without further purification. Acrylic acid (AA) with purity ≥ 99% got from Elf chem.co, France. Maleic acid (MA) of purity 99% obtained from Loba Chemie India. Copper sulphate (CuSO4.7H2O) 98% purchased from Merck, Egypt. Methylene bis-acrylamide (DAM) was supplied from (Merck, Germany). Extra pure cobalt (II) chloride (CoCl2.6H2O) was purchased from BDH (UK). Eu2O3 salts got from Merck Co., Germany. The 60Co and 152+154Eu isotopes were produced by neutron irradiation of the corresponding salt in the Second Egyptian Research Reactor (ET-RR-2) at Inshas, Egyptian Atomic Energy Authority with initial activity 100 KBq/L. A 300 mg L− 1 stock solutions of Co (II) and Eu (III) prepared by dissolving a certain amount of Co (II) and Eu (III) salts in double distilled water then spiked with the 60Co and 152+154Eu radionuclides, respectively. These solutions kept as a stock radioactive solution for all experiments.
Preparation of CuO Nps and PAACMA/CuO Nanocomposite
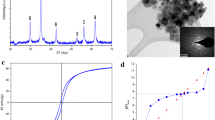
Aspergillus terreus was cultivated in Czapek-Dox broth medium [23], and CuO Nps was synthesised by hydrolysis of CuSO4 via the supernatant of the fungus [23]. The CuO Nps collected and washed with distilled water before drying for 24 h at 60 oC and calcined for 2 h at 600 oC (Fig. 1).
The calcined CuO Nps copolymerized with acrylic acid (AA) and maleic acid (MA) in the presence of the cross linker methylene bis-acrylamide (DAM). After mixing 80 mL of deoxygenated water with 18 mL of AA, 2 g MA, 0.5 g DAM, and 0.5–2 g of calcined CuO NPs, the mixture magnetically stirred for 2 h at room temperature. The mixture exposed to gamma radiation at a dose rate of 689.538 Gy/h using a cobalt-60 gamma cell 220. After polymerization, the materials were cut into small pieces and soaked in acetone for 2 h to remove water, unreacted polymer, and impurities before being dried for 24 h in a vacuum oven at 333 K and sieved to size (250 mm). The successful preparation of PAACMA/CuO nanocomposites with various molar compositions is shown in Table 1. A predicted polymerization mechanism is depicted in Fig. 2.
Instruments
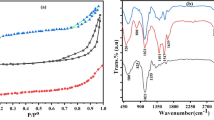
The morphology of the particles analysed using a scanning electron microscope (SEM, Philips XL 30 ESEM), which combined with energy-dispersive X-ray spectroscopy and electron backscatter diffraction (25–30 keV accelerating voltage, 1–2 mm beam diameter and 60–120 s counting time). CuO nanoparticles investigated using a transmission electron microscope (TEM), model JEM2100, manufactured by Jeol.s.b (Japan). Fourier transform infrared (FT-IR) spectra revealed the presence of functional groups (KBr pellet technique on a Perkin Elmer 1600 FTIR Spectrophotometer in wave number range 400–4000 cm− 1). The all characterization was performed at 20 oC.
Sorption Studies
The sorption of 60Co (II) and 152+154Eu (III) onto CuO Nps and PAACMA/CuO nanocomposite was studied. Reaction parameters such as pH (1–7), contact time (5 min − 48 h), metal ion concentration (50–300 mg L− 1) and ionic strength were investigated in order to achieve optimal sorption conditions. After contacting 0.1 g of the sorbent with 10 mL of the 60Co (II) and 152+154Eu (III) solutions, samples were filtered and separated from the solution. The sorption efficiency of 60Co (II) and 152+154Eu (III) radionuclides was calculated using Eq. (1):
The sorbed amount q (mg g− 1) was calculated using Eq. (2).
Where Ao and Ae represent the initial and equilibrium concentrations of the radionuclides 60Co (II) and 152+154Eu (III), m represents the mass of the nanocomposite (g), and V represents the volume of solution (L). Equation (3) was used to calculate the distribution coefficient Kd (mL g− 1).
Kinetic Modelling
The pseudo first order, pseudo second order, and Elovich models were used to propose a mechanism for the sorption reaction. Table 2 displays the linear and nonlinear equation forms of the selected kinetic modelling.
Where qe and qt are the sorbed amounts of radionuclides (mg g− 1) at equilibrium time and at any time t, respectively; k1 (min− 1) is the pesudo first rate constant, k2 (g mg− 1 min− 1) is the pseudo second order rate constant, α, β are the Elovich constants. α (mg g− 1 min− 1) represents the rate of chemisorptions at zero coverage, whereas β (g mg− 1) represents the extent of surface coverage and chemisorption activation energy.
Adsorption Isotherm Modelling
Langmuir, Freundlich, Dubinin-Radushkevich, and Temkin isotherms were used in this work. Table 3 depicts the linear and non-linear adsorption isotherm modelling forms.
Where qmax is the maximum monolayer sorption capacity (mg g− 1), b is the sorption free energy constant (b, α, e−ΔG/RT), and Ce is the equilibrium metal ion concentration. The separation factor calculated from the Langmuir constant, RL, indicates whether the isotherm is linear (RL = 1), irreversible (RL = 0), unfavourable (RL > 1), or favourable (0 < RL < 1), where Kf denotes Freundlich constants, and n denotes sorption capacity and intensity. qmDR is monolayer capacity for the D-R model, βDR is a constant related to apparent adsorption energy, ε is the polanyi potential, kT is the maximal binding energy (L mol− 1), bT is the sorption heat, R is the universal gas constant (8.314 JK− 1 mol− 1), and T is the absolute temperature (K).
Thermodynamic Parameters
The thermodynamic parameters such as standard Gibbs free energy (∆Go), enthalpy (∆Ho), and entropy (∆So) were used to determine the spontaneity, nature, and adsorbent suitability of the adsorption process [41]. Equations (20, 21, 22) were used to calculate thermodynamic parameters using temperature-dependent adsorption isotherms.
where Kd is the distribution coefficient, mL g− 1; R represents the general gas constant (8.314 JK− 1 mol− 1), T is the absolute temperature (K). ∆Ho and ∆So are obtained from the slope and intercept of the ln Kd against 1/T graph, respectively.
Desorption Studies
The 60Co (II) and 152+154Eu (III) solutions were replaced with 10 mL of 0.1 M desorbing agents, including FeCl3, AlCl3, EDTA, HCl, and NaOH, following the sorption experiments. The concentrations of 60Co (II) and 152+154Eu (III) were then determined as previously mentioned, and the desorption efficiency was calculated using Eq. (23). This was followed by 24 h of shaking.
Error Functions
The suitability of a model equation for experimental results was typically evaluated using an error function. The difference between theoretically predicted and actual experimental data values was computed by error functions. It is possible to examine error functions using quantitative or qualitative criteria [42]. It is frequently preferable to use both qualitative and quantitative criteria. The coefficient of determination (R2) [29] and corrected Information about Akaike (AICc) [30] error functions were used for quantitative criteria, while residual error plots were used for qualitative evaluation of the kinetic and adsorption isotherm modelling.
Where qexp (mg g-1) is the number of sorbed radionuclides obtained from Eq. (2), qcalc (mg g-1) is the number of sorbed radionuclides obtained from the model, n is the number of experimental data points, and p is the number of modelling parameters. The model with the highest R2 and the lowest value of AIKC was the best fit.
Results and Discussions
Characterization of the Composite
Zero Point Charge
The pH of the zero point charge (pHZPC) used as a base point for determining the type of sorbent because the surface charge is zero at a certain pH level. The sorbent surface charge is positive before the pHZPC, indicating that it works as an anionic exchanger, and negative after pHZPC, indicating that it works as a cationic exchanger. The efficiency of the sorbents was determined by the value pHZPC over a wide pH range. The zero point charge was determined by mixing 0.1 g of P4AACMA/CuO nanocomposite at 20 oC with 10 mL of 0.1 M NaCl and shaking the solution for 24 h at 20 oC in pH intervals ranging from 1 to 11. Finally, after shaking, the pH was measured, and the zero point charge was calculated by graphing the initial pH against the pH difference (pHf - pHi). The plot intersected the x-axis (pHi) at the zero point charge [43]. According to Fig. 3, the pHZPC was 2.9, implying that the P4AACMA/CuO nanocomposite can be used effectively for 60Co (II) and 152+154Eu (III) at pH levels higher than this value (pH >2.9).
FT-IR Analysis
FT-IR spectrum of biosynthesized nano CuO are represented in Fig. 4. A small broad peak was observed at 3480 cm− 1 assigned to the O–H group stretching vibration for hydrogen bonding of physically adsorbed water or moisture content [44]. Furthermore, the band was observed at 1630 cm− 1 assigned to the bending vibration of O–H group combined with copper atoms [45]. In the lower wavenumber region (< 600 cm− 1), two peaks are shown in spectrum. These are the characteristics peaks of Cu–O bond vibrations, confirming the synthesis of CuO nanoparticles [46]. The spectrum of the P4AACMA/CuO nanocomposite at 20 oC had a broad band at 3421 cm− 1, which corresponds to OH stretching for the COOH group. The band at 2927 cm− 1 represented C–H asymmetric vibration, while the band at 1736 cm− 1 represented the C=O group. The 1457 cm− 1 band represented the deformation of C–H bending vibration [47]. The C–O group is represented by the band at 1249 cm− 1. The presence of CuO in the polymeric matrix was confirmed by the bands at 1106, 876, 507, and 469 cm− 1[46].
Morphological Analysis
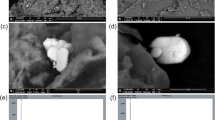
Figure 5 shows the TEM micrographs of CuO NPs to investigate the nano character of CuO particles. The crystalline nanoparticles were well dispersed and had a size of less than 50 nm with spherical and porous structure. The SEM micrograph of CuO NPs clearly showed rough agglomeration of nanostructural homogeneities with spherical morphologies of CuO nanoparticles. The SEM micrograph of P4AACMA/CuO nanocomposite show variation in morphological features and appeared rough and entangled structure and agglomeration with intergranular micropores and spherical morphologies of CuO nanoparticles are distributed randomly between the polymeric matrix. Incorporation of CuO Nps into the polymeric matrix caused cavities and roughness of the P4AACMA/CuO nanocomposite resulting in a heterogeneous framework of the synthesised nanocomposite [23].
Sorption Studies
Table 4 showed the sorption efficiencies of nano-CuO and PAACMA/CuO nanocomposites with varying CuO concentrations toward 60Co (II) and 152+154Eu (III). CuO Nps, on the other hand, had low sorption efficiencies towards both radionuclides studied. The sorption efficiencies of PAACMA/CuO composites enhanced as the mol percentage of nano CuO increased. This was caused by ionic polymer adsorption on the surface of the nano CuO oxide particle, which led to a high surface charge density and an increase in the electrostatic attraction between the nanopolymer and the adsorbed 60Co (II) ions or 152+154Eu (III) [48]. When compared to the other PAACMA/CuO nanocomposites compositions, P4AACMA/CuO with composition (80% AA, 8.89% MA, 8.88% CuO and 2.23% DAM) exhibited the highest sorption efficiency toward 60Co (II) ions and 152+154Eu (III) ions, made it the optimum composition of choice for the sorption study.
Optimization of the Sorption Parameters
Effect of pH
Figure 6a showed the results of an investigation into how pH affects the sorption efficiencies of the P4AACMA/CuO nanocomposite towards the 60Co (II) and 152+154Eu (III). The nanocomposite at pH 3 only adsorbed 33.47% of 60Co (II) and 67.04% of 152+154Eu (III) despite an initial metal ion concentration of 100 mg L− 1. 60Co (II) and 152+154Eu (III) sorption increased to 38.52 and 89.86%, respectively, when the pH was raised to 4. At pH 6, 60Co (II) was eliminated in 96% of instances while 152+154Eu (III) was eliminated in 91.82% of instances. The main function group of the P4AACMA/CuO nanocomposite was COOH that may have higher pH deprotonation. Additionally, because the surface charge of P4AACMA/CuO nanocomposite changed to be negative in accordance with pHZPC (pH 2.9), positively charged metal ions and its surface functional groups tend to attract each other electrostatically.
The precipitation curves of Co (II) and Eu (III) in the absence of the prepared nanocomposite that served as a blank, shown in Fig. 6b, showed that a significant precipitated quantity of Co (II) began to appear after pH 4.5 and of Eu (III) after pH 4. Figure 6c and d depicted the speciation diagrams of Co (II) and Eu (III) at various pH values as determined by the Hydra/Medusa chemical equilibrium software [49]. Co (II) is the most dominant species in the charts up to pH 4.5, then Co (OH)2 species appears after pH 4.5 and becomes dominant at pH 6. The trivalent species Eu (III) predominates up to pH 6, with the positive species EuOH2+ and Eu(OH)2+ appearing at pH ranges 3–5. At pH values above 6, Eu(OH)3 predominated, with precipitation accounting for the vast majority of Eu (III) removal. To avoid precipitation, the sorption experiments were carried out at pH 4.5 for Co (II) and pH 3.53 for Eu (III). The sorbed amounts of Co (II) at pH 4.5 were 5.150 mg L− 1. In addition, the amount of Eu (III) sorbed increased from 0.415 mg L− 1 at pH 1.5 to 8.705 mg L− 1 at pH 3.53. Finally, the results revealed that Eu (III) had higher sorption efficiency onto P4AACMA/CuO than Co (II). This could be due to the higher electrostatic interaction of Eu (III) as trivalent ions compared to Co (II) as divalent ions [50].
Time
Figure 7 depicted the effect of contact time on the sorption of Co (II) and Eu (III) onto P4AACMA/CuO nanocomposite from 5 min to 48 h. The equilibrium state was appointed after contacting time 24 h, as the results indicated no further change in the sorbed amounts for both elements. The sorbed amounts of the two studied elements increased substantially because the number of available active sites on the surface of P4AACMA/CuO nanocomposite was initially large enough to overcome the resistance to transfer of Co (II) and Eu (III) from aqueous phase to P4AACMA/CuO nanocomposite surface [50]. The increase in sorbed amount slowed gradually from 30 min to the saturation state at 24 h as an equilibrium time. This is due to a decrease in the number of active sites of the caused by metal ion occupancy, so there were no more available active sites on the P4AACMA/CuO nanocomposite surface after the equilibrium time. At the equilibrium state, the sorbed amounts of Co (II) and Eu (III) onto P4AACMA/CuO nanocomposite were 5.1505 and 8.705 mg g− 1, respectively.
Effect Co (II) and Eu (III) Concentrations
Figure 8 depicted the amount of metal ion sorbed as a function of initial concentration, which ranges from 50 to 300 mg L− 1 and labelled with the appropriate radioactivity of 60Co (II) and 152+154Eu (III). There is a relationship between metal ion concentration and sorbed amount on composite, which means that increasing the concentration of Co (II) and Eu (III) in the solution increased the sorbed amount of metal ions. The driving force of Co (II) and Eu (III) at the surface interface has increased as the number of effective interactions or collisions between initial metal ions and active sites on the surface of the P4AACMA/CuO nanocomposite has increased. Because Eu (III) has a higher charge density than Co (II), Eu (III) had higher sorption efficiency than Co (II). The sorbed amount of Co (II) increased from 4.176 to 11.939 mg g− 1 at 300 mg L− 1, while it increased from 4.55 to 18.16 mg g− 1 over the same initial metal ion concentration range for Eu (III).
Ionic Strength
The impact of ionic strength on the sorption reaction was studied by incorporating 0.1 g of the nanocomposite with 10 mL of the sorbents at pH 4.5 for 60Co (II) and 3.53 for 152+154Eu (III) and ranging the concentration of NaCl from 0.02 to 0.2 mol L− 1. Ionic strength had a significant effect on the sorption reaction of 60Co (II) and 152+154Eu (III) ions onto P4AACMA/CuO nanocomposite, as shown in Fig. 9. The sorbed amounts of 60Co (II) and 152+154Eu (III) ions decreased from 5.1505 to 8.704 mg g− 1 to 4.06 and 5.99 mg g− 1, respectively, in the presence of 0.02 mol L− 1 NaCl. The sorbed amounts of the radionuclides 60Co (II) and 152+154Eu (III) decreased to 0.442 mg g− 1 and 3.485 mg g− 1, respectively, at the concentration of 0.2 mol L− 1 NaCl. The competition between Na (I) and 60Co (II) or 152+154 Eu (III) ions in the solution, as well as the electrostatic stability of 60Co (II) and 152+154Eu (III) in the solution by Cl− anions, could be contributed to this trend. These aspects reduced the rate at which these radionuclides diffuse onto the surface of the nanocomposite. This implies that the predicted physical adsorption or ion exchange chemisorption occurred, as well as an outer-sphere interaction between the radionuclides 60Co (II) or 152+154Eu (III) and the P4AACMA/CuO nanocomposite surface [51, 52] existed.
Temperature
Figure 10 depicted the study of the effect of temperature on the sorption reaction over a temperature range of 20 to 50 oC. The sorption of 60Co (II) and 152+154Eu (III) radionuclides were affected by temperature, suggesting a chemical mechanism for the reaction [45]. The findings demonstrated that increasing temperature accelerated the rate of the sorption reaction due to an increase in radionuclide kinetic energy, which led to an increase in radionuclide diffusion at the surface of the nanocomposite, thereby enhanced the sorption process. Increased sorption on the surface of the nanocomposite and promoted deprotonation of the active group were additional effects of increased temperature [53].
Kinetic Modelling
The rate and pathways of radionuclide adsorption on the nanocomposite were determined using kinetic modelling. Three kinetic models were used to determine the correct mechanism for the sorption reaction: pseudo first order, pseudo second order, and the Elvoich model. Figure 11 depicted a linear plot examination. In addition to the coefficients of determination (R2) and AICc values, the more fitted model can be identified by comparing the calculated qt to the experimental qt. The Elvoich model’s coefficients and can be used to distinguish between physisorption and chemisorption reactions by comparing their coefficients α and β.
The results in Table 5 showed that the pseudo second order model had good fitting results, such as higher coefficients of determination, lower AICc values, and consistency with the equilibrium sorbed amount (qtexp). The residual error plots were examined, and the pseudo second order employed the lowest residual error possible, as shown in Fig. 11e, f. This refers to the chemisorption nature of the reaction, which occurs via electrostatic attraction of the 60Co (II) and 152+154Eu (III) radionuclides and negatively charged surface of the P4AACMA/CuO nanocomposite, which was consistent with the pHZPC results. Furthermore, the Elvoich model expressed the initial adsorption rate and the desorption constant. The highest and lowest parameter values for 60Co (II) and 152+154Eu (III) confirmed the sorption reaction’s chemisorption nature.
Isotherm Modelling
The isotherm model was used to determine the interaction of 60Co (II) and 152+154Eu (III) with the surface of P4AACMA/CuO nanocomposite. Figure 12 depicted the residual error plots of the investigated models with linear and non-linear regression plots of Langmuir, Freundlich, Temkin, and Dubinin-Radushkevich (D-R) isotherm modelling. The linear and non-linear regression results were listed in Table 6. For both linear and nonlinear regression, the residual error plots had the lowest D-R values. The highest R2, in linear regression revealed that the sorption mechanism was regulated by the Langmuir isotherm and D-R model regulated the sorption reaction in non-linear regression. Thus, linear regression indicated that monolayer coverage of Co (II) and Eu (III) causes sorption onto P4AACMA/CuO nanocomposites. Chemisorption was responsible for the sorption of Co (II) and Eu (III) ions onto P4AACMA/CuO nanocomposites, as E values were greater than 8 kJ mol− 1 in non-linear regression [54]. The D-R mechanism outperformed the Langmuir isotherm because it did not consider a homogeneous surface or constant adsorption potential. The sorption mechanism was controlled by the D-R mechanism in terms of AICc for both linear and nonlinear regression because D-R fitting had the lowest AICc values. Non-linear regression was found to be more accurate in determining the best isotherm [55].
Thermodynamic Studies
The plot of lnKd versus 1/T for the sorption of 60Co (II) and 152+154Eu (III) onto P4AACMA/CuO nanocomposite shown in Fig. 13. Table 7 showed the results of ∆Go, ∆Ho and ∆So. The free energy change ∆Go obtained during the sorption reaction at 293, 303, 313, and 323 K was all negative, indicating that the sorption process was spontaneous and favourable. Furthermore, an increase in negative ∆Go values by increase in temperature indicated a greater driving force for metal ion binding. The presence of a positive value for ∆Ho indicated that the sorption process was endothermic. The increased randomness at the 60Co (II) and 152+154Eu (III) molecules onto the P4AACMA/CuO nanocomposite interface during the sorption process was indicated by a positive value of ∆So.
Desorption Studies
Figure 14 depicted the results of desorption of 60Co (II) and 152+154Eu (III) using a different desorbing reagents. The results indicated that 55.53% and 95.04% of 60Co (II) and 152+154Eu (III), respectively were quantitatively desorbed from the loaded P4AACMA/CuO nanocomposite with 0.1 mol L− 1 EDTA solution. 0.1 mol L− 1 NaOH had poor desorbing percentage due to the deprotonation of the surface [56]. Hence, 60Co (II) and 152+154Eu (III) find it was difficult to be detached from the adsorbent. However, HCl had the ability to cover loaded P4AACMA/CuO nanocomposite surface with [H+] and hence, 60Co (II) and 152+154Eu (III) detached easily [44]. However, EDTA had higher desorption efficiency than HCl due to the strong complexation of EDTA. For AlCl3 and FeCl3 used as desorbing agents by ion exchange but the difference in ionic size among [H+], Al(III) and Fe(III) caused the difference in desorption efficiencies. The order of desorbing efficiencies was EDTA > HCl > AlCl3 > FeCl3 > NaOH.
Comparative Studies
Table 8 summarized the adsorbent materials [33, 38, 44, 57,58,59,60,61,62] to that of the P4AACMA/CuO nanocomposite for the adsorption of 60Co (II) and 152+154Eu (III) radionuclides. As a result, P4AACMA/CuO nanocomposite can be considered as a prospective low-cost adsorbent for 60Co (II) and 152+154Eu (III) radionuclides from radioactive waste solution.
Conclusion
A nanocomposite of polyacrylic acid/maleic acid with nano copper oxide (PAACMA/CuO) was successfully prepared and well characterized. The prepared PAACMA/CuO nanocomposite was used for sorption of 60Co (II) and 152+154Eu (III) radionuclides from aqueous solutions. CuO was synthesised in nano-size less than 50 nm by hydrolysis of its salt in the presence of the fungus Aspergillus terreus and then cross linked with acrylic acid and maleic acid via free radical polymerization. P4AACMA/CuO nanocomposite with acrylic acid (80%), maleic acid (8.89%), CuO (8.88%), and 2.23% cross linker (DAM) was chosen for batch testing. The best sorption conditions (pH 4.5 for 60Co and pH 3.53 for 152+154Eu, 24 hours equilibrium time at 20 oC) were used. The reaction kinetic was controlled by pseudo second order based on residual plots, (R2), and (AICc). Due to R2, the sorption reaction mechanism was regulated with D-R model for non-linear regression, and with Langmuir model for linear regression. While the D-R model was used to control the sorption reaction mechanism based on residual error plots and AICc results for linear and non-linear regression. The P4AACMA/CuO nanocomposite had a monolayer adsorption capacity of 11.04 mg g-1 for Co (II) and 21.54 mg g-1 for Eu (III). According to desorption studies, EDTA at 0.1 mol L-1 concentration could recover 55.46% and 95.044% of the sorbed radionuclides 60Co (II) and 152+154Eu (III), respectively. Thermodynamically, the sorption process was endothermic in nature and took place spontaneously. Finally, the results indicate that the P4AACMA/CuO nanocomposite has great potential to be used as an economic and effective sorbent for preconcentration and recovery of 60Co (II) and 152+154Eu (III) from aqueous solutions. It was recommended to use more than one error function as a deciding factor when selecting the best isotherm. For superior advancements in the field of adsorption science, non-linear regression was preferred over linear regression.
Data Availability
All the data used for this work are publicly available.
References
Mekawy ZA, Moussa SI, Mousa AM et al (2022) Sorption of 60Co (II) from aqueous solutions onto biosynthesized zinc oxide nanocomposites. J Radioanal Nucl Chem 331:2331–2347. https://doi.org/10.1007/s10967-022-08292-3
Attallah MF, Helal AA, Hamed MM, Allan KF (2022) Elaboration of composite based on the incorporation of marble particles into polymeric framework for the removal of Co (II) and Eu (III). Radiochim Acta 110:121–131
Zhang H, Zhu M, Du X et al (2021) Removal of cesium from radioactive waste liquids using geomaterials. Appl Sci 11:8407
Soliman S, Mohamed ME (2022) Removal of radioactive waste from water using polymernanocomposites. In: Shalan AE, Makhlouf ASH, Lanceros-Méndez S (eds) Advances in nanocompositematerials for environmental and energy harvesting applications. Springer, Cham, pp 473–490
Laraia M (2015) Radioactive contamination and other environmental impacts of wastefrom nuclear and conventional power plants, medical and other industrialsources. In: van Velzen L (ed) Environmental remediation andrestoration of contaminated nuclear and norm sites. Elsevier, Amsterdam, pp 35–56
López M, Martín M (2011) Medical management of the acute radiation syndrome. Rep Pract Oncol Radiother 16:138–146
Mansur M, Mushtaq A (2011) Separation of yttrium-90 from strontium-90 via colloid formation. J Radioanal Nucl Chem 288:337–340
Attallah MF, Rizk SE, Shady SA (2018) Separation of 152+ 154Eu, 90Sr from radioactive waste effluent using liquid–liquid extraction by polyglycerol phthalate. Nucl Sci Tech 29:1–9
Jabbar KQ, Barzinjy AA, Hamad SM (2022) Iron oxide nanoparticles: preparation methods, functions, adsorption and coagulation/flocculation in wastewater treatment. Environ Nanatechnol Monit Manag 17:100661
Aragaw TA, Bogale FM, Aragaw BA (2021) Iron-based nanoparticles in wastewater treatment: a review on synthesis methods, applications, and removal mechanisms. J Saudi Chem Soc 25:101280
Osmanlioglu EA (2018) Decontamination of radioactive wastewater by two-staged chemical precipitation. Nuclear Eng Technol 50(6):886–889. https://doi.org/10.1016/j.net.2018.04.009
Hassan HS, Madcour WE, Elmaghraby EK (2019) Removal of radioactive cesium and europium from aqueous solutions using activated Al2O3 prepared by solution combustion. Mater Chem Phys 234:55–66
Younssi SA, Breida M, Achiou B (2018) Alumina membranes for desalination and water treatment. In: Eyvaz M, Yüksel E (eds) Desalination and water treatment. InTech, London
Dutta S (2020) Wastewater treatment using TiO2-basedphotocatalysts. In: Hussain CM, Mishra AK (eds) Handbook of smart photocatalytic materials. Elsevier, Amsterdam, pp 303–323
Xu Z, Li Q, Gao S, Shang JK (2010) As (III) removal by hydrous titanium dioxide prepared from one-step hydrolysis of aqueous TiCl4 solution. Water Res 44:5713–5721
Elbasuney S, Elsayed MA, Mostafa SF, Khalil WF (2019) MnO2 nanoparticles supported on porous Al2O3 substrate for wastewater treatment: synergy of adsorption, oxidation, and photocatalysis. J Inorg Organomet Polym Mater 29:827–840
Ibrahim HA, Hassan HS, Mekhamer HS, Kenawy SH (2019) Diffusion and sorption of Cs+ and Sr2+ ions onto synthetic mullite powder. J Radioanal Nucl Chem 319:1–12
Hang C, Li Q, Gao S, Shang JK (2012) As (III) and as (V) adsorption by hydrous zirconium oxide nanoparticles synthesized by a hydrothermal process followed with heat treatment. Ind Eng Chem Res 51:353–361
Hua M, Zhang S, Pan B et al (2012) Heavy metal removal from water/wastewater by nanosized metal oxides: a review. J Hazard Mater 211:317–331
Yılmaz D, Gürol A (2021) Efficient removal of iodine-131 from radioactive waste by nanomaterials. Instrum Sci Technol 49:45–54
Zhang X, Liu Y (2020) Nanomaterials for radioactive wastewater decontamination. Environ Sci Nano 7:1008–1040
Singh J, Dutta T, Kim K-H et al (2018) ‘Green’synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J Nanobiotechnol 16:1–24
Mousa AM, Aziz OAA, Al-Hagar OEA et al (2020) Biosynthetic new composite material containing CuO nanoparticles produced by aspergillus terreus for 47Sc separation of cancer theranostics application from irradiated ca target. Appl Radiat Isot 166:109389
Chipara M, Lau AKT, Aliofkhazraei M et al (2015) Polymer-based nano/composites: theory, synthesis, modifications, and properties. J Nanomater. https://doi.org/10.1155/2015/603907
Jang Y-J, Liu S, Yue H et al (2020) Hydrophilic biocompatible poly (acrylic acid-co-maleic acid) polymer as a surface-coating ligand of ultrasmall Gd2O3 nanoparticles to obtain a high r1 value and T1 MR images. Diagnostics 11:2
Yaneva ZL, Koumanova BK, Georgieva NV (2013) Linear and nonlinear regression methods for equilibrium modelling of p-nitrophenol biosorption by Rhizopus oryzae: comparison of error analysis criteria. J Chem. https://doi.org/10.1155/2013/517631
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Qureshi SS, Memon SA, Ram N et al (2022) Rapid adsorption of selenium removal using iron manganese-based micro adsorbent. Sci Rep 12:1–13
Subramanyam B, Das A (2014) Linearised and non-linearised isotherm models optimization analysis by error functions and statistical means. J Environ Heal Sci Eng 12:1–6
Ibrahim HA, Abdel Moamen OA, Monem NA, Ismail IM (2018) Assessment of kinetic and isotherm models for competitive sorption of Cs+ and Sr2+ from binary metal solution onto nanosized zeolite. Chem Eng Commun 205:1274–1287
Dakroury GA, El-Shazly EAA, Hassan HS (2021) Preparation and characterization of ZnO/Chitosan nanocomposite for cs(I) and Sr(II) sorption from aqueous solutions. J Radioanal Nucl Chem 330:159–174
Dakroury GA, El-Shazly EAA, Hassan HS (2022) Sorption of lead (II) and strontium (II) ions from aqueous solutions onto non-living Chlorella Vulgaris Alga/Date pit activated carbon composite. Carbon Lett 32:495–512
El-Shazly EAA, Dakroury GA, Someda HH (2021) Kinetic and isotherm studies for the sorption of 134Cs and 60Co radionuclides onto supported titanium oxide. J Radioanal Nucl Chem 330:127–139
Dakroury GA, Abo-Zahra Sh F (2020) The use of titanium oxide/polyethylene glycol nanocomposite in sorption of 134Cs and 60Co radionuclides from aqueous solutions. J Radioanal Nucl Chem 324:1351–1364. https://doi.org/10.1007/s10967-020-07167-9
Dakroury GA, Abo-Zahra SF, Hassan HS (2020) Utilization of olive pomace in nano MgO modification for sorption of Ni (II) and Cu (II) metal ions from aqueous solutions. Arab J Chem 13:6510–6522
Kisiela-Czajka AM, Dziejarski B (2022) Linear and non-linear regression analysis for the adsorption kinetics of SO2 in a fixed carbon bed reactor—A case study. Energies 15:633. https://doi.org/10.3390/en15020633
Dakroury GA, Ali SM, Hassan HS (2021) Assessment of adsorption performance of chitosan/ZrO2 biosorbent composite towards cs (I) and Co (II) metal ions from aqueous solution. J Polym Res 28:1–17
Dakroury GAR, Abo-Zahra SF, Hassan HS, Ali HEA (2020) Improvement of the sorption behavior of aluminum silicate composite toward 134Cs and 60Co radionuclides by non-living biomass of Chlorella vulgaris. Environ Sci Pollut Res 27:21109–21125
Nebaghe KC, El Boundati Y, Ziat K et al (2016) Comparison of linear and non-linear method for determination of optimum equilibrium isotherm for adsorption of copper (II) onto treated Martil sand. Fluid Phase Equilib 430:188–194
Dakroury GA, Maree RM, El-Shazly EAA, Allan KF (2022) Synthesize of poly (acrylamide-co-itaconic/TiO2) nanocomposite for ce (III) sorption from Monazite Leachate. J Polym Environ 30:1942–1958
Huang W, Chen J, He F et al (2015) Effective phosphate adsorption by Zr/Al-pillared montmorillonite: insight into equilibrium, kinetics and thermodynamics. Appl Clay Sci 104:252–260
Chen C (2013) Evaluation of equilibrium sorption isotherm equations. Open Chem Eng J. https://doi.org/10.2174/1874123101307010024
Hassan HS, Attia L, Dakroury GA (2020) Exploration of the parameters affecting the radioactive europium removal from aqueous solutions by activated carbon-epoxy composite. Appl Radiat Isot 164:109278
Sheha RR, Mekawy ZA, Someda HH et al (2020) Assessing the sorptive ability of synthesized graphene oxide-metal oxide composite to remove certain lanthanides. Clean - Soil Air Water 48:1–13. https://doi.org/10.1002/clen.202000348
Sharaf El-Deen SEA, Moussa SI, Mekawy ZA et al (2017) Evaluation of CNTs/MnO2 composite for adsorption of 60Co (II), 65Zn(II) and cd(II) ions from aqueous solutions. Radiochim Acta 105:43–55. https://doi.org/10.1515/ract-2016-2624
Vishveshvar K, Krishnan A, Haribabu K, Vishnuprasad S (2018) Green synthesis of copper oxide nanoparticles using Ixiro coccinea plant leaves and its characterization. Bionanoscience 8:554–558
Grabowska B, Cukrowicz S, Kurleto-Kozioł Ż et al (2019) Studies of poly (Acrylic Acid-co-Maleic Acid) sodium salt intercalated montmorillonite. Arch Foundry Eng. https://doi.org/10.24425/afe.2019.129632
Joshi AC, Rufus AL, Velmurugan S (2018) Poly(acrylic acid-co-maleic acid), a polymer dispersant for the control of oxide deposition over nuclear steam generator surfaces. J Nucl Mater 498:421–429
Puigdomenech I (2013) Make equilibrium diagrams using sophisticated algorithms (MEDUSA), Inorganic Chemistry. Royal Institute of Technology, Stockholm Sweden, https://www.kemi.kth.se/medusa, https://sites.google.com/site/chemdiagr
Ali IM (2019) 152/154 Eu (III) ions sorption on stannic silicate granules: a radiotracer study. Chem Sci 8:180–194
Chen Z, He J, Chen L, Lu S (2016) Sorption and desorption properties of Eu (III) on attapulgite. J Radioanal Nucl Chem 307:1093–1104
Yao T, Xiao Y, Wu X et al (2016) RETRACTED: adsorption of Eu (III) on sulfonated graphene oxide. Combined macroscopic and modeling techniques. J Mol Liq 215:443–448
Jiang L, Yu H-T, Pei L, Hou X (2018) The effect of temperatures on the synergistic effect between a magnetic field and functionalized graphene oxide-carbon nanotube composite for Pb2+ and phenol adsorption. J Nanomater. https://doi.org/10.1155/2018/9167938
Hu Q, Zhang Z (2019) Application of Dubinin–Radushkevich isotherm model at the solid/solution interface: a theoretical analysis. J Mol Liq 277:646–648
Kumar KV, Porkodi K, Rocha F (2008) Isotherms and thermodynamics by linear and non-linear regression analysis for the sorption of methylene blue onto activated carbon: comparison of various error functions. J Hazard Mater 151:794–804
Indah S, Helard D, Binuwara A (2018) Studies on desorption and regeneration of natural pumice for iron removal from aqueous solution. Water Sci Technol 2017:509–515
Rizk HE, Attallah MF, Ali AMI (2017) Investigations on sorption performance of some radionuclides, heavy metals and lanthanides using mesoporous adsorbent material. J Radioanal Nucl Chem 314:2475–2487
Mahmoud MR, Rashad GM, Metwally E et al (2017) Adsorptive removal of 134Cs+, 60Co2+ and 152+ 154Eu3+ radionuclides from aqueous solutions using sepiolite: single and multi-component systems. Appl Clay Sci 141:72–80
Hamed MM, Attallah MF, Shehata FA (2012) Synthesis, characterization and sorption behavior of some radionuclides on zirconium tungstate ion exchanger. Arab J Nucl Sci Appl 45:37–50
Yao W, Wu Y, Pang H et al (2018) In-situ reduction synthesis of manganese dioxide@ polypyrrole core/shell nanomaterial for highly efficient enrichment of U (VI) and Eu (III). Sci China Chem 61:812–823
Negrea A, Gabor A, Davidescu CM et al (2018) Rare earth elements removal from water using natural polymers. Sci Rep 8:1–11
Dakroury GA, Abo-Zahra SF, Hassan HS et al (2020) Utilization of silica–chitosan nanocomposite for removal of 152+154Eu radionuclide from aqueous solutions. J Radioanal Nucl Chem 323:439–455. https://doi.org/10.1007/s10967-019-06951-6
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, Material preparation, data collection and analysis. All authors read and approved the final manuscript>
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research Involving Human and Animals Rights
Not applicable.
Ethical Approval
The authors confirm that the manuscript has been read and approved by all authors. The authors declare that this manuscript has not been published and not under consideration for publication elsewhere.
Consent to Participate
All of the authors consented to participate in the drafting of this manuscript.
Consent for Publication
All of the authors consent to publish this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moussa, S.I., Mekawy, Z.A., Dakroury, G.A. et al. Linear and Non Linear Recognition for the Sorption of 60Co and 152+154Eu Radionuclides onto Bio CuO Nanocomposite. J Polym Environ 31, 2148–2165 (2023). https://doi.org/10.1007/s10924-022-02735-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02735-4