Abstract
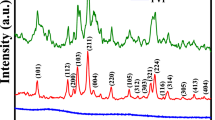
Various environmental concerns have emerged today as a result of the developing industrial revolution. The use of hazardous oxidizing agents and organic dyes is one of the biggest problems facing the textile industry today. This approach needs effective and affordable system to degrade such organic pollutants from the point sources. In this work, MnCr2O4 nanoparticle is synthesized using a green method for a crystal violet dye removal from wastewater. Three nanoparticle samples (CMO-A, CMO-B and CMO-C) were synthesized via green synthesis using bitter leaf extract and different concentration (0.3 M, 0.4 M, and 0.5 M) of KMnO4. The structural, morphological, optical properties, and photocatalytic activity of the synthesized MnCr2O4 spinel were studied. X-ray diffraction (XRD) was used to examine the crystal structure and the MnCr2O4 spinel exhibits cubic symmetry (Fd3m). The lattice parameters, crystallite size, microstrain, and dislocation density of the produced nanoparticles were also assessed using the diffraction data. The bandgap energy of the MnCr2O4 spinel decreased from 1.96 to 1.81 eV as the concentration of Mn ion increases from 0.3 to 0.5 M. The MnCr2O4 spinel showed good absorbance of light in visible range and also showed excellent photodegradation of crystal violet dye solution, with a record of 62.6%, 68.4%, and 74.9% degradation efficiency for CMO-A, CMO-B, and CMO-C, respectively, after 130 min of irradiation time.







Similar content being viewed by others
Data availability
The data that support the findings of this research work are available from the corresponding author upon reasonable request.
References
S. Aman, S. Gouadria, F.F. Alharbi et al., Novel Sr-based Al2O4 spinel material an environmental friendly electrode for supercapacitor application. Appl. Phys. A 129, 347 (2023). https://doi.org/10.1007/s00339-023-06591-4
M. Waheed, K. Jabbour, S. Houda, F.M.A. Alzahrani, K.M. Katubi, S. Riaz, M.S. Al-Buriahi, Fabrication of mesoporous Er doped ZnMnO3 nanoflake via sol gel approach for energy storage application. Ceram. Int. (2023). https://doi.org/10.1016/j.ceramint.2022.11.328
M. Abudllah, M. Al Huwayz, N. Alwadai et al., Facile fabrication of ternary CuO/CuS/ZnS for photodegradation of methylene blue. J. Korean Ceram. Soc. 60, 569–580 (2023). https://doi.org/10.1007/s43207-023-00287-4
O.S. Okwundu et al., Heavy metal sorption using thiolated oils of elaeis guineensis and glycine max. Metall. Mater. Eng. 26(3), 317–327 (2020). https://doi.org/10.30544/538.5
R. Yang et al., MnO2-Based materials for environmental applications. Adv. Mater. 33(9), 1–53 (2021). https://doi.org/10.1002/adma.202004862
O.S. Okwundu, C.O. Ugwuoke, A.C. Okaro, Recent trends in non-faradaic Supercapacitor Electrode materials. Metall. Mater. Eng. 25, 105–138 (2019). https://doi.org/10.30544/417
C.O. Ugwuoke, S. Ezugwu, S.L. Mammah, A.B.C. Ekwealor, F.I. Ezema, The Application of carbon and graphene quantum dots to emerging optoelectronic devices, in Electrode materials in energy storage and conversion. (Taylor & Francis, UK, 2021), pp.421–436. https://doi.org/10.1201/9781003145585-22
I.K. Konstantinou, T.A. Albanis, TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: a review. Appl. Catal. B Environ. 49(1), 1–14 (2004). https://doi.org/10.1016/j.apcatb.2003.11.010
V.S. Kirankumar, S. Sumathi, A review on photodegradation of organic pollutants using spinel oxide. Mater. Today Chem. 18, 100355 (2020)
S. Guo, H. Lin, K. Zheng, Z. Xiao, F. and, Li, Sulfanilic acid-modified P25 TiO2 nanoparticles with improved photocatalytic degradation on Congo Red under visible light. Dye. Pigment. no. 92, 1278–1284 (2012). https://doi.org/10.1016/j.dyepig.2011.09.004
A.M. Alenad, M.S. Waheed, S. Aman, N. Ahmad, A.R. Khan, R.Y. Khosa, T.A.M. Taha, Visible light driven Ni doped hematite for photocatalytic reduction of noxious methylene blue. Mater. Res. Bull. 165, 112306 (2023). https://doi.org/10.1016/j.materresbull.2023.112306
H.A. Alburaih, S. Aman, N. Ahmad, S.R. Ejaz, R.Y. Khosa, A.G. Abid, T.A. Taha, Synergistic photodegradation of methylene blue by Sm doped Fe2O3 photocatalyst under sunlight. Chin. J. Phys. 83, 637–649 (2023). https://doi.org/10.1016/j.cjph.2022.08.017
S. Das, A. Samanta, S. Jana, Light-assisted synthesis of hierarchical flower-Like MnO2 nanocomposites with solar light induced enhanced photocatalytic activity. ACS Sustain. Chem. Eng. (2017). https://doi.org/10.1021/acssuschemeng.7b02003
H. Zhang et al., Visualization of the formation and 3D porous structure of Ag doped MnO2 aerogel monoliths with high photocatalytic activity. ACS Sustain. Chem. Eng. (2016). https://doi.org/10.1021/acssuschemeng.6b00578
Y. Yang et al., UV-visible-infrared light-driven photothermocatalytic abatement of CO on Cu doped ramsdellite MnO2 nanosheets enhanced by a photoactivation effect. “Applied Catal. B Environ. (2017). https://doi.org/10.1016/j.apcatb.2017.11.017
C.O. Ugwuoke, P.C. Tagbo, O.S. Okwundu, C.A. Okaro, S. Ezugwu, F.I. Ezema, “Low-Temperature Processed Metal Oxides and Ion-Exchanging Surfaces as pH Sensor,” in Chemically deposited nanocrystalline metal oxide thin films, synthesis, characterization, and applications, 2021, pp. 821–861
S.O. Aisida et al., Biogenic synthesis of iron oxide nanorods using Moringa oleifera leaf extract for antibacterial applications. Appl. Nanosci. 10, 305–315 (2020)
N. Madubuonua et al., Biosynthesis of iron oxide nanoparticles via a composite of Psidium guavaja-Moringa oleifera and their antibacterial and photocatalytic study. J. Photochem. Photobiol B Biol. 199, 111601 (2019)
S.O. Aisida et al., Biogenic synthesis and antibacterial activity of controlled silver nanoparticles using an extract of Gongronema Latifolium. Mater. Chem. Phys. 237, 121859 (2019)
C.A. Okaro, O.S. Okwundu, P.C. Tagbo, C.O. Ugwuoke, S. Ezugwu, F.I. Ezema, Nanostructured Metal Oxide-Based Electrode Materials for Ultracapacitors, in Chemically Deposited Nanocrystalline Metal Oxide Thin Films. (Springer Nature, Germany, 2021), pp.561–599. https://doi.org/10.1007/978-3-030-68462-4_22
X. Hao et al., Mild aqueous synthesis of urchin-like MnOx hollow nanostructures and their properties for RhB degradation. Chem. Eng. J. (2013). https://doi.org/10.1016/j.cej.2013.06.007
R.T. Rasheed, H.S. Mansoor, R.R. Al-shaikhly, Synthesis and catalytic activity studies of α-MnO2 nanorodes, rutile TiO2 and its composite prepared by hydrothermal method. AIP Conference Proceedings 2213, 020122 (2020)
W.H. Kuan, C.Y. Chen, C.Y. Hu, Removal of methylene blue from water by γ-MnO 2. Water Sci. Technol. (2011). https://doi.org/10.2166/wst.2011.262
P. Cui, Y. Chen, G. Chen, Degradation of low concentration methyl orange in aqueous solution through sonophotocatalysis with simultaneous recovery of photocatalyst by ceramic membrane microfiltration. Industial Eng. Chem. Res (2011). https://doi.org/10.1021/ie100832q
C. Hou, B. Hu, J. Zhu, Photocatalytic degradation of methylene blue over TiO2 pretreated with varying concentrations of NaOH. Catalysts 8, 575 (2018). https://doi.org/10.3390/catal8120575
M. Rai, C. Dos-Santos, Nanotechnology Applied To Pharmaceutical Technology (Springer, Switzerland, 2017)
A. Fujishima, X. Zhang, D.A. Tryk, TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep (2008). https://doi.org/10.1016/j.surfrep.2008.10.001
C.O. Ugwuoke, S. Ezugwu, S.L. Mammah, A.B.C. Ekwealor, M. Suguyima, F.I. Ezema, “Physical Methods to Fabricate TiO2 QDs for Optoelectronics Applications,” in Electrode materials in energy storage and conversion, 2021, pp. 321–338. https://doi.org/10.1201/9781003145585-15
S. Chandrasekaran, C. Bowen, P. Zhang, Z. Li, Q. Yuan, X. Ren, L. Deng, Spinel photocatalysts for environmental remediation, hydrogen generation, CO2 reduction and photoelectrochemical water splitting. J. Mater. Chem. A 6, 11078–11104 (2018)
S.K. Rawal, R. Chandra, “Wettability And Optical Studies Of Films prepared from Power Variation Of Co-Sputtered Cr And Zr Targets By Sputtering,” in 2nd International Conference on Innovations in Automation and Mechatronics Engineering, ICIAME 2014, 2014, vol. 14, pp. 304–311. https://doi.org/10.1016/j.protcy.2014.08.040
A. Zekaik, H. Benhebal, B. Benrabah, Synthesis and characterization of Cu doped chromium oxide (Cr2O3) thin films. High Temp. Mater. Proc 38, 806–812 (2019)
J.T. Anandhi, S.L. Rayer, T. Chithambarathanu, F.T.I.R. “Synthesis, Studies, Optical properties of aluminium doped chromium oxide nanoparticles by microwave irradiation at different concentrations. Chem. Mater. Eng. 5(2), 43–54 (2017). https://doi.org/10.13189/cme.2017.050204
J. Sackey, R. Morad, A.K.H. Bashir, L. Kotsedi, C. Kaonga, M. Maaza, Bio-synthesised black a-Cr2O3 nanoparticles; experimental analysis and density function theory calculations. J. Alloys Compd. 850, 156671 (2021). https://doi.org/10.1016/j.jallcom.2020.156671
P. Mohanty, A.R.E. Prinsloo, B.P. Doyle, E. Carleschi, C.J. Sheppard, Structural and magnetic properties of (Co1–xNix)Cr2O4 (x = 0.5, 0.25) nanoparticles. AIP Adv. (2018). https://doi.org/10.1063/1.5006568
P. Mohanty, C.J. Sheppard, A.R.E. Prinsloo, Field induced magnetic properties of Ni doped CoCr2O4. AIP Adv. 2115, 030195 (2019)
H. Mohamed et al., Comprehensive study on morphological, structural and optical properties of Cr2O3 nanoparticle and its antibacterial activities. J. Mater. Sci. Mater. Electron. 0(0), 0 (2019). https://doi.org/10.1007/s10854-019-01125-2
N. Stüsser, M. Reehuis, M. Tovar, B. Klemke, A. Hoser, J. Hoffmann, Spin reorientation by Ni doping in Cu1 – xNixCr2O4 spinels with x = 0 and 0. 1, and evidence for canted magnetic cr order above the onset of a ferromagnetic cu. J. Magn. Magn. Mater. 506, 166683 (2020). https://doi.org/10.1016/j.jmmm.2020.166683
J. Singh, V. Verma, R. Kumar, R. Kumar, Influence of Mg2+-substitution on the optical band gap energy of Cr2 – xMgxO3 nanoparticles. Results Phys. 13, 102106 (2019). https://doi.org/10.1016/j.rinp.2019.02.042
N. Shinde, M. Lokhande, A. C., C.D. Lokhande, A green synthesis method for large area silver thin film containing nanoparticles. J. Photochem. Photobiol., B 136, 19–25 (2014). https://doi.org/10.1016/j.jphotobiol.2014.04.011
N.M. Shinde, A.C. Lokhande, J.S. Bagi, C.D. Lokhande, Biosynthesis of large area (30×30 cm2) silver thin films. Mater. Sci. Semicond. Process. vol. 22, 28–36 (2014). https://doi.org/10.1016/j.mssp.2014.01.011
T. Siyao, Y. Xiaocai, Y. Danni, W. Liping, L. Jiaqi, Z. Wanting, Study on degradation of diesel pollutants in seawater by composite photocatalyst MnO2/ZrO2. Water Sci. Technol. (2020). https://doi.org/10.2166/wst.2020.316
S. Zhou, Z. Du, X. Li, Y. Zhang, Degradation of methylene blue by natural manganese oxides: kinetics and transformation products. R Soc. Open. Sci. 6, 190351 (2019). https://doi.org/10.1098/rsos.190351
D. Mondal, S. Das, B. Kumar, D. Bhattacharya, Size engineered Cu-doped α -MnO2nanoparticles for exaggerated photocatalytic activity and energy storage application. Mater. Res. Bull (2019). https://doi.org/10.1016/j.materresbull.2019.03.023
M. Touqeer et al., New Co-MnO based Nanocrsytallite for photocatalysis studies driven by visible light. J. Taibah Univ. Sci. (2020). https://doi.org/10.1080/16583655.2020.1846966
A. Afzal, S. Atiq, M. Saleem, S.M. Ramay, S. Naseem, S.A. Siddiqi, Structural and magnetic phase transition of sol–gel-synthesized Cr2O3 and MnCr2O4 nanoparticles. J. Sol-Gel Sci. Technol. 80(1), 96–102 (2016). https://doi.org/10.1007/s10971-016-4066-4
E.T. Sibanda, A.R.E. Prinsloo, C.J. Sheppard, P. Mohanty, Size effect on magnetic properties of MnCr2O4 nanoparticles. J. Magn. Magn. Mater. 558, 169486 (2022). https://doi.org/10.1016/j.jmmm.2022.169486
N. Madubuonu et al., Bio-inspired iron oxide nanoparticles using Psidium guajava aqueous extract for antibacterial activity. Appl. Phys. A 126(72), 1–8 (2020)
S.O. Aisida et al., Biosynthesis of silver oxide nanoparticles using leave extract of Telfairia Occidentalis and its antibacterial activity. Mater. Today Proc 36, 208–213 (2021)
X. Zeng, B. Li, R. Liu, X. Li, T. Zhu, Investigation of promotion effect of Cu doped MnO2 catalysts on ketone-type VOCs degradation in a one-stage plasma-catalysis system. Chem. Eng. J. (2019). https://doi.org/10.1016/j.cej.2019.123362
M. Hamza et al., Catalytic removal of alizarin red using chromium manganese oxide nanorods: degradation and kinetic studies. Catalysts. 10, 1150 (2020). https://doi.org/10.3390/catal10101150
R.O. Ijeh, C.O. Ugwuoke, E.B. Ugwu, S.O. Aisida, F.I. Ezema, Structural, optical and magnetic properties of Cu-doped ZrO2 films synthesized by electrodeposition method. Ceram. Int. 48(4), 4686–4692 (2022). https://doi.org/10.1016/j.ceramint.2021.11.004
O.R. Alara, N.H. Abdurahman, C. Ishmael, N.A. Kabbashi, Extraction and characterization of bioactive compounds in Vernonia amygdalina leaf ethanolic extract comparing Soxhlet and microwave-assisted extraction techniques. J. Taibah Univ. Sci. 13(1), 414–422 (2019). https://doi.org/10.1080/16583655.2019.1582460
N. Bala et al., Green Synthesisof zinc oxide nanoparticles using Hibiscus subdariffa leaf etract: effect of temperature on synthesis, anti-bacterial activity and antidiabetic activity. RSC Adv. 5, 4993–5003 (2014)
R. Dumitru et al., “Synthesis, characterization of nanosized ZnCr2O4 and its photocatalytic performance in the degradation of humic acid from drinking water. Catalysts (2018). https://doi.org/10.3390/catal8050210
M.M. Abdullah, F.M. Rajab, S.M. Al-Abbas, “Structural and optical characterization of Cr2O3 nanostructures: Evaluation of its dielectric properties,” vol. 027121, no. October 2013, 2014, https://doi.org/10.1063/1.4867012
I.L. Ikhioya, C.O. Ugwuoke, R.M. Obodo, D.N. Okoli, M. Maaza, F.I. Ezema, Influence of precursor pH on Bi doped ZnSe material via electrochemical deposition technique. Appl. Surf. Adv. 9, 100232 (2022). https://doi.org/10.1016/j.apsadv.2022.100232
E. Jafarnejad, S. Khanahmadzadeh, F. Ghanbary, M. Enhessari, Synthesis, characterization and optical band gap of Pirochromite (MgCr2O4) nanoparticles by Stearic Acid Sol-Gel Method. Curr. Chem. Lett. 5, 173–180 (2016). https://doi.org/10.5267/j.ccl.2016.7.001
M. Tian et al., Facile synthesis of rod-like TiO2-based composite loaded with g-C3N4 for efficient removal of high-chroma organic pollutants based on adsorption-photocatalysis mechanism. Inorg. Chem. Commun. 141, 109517 (2022). https://doi.org/10.1016/j.inoche.2022.109517
I. Okeke, K. Agwu, A. Ubachukwu, M. Maaza, F. Ezema, Impact of Cu doping on ZnO nanoparticles phyto-chemically synthesized for improved antibacterial and photocatalytic activities. J. Nanopart. Res. 22, 272 (2020)
A. Kumar Paul, G. Madras, S. Natarajan, Adsorption-desorption and photocatalytic properties of inorganic-organic hybrid cadmium thiosulfate compounds. Phys. Chem. Chem. Phys. 11(47), 11285–11296 (2009). https://doi.org/10.1039/b913407g
E. Gao et al., Insights on the mechanism of enhanced selective catalytic reduction of NO with NH3 over Zr-doped MnCr2O4: A combination of in situ DRIFTS and DFT. Chem. Eng. J (2019). https://doi.org/10.1016/j.cej.2019.123956
S. Chiam, S. Pung, F. Yeoh, Recent developments in MnO2 -based photocatalysts for organic dye removal: a review. Environ. Sci. Pollut Res. (2020). https://doi.org/10.1007/s11356-019-07568-8
M.A. Rauf, S.S. Ashraf, Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem. Eng. J. 151, 1–3 (2009). https://doi.org/10.1016/j.cej.2009.02.026
C. Boon, L. Yong, A. Wahab, A review of ZnO nanoparticles as solar photocatalysts: synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 81, 536–551 (2018). https://doi.org/10.1016/j.rser.2017.08.020
I.L. Ikhioya, C.O. Ugwuoke, D.N. Okoli, A.J. Ekpunobi, M. Maaza, F.I. Ezema, Effect of cobalt on the photovoltaic properties of zinc selenide thin film deposited on fluorine-doped tin oxide (FTO) via electrochemical deposition technique. Curr. Res. Green. Sustain. Chem. (2022). https://doi.org/10.1016/j.cogsc.2022.100630
W. Yuan, X. Liu, L. Li, Synthesis, characterization and photocatalytic activity of cubic-like CuCr2O4 for dye degradation under visible light irradiation. Appl. Surf. Sci (2014). https://doi.org/10.1016/J.APSUSC.2014.07.158
S. Taghavi Fardood et al., Facile green synthesis, characterization and visible light photocatalytic activity of MgFe2O4@CoCr2O4 magnetic nanocomposite. J. Photochem. Photobiol A Chem. 423, 3–5 (2022). https://doi.org/10.1016/j.jphotochem.2021.113621
P. de Cubas, A.W. Semkiw, F.C. Monteiro, P. Los Weinert, J.F.H.L. Monteiro, S.T. Fujiwara, Synthesis of CuCr2O4 by self-combustion method and photocatalytic activity in the degradation of Azo Dye with visible light. J. Photochem. Photobiol. A Chem (2019). https://doi.org/10.1016/j.jphotochem.2020.112797
F. Beshkar, O. Amiri, M. Salavati-Niasari, F. Beshkar, Novel dendrite-like CuCr2O4 photocatalyst prepared by a simple route in order to remove of azo dye in textile and dyeing wastewater. J. Mater. Sci. Mater. Electron. 26(10), 8182–8192 (2015). https://doi.org/10.1007/s10854-015-3479-0
R. Bajaj, M. Sharma, D. Bahadur, Visible light-driven novel nanocomposite (BiVO4/CuCr2O4) for efficient degradation of organic dye. Dalt Trans. 42, 6736–6744 (2013). https://doi.org/10.1039/c2dt32753h
Funding
FIE acknowledges the grant by TETFUND under contract number.
TETFUND/DR&D/CE/UNI/NSUKKA/RP/VOL.I and also acknowledges the support received from the Africa Centre of Excellence for Sustainable Power and Energy Development (ACE-SPED), University of Nigeria, Nsukka. We thank Engr. Emeka Okwuosa for the generous sponsorship of April 2014, July 2016, July 2018, and July 2021 conferences/workshops on applications of nanotechnology to energy, health &. Environment and for providing some research facilities.
Author information
Authors and Affiliations
Contributions
COU carried out the experimental work and drafted the manuscript. AGT revised the manuscript. ROI and HEN analyze experimental result. EIU, SM and AA read the approved submitted manuscript. SE, FIE supervised the project. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ugwuoke, C.O., Temam, A.G., Ijeh, R.O. et al. Green synthesis of MnCr2O4 nanoparticles using Vernonia amygdalina (bitter leaf) for photocatalytic crystal violet dye degradation. J Mater Sci: Mater Electron 34, 2111 (2023). https://doi.org/10.1007/s10854-023-11499-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-11499-z