Abstract
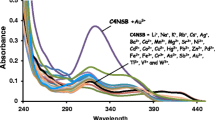
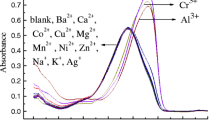
We have developed a chemosensor using calix[4]arene, which features a thiosemicarbazone binding/sensing unit and a naphthalene chromogenic group. Our objective was to understand the intricate binding affinity of these chemosensors towards a diverse range of anions and cations using UV–Visible, HNMR and IR spectroscopic techniques. We showed that this chemosensor can form complexes with Ag(I) or Cu (II) and to detect CN− or F− ions by deprotonation of thiosemicarbazone. To understand the behavior of these interactions, our analysis provides information on the interaction patterns between the receptors and the ions. The sulfur and imine nitrogen on the thiosemicarbazone substituent are vital sites of engagement for cation ions, as evidenced by the observed changes in IR. HNMR studies for interaction with anions indicate the involvement of the thiosemicarbazone hydrogens. Interactions taking place during complex formation with cations lead to changes in the color of the solution or solid complex, easy to follow by UV–Vis spectrocopy. Our study improves the understanding of molecular recognition of this chemosensor, highlighting its potential for ion- selective detection.







Similar content being viewed by others
Data availability
Freely available.
References
Neri, P., Sessler, J.L., Wang, M.-X.: Calixarenes and Beyond. Springer, Cham (2016)
Vicens, J., Harrowfield, J., Baklouti, L.: Calixarenes in the Nanoworld. Springer, Dordrecht (2007)
Español, E.S., Villamil, M.M.: Calixarenes: generalities and their role in improving the solubility, biocompatibility, stability, bioavailability, detection, and transport of biomolecules. Biomolecules 9, 90 (2019)
Atwood, J.L., Gokel, G.W., Barbour, L.J.: Comprehensive Supramolecular Chemistry II, 2nd edn. Elsevier, Oxford (2017)
Durmaz, M., Halay, E., Bozkurt, S.: Recent applications of chiral calixarenes in asymmetric catalysis. Beilstein J. Org. Chem. 14, 1389–1412 (2018)
Baldini, L., Sansone, F., Casnati, A., Ungaro, R.: Supramolecular Chemistry: From Molecules to Nanomaterials. Wiley, Chichester (2012)
Hosseinzadeh, R., Nemati, M., Zadmard, R., Mohadjerani, M.: Amidofluorene-appended lower rim 1,3-diconjugate of calix[4]arene: synthesis, characterization, and highly selective sensor for Cu2+. Beilstein J. Org. Chem. 12, 1749–1757 (2016)
Mutihac, L., Lee, J.H., Kim, J.S., Vicens, J.: Recognition of amino acids by functionalized calixarenes. Chem. Soc. Rev. 40, 2777–2796 (2011)
Helttunen, K., Shahgaldian, P.: Self-assembly of amphiphilic calixarenes and resorcinarenes in water. New J. Chem. 34, 2704–2714 (2010)
De Rosa, M., La Manna, P., Soriente, A., Gaeta, C., Talotta, C., Neri, P.: Exploiting the hydrophobicity of calixarene macrocycles for catalysis under “on-water” conditions. RSC Adv. 6, 91846–91851 (2016)
Simões, J.B., da Silva, D.L., de Fátima, A., Fernandes, S.A.: Calix[n]arenes in action: useful host-guest catalysis in organic chemistry. Curr. Org. Chem. 16, 949–971 (2012)
Zhou, Y., Li, H., Yang, Y.-W.: Controlled drug delivery systems based on calixarenes. Chin. Chem. Lett. 26, 825–828 (2015)
Wu, Y., Feng, J., Hu, G., Zhang, E., Yu, H.-H.: Colorimetric sensors for chemical and biological sensing applications. Sensors 23(5), 2749 (2023)
Liu, B., Zhuang, J., Wei, G.: Recent advances in the design of colorimetric sensors for environmental monitoring. Environ. Sci. Nano 7, 2195–2213 (2020)
Ferreira, J.F., Bagatin, I.A.: A Cr(VI) selective probe based on a quinoline-amide calix[4]arene. Spectrochim. Acta A 189, 44–50 (2018)
Quiroga-Campano, C., Gómez-Machuca, H., Moris, S., Jara, P., De la Fuente, J.R., Pessoa-Mahana, H., Jullian, C., Saitz, C.: Synthesis of bifunctional receptor for fluoride and cadmium based on calix[4]arene with thiourea moieties. J. Mol. Struct. 1141, 133–141 (2017)
Athar, M., Lone, M.Y., Jha, P.C.: Recognition of anions using urea and thiourea substituted calixarenes: a density functional theory study of non-covalent interactions. Chem. Phys. 501, 68–77 (2018)
Quiroga-Campano, C., Gómez-Machuca, H., Moris, S., Pessoa-Mahana, H., Jullian, C., Saitz, C.: Synthesis of calix[4]arenes bearing thiosemicarbazone moieties with naphthalene groups: highly selective turn off/on fluorescent sensor for Cu(II) recognition. J. Mol. Struct. 1225, 129125 (2021)
Bhowmick, R., Alam, R., Mistri, T., Das, K.K., Katarkar, A., Chaudhuri, K., Ali, M.: A thiosemicarbazone based chemo and fluorogenic sensor for Zn2+ with CHEF and ESIPT behaviour: computational studies and cell imaging application. RSC Adv. 6, 11388–11399 (2016)
Tang, L., Huang, Z., Zheng, Z., Zhong, K., Bian, Y.: A New Thiosemicarbazone-based fluorescence “Turn-on” Sensor for Zn2+ recognition with a large stokes shift and its application in live cell imaging. J. Fluoresc. 26, 1535–1540 (2016)
Tang, L., Zhou, P., Zhang, Q., Huang, Z., Zhao, J., Cai, M.: A simple quinoline derivatized thiosemicarbazone as a colorimetic and fluorescent sensor for relay recognition of Cu2+ and sulfide in aqueous solution. Inorg. Chem. Commun. 36, 100–104 (2013)
Kim, J.S., Kim, H.J., Kim, H.M., Kim, S.H., Lee, J.W., Kim, S.K., Cho, B.R.: Metal ion sensing novel calix[4]crown Fluoroionophore with a two-photon absorption property. J. Org. Chem. 71, 8016–8022 (2006)
Wang, X.M., Yan, H., Feng, X.L., Chen, Y.: 1-Pyrenecarboxaldehyde thiosemicarbazone: a novel fluorescent molecular sensor towards mercury (II) ion. Chin. Chem. Lett. 21, 1124–1128 (2010)
Bhatti, A.A., Oguz, M., Memon, S., Yilmaz, M.: Dual fluorescence response of newly synthesized naphthalene appended calix[4]arene derivative towards Cu2+ and I−. J. Fluoresc. 27, 263–270 (2017)
Senthilvelan, A., Ho, I.-T., Chang, K.-C., Lee, G.-H., Liu, Y.-H., Chung, W.-S.: Cooperative recognition of a copper cation, and anion by a calix[4]arene substituted at the lower rim by a β-amino-α, β-unsaturated ketone. Chem. Eur. J. 15, 6152–6160 (2009)
Cao, X., Luo, L., Zhang, F., Miao, F., Tian, D., Li, H.: Synthesis of a deep cavity calix[4]arene by fourfold Sonogashira cross-coupling reaction and selective fluorescent recognition toward p-nitrophenol. Tetrahedron Lett. 55, 2029–2032 (2014)
Chawla, H.M., Goel, P., Munjal, P.: A new metallo-supramolecular sensor for recognition of sulfide ions. Tetrahedron Lett. 56, 682–685 (2015)
Liu, J.-M., Bu, J.-H., Zheng, Q.-Y., Chen, C.-F., Huang, Z.-T.: Highly selective fluorescent sensing of Pb2+ by a new calix[4]arene derivative. Tetrahedron Lett. 47, 1905–1908 (2006)
Memon, S., Bhatti, A.A., Bhatti, A.A., Ocak, U., Ocak, M.: Calix [4]arene based highly efficient fluorescent sensor for Au3+ and I−. J. Fluoresc. 25, 1507–1515 (2015)
Bok, J.H., Kim, H.J., Lee, J.W., Kim, S.K., Choi, J.K., Vicens, J., Kim, J.S.: Selective metal detection in an unsymmetrical 1,3-alternate calix[4]biscrown chemosensor. Tetrahedron Lett. 47, 1237–1240 (2006)
Liu, Y., Li, Z., Guo, D.-S.: Conformational transition effects of anion recognition by calix[4]arene derivatives. Supramol. Chem. 21, 465–472 (2009)
Liu, J.-M., Zheng, Q.-Y., Yang, J.-L., Chen, C.-F., Huang, Z.-T.: A new fluorescent chemosensor for Fe3+ and Cu2+ based on calix[4]arene. Tetrahedron Lett. 43, 9209–9212 (2002)
Jäschke, A., Kischel, M., Börner, M., Carlotto, S., Armelao, L., Luneau, D., Kersting, B.: Synthesis, structure and magnetic properties of some copper(II) complexes supported by pendant calix[4]arene ligands. Eur. J. Inorg. Chem. (2024). https://doi.org/10.1002/ejic.202300564
Quiroga-Campano, C., Gómez-Machuca, H., De la Fuente, J.R., Pessoa-Mahana, H., Jullian, C., Saitz, C.: Study by fluorescence of calix[4]arenes bearing heterocycles with divalent metals: highly selective detection of Pb2+. J. Incl. Phenom. Macrocycl. Chem. 79, 161–169 (2014)
Gómez-Machuca, H., Quiroga-Campano, C., De la Fuente, J.R., Pessoa-Mahana, H., Dobado, J.A., Jullian, C., Saitz, C.: Study by fluorescence of calix[4]arenes bearing heterocycles with anions: highly selective detection of iodide. J. Incl. Phenom. Macrocycl. Chem. 80, 369–375 (2014)
Gómez-Machuca, H., Quiroga-Campano, C., Jullian, C., Saitz, C.: Bifunctional receptor based on calix[4]arene with chromone groups as an efficient colorimetric sensor for Co2+, Cu2+, CN− and F−. ChemistrySelect (2022). https://doi.org/10.1002/slct.202202581
Chawla, H.M., Pant, N., Kumar, S., Mrig, S., Srivastava, B., Kumar, N., Black, DSt.C.: Synthesis and evaluation of novel tetrapropoxycalix[4]arene enones and cinnamates for protection from ultraviolet radiation. J. Photochem. Photobiol. B. 105, 25–33 (2011)
Kelderman, E., Verboom, W., Engbersen, J.F.J., Reinhoudt, D.N., Heesink, G.J.T., van Hulst, N.F., Derhaeg, L., Persoons, A.: Nitrocalix[4]arenes as molecules for second-order nonlinear optics. Angew. Chem. Int. Ed. 31(8), 1075–1077 (1992)
van Wageningen, A.M.A., Snip, E., Verboom, W., Reinhoudt, D.N., Boerrigter, H.: Synthesis and application of iso(thio)cyanate-functionalized calix[4]arenes. Liebigs Ann. 1997(11), 2235–2245 (1997)
Job, P.: Formation and stability of inorganic complexes in solution. Ann. Chim. 9(22), 113–203 (1928)
Deng, T., Chen, J., Yu, H., Yang, P., Jian, Y., Li, G., Zhou, X., Shen, H., Gui, J.: Adenosine triphosphate-selective fluorescent turn-on response of a novel thiazole orange derivative via their cooperative co-assembly. Sens. Actuators B Chem. 209, 735–743 (2015)
Zhang, H., Li, H., Sun, S., Tan, L., Shen, H., Lin, B., Yang, P.: N-embedded cubarene: a quadrangular member of the macrocycle family. Org. Lett. 25, 2078–2083 (2023)
Qazi, M., Ocak, Ü., Ocak, M., Memon, S., Solangi, I.: Bifunctional calix[4]arene sensor for Pb(II) and Cr2O72− ions. J. Fluoresc. 23(3), 575–590 (2013)
Qazi, M.A., Qureshi, I., Memon, S.: A highly copper selective chromogenic calix [4]arene derivative. New J. Chem. 34(11), 2579–2586 (2010)
Samanta, S., Manna, U., Ray, T., Das, G.: An aggregation-induced emission (AIE) active probe for multiple targets: a fluorescent sensor for Zn2+ and Al3+ & a colorimetric sensor for Cu2+ and F-. Dalton Trans. 44(43), 18902–18910 (2015)
Santos-Figueroa, L.E., Moragues, M.E., Raposo, M.M.M., Batista, R.M.F., Costa, S.P.G., Ferreira, R.C.M., Sancenon, F., Martinez-Manez, R., Ros-Lis, J.V., Soto, J.: Synthesis, and evaluation of thiosemicarbazones functionalized with furyl moieties as new chemosensors for anion recognition. Org. Biomol. Chem. 10(36), 7418–7428 (2012)
Shang, X., Yang, Z., Fu, J., Zhao, P., Xu, X.: The synthesis and anion recognition property of symmetrical chemosensors involving thiourea groups: theory and experiments. Sensors. 15(11), 28166 (2015)
Wells, M. A., Jelinska, C., Hosszu, L.L.P., Craven, C.J., Clarke, A. R., Collinge, J., Waltho, J. P., Jackson, G. S. Multiple forms of copper (II) co-ordination occur throughout the disordered N-terminal region of the prion protein at pH 7.4. Biochem. J. 400(3), 501–510 (2006)
Sarkar, A., Bhattacharyya, S., Mukherjee, A.: Colorimetric detection of fluoride ions by anthraimidazoledione based sensors in the presence of Cu(ii) ions. Dalton Trans. 45(3), 1166–1175 (2016)
Orojloo, M., Amani, S.: A highly selective chemosensor for naked-eye detection of fluoride and aluminium ions based on a new schiff base derivative. Aust. J. Chem. 69(8), 911–918 (2016)
Gao, Y., Shu, J., Zhang, C., Zhang, X., Chen, H., Yao, K.: A fluorescence on-off sensor for Cu2+ and its resultant complex as an off-on sensor for Cr3+ in aqueous media. RSC Adv. 5(91), 74629–74637 (2015)
Acknowledgements
Our thanks are to FONDECYT Grants: 1151310 and to CONICYT (Beca Doctorado Nacional 21130501 and 21130502) for support. Dr. Horacio Gomez-Machuca thanks are to ANID for postdoctoral project FONDECYT grants: 3210140. We would like to dedicate this work to the memory of Dr. Carolina Jullian Matthaei.
Funding
None.
Author information
Authors and Affiliations
Contributions
H. G.-M. wrote the main manuscript text and carried out the methodology and investigation. H. G.-M prepared figures, tables and suplementary information. C. S. and H. G.-M. were involved in conceptualization, C. Q.-C. supervised the experiments in spectroscopic techniques, especially NMR. H. P.-M., C. S., and C. Q.-C. reviewed, edited, and supervised the manuscript. C. S. was in charge of project management and was the main source of fund acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Provided upon request.
Informed consent
Provided upon request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gómez-Machuca, H., Quiroga-Campano, C., Pessoa-Mahana, H. et al. Ion sensing with a calix[4]arene bifunctional receptor with thiosemicarbazone moieties and naphthalene chromophore. J Incl Phenom Macrocycl Chem 104, 161–170 (2024). https://doi.org/10.1007/s10847-024-01239-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-024-01239-z